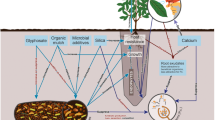
Abstract
The ability of organic, microbial or mineral-based soil additives to suppress root rot caused by Phytophthora cinnamomi was compared with disease reduction resulting from the use of the fungicides phosphite or metalaxyl. The effect of glyphosate (commonly used for weed control) on plant health was also examined. Avocado plants were grown in a glasshouse in pots with soils collected under mature commercial avocado trees. To simulate ‘orchard soil’ conditions, chicken manure, wood mulch, and mulch from beneath 20-year-old trees in an avocado orchard were added to the pots. The effect of P. cinnamomi on plant growth and visible root damage was assessed using plants grown under these ‘orchard’ soil conditions, and treatments with further additives (two microbial soil conditioners, one organic and two mineral-based mulches). In two of three experiments, infestation of soil with P. cinnamomi resulted in no significant reduction on fine root dry weight for plants sprayed with phosphite, or treated with a silicate-based mulch. However, when a combination of these two treatments gave no additive effect. In one experiment, a microbial-based conditioner was also beneficial. Phosphite was preferable to metalaxyl as a chemical treatment, as the latter reduced shoot dry weight by 25% and fine root dry weight by 30% of that in non-inoculated plants. Glyphosate treatment of wheat seedlings growing in the pots with the avocados also reduced shoot dry weight (20%) and fine root dry weight (20%) of non-inoculated avocados. These observations need to be confirmed under field conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, Phytophthora root rot caused by the oomycete Phytophthora cinnamomi is the most serious disease of Persea americana (avocado). This pathogen attacks the feeder roots of avocado trees, and, if left untreated, can result in the death of trees and high economic losses (Reeksting et al. 2016; Ramirez-Gil et al. 2017). Management strategies include preventing pathogen spread by controlling the movement of people, livestock and vehicles, the use of chemicals, soil additives, and the development of P. cinnamomi-tolerant cultivars or rootstocks (D’Souza et al. 2005). The most robust and environmentally benign methods are integrated management approaches (Pegg and Whiley 1987; Wolstenholme and Sheard 2010) including the selection of tolerant varieties, application of organic fertilizers and mulches, the addition of cultured microbial antagonists, inorganic nutrition and liming, irrigation management and fungicide application (Wolstenholme and Sheard 2010; Pegg 2010). Phosphite is the most commonly used chemical for control of Phytophthora root rot caused by P. cinnamomi in agricultural, forest, horticultural, and natural ecosystems worldwide (Pegg et al. 1987; Hardy et al. 2001; Shearer and Fairman 2007; King et al. 2010; Scott et al. 2015; Ramirez-Gil et al. 2017; Masikane et al. 2020). It is an effective chemical for control of this disease as it stimulates the plant’s defence mechanisms and may also impact directly on the pathogen (Guest and Bompeix 1990). However, prolonged use of phosphite increases the potential for developing phosphite resistance in the pathogen (Dobrowolski et al. 2008). Moreover, agrochemicals may negatively affect the environment through detrimental effects on beneficial soil microbes and disruption of soil ecology (Hardy et al. 2001; Gill and Garg 2014; Xi et al. 2020).
Commercial microbial soil additives/conditioners comprising proprietary mixtures of microbial species are being developed, with the global interest in these products growing at 10% each year (Berg 2009; Song et al. 2012). The most promising biocontrol beneficial microbial organisms, effective against several foliar and soilborne diseases, are the Proteobacteria (e.g. Bacillus spp.), Actinobacteria (e.g. Streptomyces spp.), the fluorescent Pseudomonads (e.g. Firmicutes), and fungi (e.g. non-pathogenic Fusarium spp. and Trichoderma spp.) (Raaijmakers et al. 2009; Bhattacharjee and Dey 2014).
Mineral soil conditioners, particularly silicate-based mulches, have also shown encouraging outcomes for crop disease control and crop growth (Pozza et al. 2015; Tubana et al. 2016). They have been effective in controlling plant disease in many crops, including powdery mildew of wheat, barley, bitter gourd and cucumber; macrospora leaf spot of maize, anthracnose of sorghum, Phytophthora root rot in soyabean (Rasoolizadeh et al. 2018, 2020; Rodrigues et al. 2015; Debona et al. 2017; Datnoff et al. 2007; Samuels et al. 1991; Menzies et al. 1992; Belanger et al. 2003; Dann and Le 2017) showed that some silicate-based products effectively controlled Calonectria ilicicola in potted avocado plants and improved the health of diseased trees in two of three field trials. Silica impacts a wide range of plant metabolic processes and affects the soil microbiota, though less is known about the latter (Rajput et al. 2021).
Little information is currently available on the comparative effectiveness of commercially available, microbial soil conditioners for management of Phytophthora in avocado. This study investigated the effectiveness of spraying with phosphite, compared with using organic mulches, microbial commercial soil conditioners, or silicate-based mineral mulches to control root damage in avocado caused by P. cinnamomi. It also investigated whether glyphosate, a commonly used herbicide, exacerbates Phytophthora root damage.
Materials and methods
Plant material
Avocados (cv. Reed) were grown from seed, initially in potting mix (Richgro Garden Products, Australia). For the first experiment, nine-month-old plants were grown in 15 L free-draining polybags; in experiment 2, the plants were 6 months old, and 7 L polybags were used; in experiment 3, plants were 18 months old and 15 L polybags were used.
Soil and growing conditions
In experiment 1, Channybearup yellow-brown clay loam soil was sourced from an avocado orchard in Manjimup, Western Australia (WA). It is moderately well-draining and has a high-water holding capacity. It was used in a 1:4 ratio of soil: coarse perlite (Perlite and Vermiculite Company, Myaree, WA). A Spearwood Sand was collected from an avocado orchard in Carabooda, WA, for the second and third experiments. It was moderately well-drained with good porosity and was mixed with river sand in experiment 2 and with potting mix (Richgro Garden Products) in experiment 3 in a ratio of 1:1. Both soils are conducive to Phytophthora as both native vegetation and avocado orchards on these soils suffer from Phytophthora dieback. However, both soils were determined to be free of P. cinnamomi through standard baiting of the soil (Aghighi et al. 2016; Simamora et al. 2018). Two inoculation tubes (25 mm dia x 20 cm long) were placed in each bag when plants were potted to minimise the chance of damaging roots during the insertion of the inoculation plugs. Plants were watered daily to container capacity. Plants were placed on benches in a completely randomized design in an evaporatively cooled glasshouse maintained at 25–27 °C. All had weekly applications of liquid ‘Thrive for Fruit’ (Yates, Australia), (4 g/L, 300-400ml/pot) for experiments 1 and 3 and half of this amount in experiment 2. Eco oil (5ml/L) (Organic crop protectant Pty. Ltd., NSW, Australia) was sprayed on foliage and the soil surface when required for insect control.
Treatments
Across the three experiments, 12 treatments were explored. Experiment 1 included treatments 1–11; experiment 2 treatments 1, 3, 4, 7, 8 and 10; experiment 3 treatments 1, 3, 7, 10 and 12 (Table 1).
Treatment 1 had no additives.
Treatments 2–12 had well-decomposed chicken manure applied monthly and jarrah (Eucalyptus marginata) wood mulch placed on the surface when transplanting the seedlings. In experiments 1 and 3, 50 g/pot of manure and 200 g/pot of mulch was used, and in experiment 2, half these amounts were applied.
Treatments 3–12 had avocado orchard mulch added at the time of transplanting (150 g/pot for experiments 1 and 3, and 75 g/pot for experiment 2). Avocado orchard mulch was collected from a 20-year-old orchard (Delroy Orchards, Pemberton, WA) in which P. cinnamomi is controlled by phosphite spray, application of decomposed jarrah wood mulch, chicken manure, and fertigation with standard fertilisers and fish emulsion. The jarrah wood mulch and the avocado orchard mulch were determined to be free of P. cinnamomi through baiting.
Treatments 4–8 each had a microbial or mineral-based soil additive applied as indicated in Table 1, and with the amounts adjusted for the two sizes of polybags.
Treatment 9 tested the effect of killing ‘weeds’ in the pots- (wheat seedlings sown at the time of transplanting the avocado plants). The stems of the avocado plants were protected from the spray by aluminium foil.
Treatments 10 and 11 compared the two fungicides: phosphite, which was used as a spray, and metalaxyl which was used as a drench as shown in Table 1.
Treatment 12 examined the effect of treating plants with both a silicate-based mineral mulch and spraying with phosphite.
Inoculum production and inoculation
Branches of live tagasaste (Chamaecytisus palmensis) (1–2 cm dia.) were cut into approximately 2 cm pieces. Two hundred plugs were placed in each of ten 2 L conical flasks. The plugs were soaked in distilled water overnight, then rinsed. Distilled water (approx. 50–70 mL, sufficient to cover the bottom of the flask) was added. The flask was plugged with a non-absorbent cotton plug and autoclaved at 121 °C for 30 min, then allowed to cool to room temperature, and the process was repeated after 24 h. Two Petri plates of P. cinnamomi (isolate MP 94 − 48 from the Centre for Phytophthora Science and Management culture collection, Genbank Accession number for ITS gene region is JX113294), cultured on a V8 (vegetable juice) agar medium for 7 days at 25 °C in the dark, were cut into 1 cm squares and aseptically added to the conical flasks. The flasks were shaken to evenly distribute the agar plugs and incubated at 25 °C in the dark. Flasks were incubated for 4 weeks and shaken weekly to obtain uniform colonisation of the inoculum plugs.
All treatments consisted of ten non-inoculated controls and ten plants inoculated with P. cinnamomi. Plants were inoculated by inserting two P. cinnamomi colonised plugs to a depth of 10 cm into each of the two holes created after removing the inoculation tubes. Non-colonised plugs were inserted to the same depth in the holes for the control plants in each treatment. Three weeks after inoculation, all the plant containers were flooded for 24 h to stimulate sporangia production and the release of zoospores.
Harvesting
Twelve weeks after inoculation with P. cinnamomi, the plants were harvested. In each experiment, shoots were excised and dried in an oven at 60 °C to constant dry weight. Roots were washed free of soil, and the visual damage caused by the pathogen was scored using the rankings 1 = healthy roots, 2 = 1–25% damage, 3 = 26–50% damage, 4 = 51–75% damage, and 5 = 76–100% damage (Fig. 1). Fine roots were separated, and the dry weight of fine and coarse roots was recorded. Total root dry weights were calculated by adding fine and coarse root dry weights. Re-isolation of P. cinnamomi was undertaken by plating a damaged root from each inoculated treatment onto NARH medium: a cornmeal agar medium with nystatin 22.72 ppm, ampicillin 100 ppm, rifampicin 10 ppm, hymexazol 50 ppm but without the PCNB in the original formulation of Huberli et al. (2000), to confirm that the inoculation caused the disease.
Data analysis
To check the effect of the different treatments on the control of damage caused by P. cinnamomi, data for shoot dry weight, total root dry weight and fine root dry weight were analysed using a linear model with terms for treatment, with and without P. cinnamomi, and their interactions. Homoscedasticity was assessed by plotting residuals vs. fitted values, and normality of residuals was assessed using q-q plots. The least significant difference test was used to assess the significance between all pairs of treatments, with/without P. cinnamomi combinations. The qualitative data on root damage for each treatment was assessed separately using Chi-square tests, and function emmeans were used to access the level of significance between the treatments. All the data analyses were conducted, and bar graphs were generated in R software (version 4.1.1).
Results
In all three experiments, all plants were alive at the time of harvest, and there was no evidence of wilt associated with the presence of Phytophthora. Shoot dry weights showed little response to soil treatments, and only plants sprayed with phosphite showed a significant drop in shoot weight after infection with P. cinnamomi(Supp Fig. S1 c-c). Phytophthora cinnamomi was reisolated from diseased roots of plants sampled from all inoculated treatments.
Effect of the addition of manure and organic mulches
For plants given no manure or mulches Phytophthora significantly reduced total root weight in in three experiments (Fig. 2a, b, c), and fine root weight in two experiments (Fig. 3a, c). When pots were provided with a combination of chicken manure, wood mulch, and avocado orchard mulch (T3) Phytophthora infestation significantly reduced fine root weight in all experiments (Fig. 3a, b, c) and total root weight in two experiments (Fig. 2b, c). Root damage was significantly higher in inoculated plants in all experiments for plants with or without the organic soil amendments (Fig. 4a, b, c). The comparative growth of non-inoculated plants with or without the organic amendments was not significantly different in any experiment, which was also observed for inoculated plants.
Total root dry weight. (a) Experiment 1: Soil treatments (T1-T12, see Table 1). (b) Experiment 2: six Soil treatments (T1, T3, T4, T7, T8, T10). (c) Experiment 3: Soil treatments (T1, T3, T7, T10 and T12). Non-inoculated avocado plants (grey bars) and those inoculated with Phytophthora cinnamomi (black bars). Vertical lines indicate SE and letters that are different indicate significant (p < 0.05) differences between treatments. * indicates a significant (p < 0.05) difference between non-inoculated and inoculated plants in the same treatment
Fine root dry weight. (a) Experiment 1: Soil treatments (T1-T12, see Table 1). (b) Experiment 2: Soil treatments (T1, T3, T4, T7, T8, T10). (c) Experiment 3: Soil treatments (T1, T3, T7, T10 and T12). Non-inoculated avocado plants (grey bars) and those inoculated with Phytophthora cinnamomi (black bars). Vertical lines indicate SE and letters that are different indicate significant (p < 0.05) differences between treatments. * indicates a significant (p < 0.05) difference between non-inoculated and inoculated plants in the same treatment
Root damage. (a) Experiment 1: Soil treatments (T1-T12, see Table 1). (b) Experiment 2: Soil treatments (T1, T3, T4, T7, T8, T10). (c) Experiment 3: Soil treatments (T1, T3, T7, T10 and T12). Non-infested avocado plants (grey bars) and those infested with Phytophthora cinnamomi (black bars). Vertical lines indicate SE and letters that are different indicate significant (p < 0.05) differences between treatments. * indicates a significant (p < 0.05). For root damage score see text
Effect of the addition of commercial microbial and organic soil conditioners
In the first experiment, the application of microbial or organic soil conditioners (T4, T5, T6) resulted in no reduction of total root weight after inoculation with Phytophthora (Fig. 2a). However, there was a significant drop in fine root dry weight, and an increase in root damage in all treatments (Figs. 3a and 4a). When the treatment with microbial conditioner 1 (T4) was repeated in experiment 2, although inoculation resulted in a significant drop in total or fine root weights, the growth of the non-inoculated plants was higher than for comparable plants in T3 (Figs. 2b and 3b).
Effect of silicate-based mineral mulches
Application of mineral mulches (T7, T8) suppressed Phytophthora in experiment 1; there was no significant drop in total or fine root dry weight after inoculation, although visible root damage was comparable to that recorded for T3 (Figs. 2a, 3a and 4a). In the third experiment, mineral mulch 1 (T7) again resulted in no reduction in root weight after inoculation (Figs. 2c and 3c), but there was a reduction in the second experiment (Figs. 2b and 3b). However, in both the second and third experiments, there was also a significant reduction in root damage resulting from applying the mineral mulch 1 (T7 compared to T3), and the overall growth of both non-inoculated and inoculated plants given mineral mulch was significantly higher than those in T3 (Fig. 4a, b).
The effect of the use of a herbicide on weeds
Although glyphosate (T9) was not sprayed directly on the avocado plants, but only applied to the wheat seedlings growing in the pots, compared to T3, non-inoculated avocados showed a 20% reduction for each of the parameters fine root dry weight total root dry weight and shoot dry weight. (Fig. 2a, Sup. Figure 1).
The effect of fungicides
In two of the three experiments, phosphite treatment resulted in no significant reduction in total or fine root weight of plants inoculated with Phytophthora although visible root damage was similar (Figs. 2a and c, 3a and c and 4a and c). Phosphite also increased root growth compared with plants in T3, irrespective of whether soil was inoculated with Phytophthora; this was observed in two of the three experiments (Figs. 2b and c and 3b and c).
Although the Metalaxyl (T11) treatment prevented a significant drop in total and fine root dry weights in inoculated, compared to non-inoculated plants, it had a detrimental effect on overall growth. Compared to plants in T3, non-inoculated avocados showed a reduction in fine root dry weight (30%), total root dry weight (30%) and shoot dry weight (25%), and the values for inoculated plants were also significantly lower than those for T3 plants (Figs. 2a and 3a, S1).
Effect of treatment with both mineral mulch and phosphite
Combining the mineral mulch and phosphite (T12) resulted in suppressing Phytophthora damage. The fine, and total root weight of the non-inoculated plants of T12 was significantly less than those plants treated with only mineral mulch (Figs. 2c and 3c).
Discussion
Applying a silicate-based mineral mulch to the soil surface improved the control of Phytophthora damage to avocado roots above that obtained through manure and organic mulches. The magnitude of the suppression was similar to that obtained through use of phosphite spray.
In these experiments, even though root weight was significantly reduced in some treatments and roots were visibly damaged in inoculated plants, all shoots were healthy, and no wilting was observed. The lack of wilting is probably because plants were grown under glasshouse conditions with daily watering and frequent fertilization. Under field conditions, a similar proportion of damaged roots could have a much greater impact on the total dry weight. In addition, if the glasshouse experiment had used younger plants with less woody roots (Rodriguez-Molina et al. 2002), or if the plants had been grown for longer after inoculation (Faber et al. 2000), there may have been a greater impact of P. cinnamomi on shoot dry weights. As P. cinnamomi has its initial impact on fine roots, it was considered that the most accurate assessment of the effectiveness of the treatments would be seen through the data on roots, particularly fine root dry weight and visible root damage.
Addition of manure and organic mulches (standard practice)
There is considerable evidence in the literature that the application of organic mulches improves the growth and disease resistance of plants, either through enhancement of the general vigour of growth, or through developing a beneficial microbial consort in the soil (Kasuya et al. 2006; Klein et al. 2007, 2016). Manure and organic mulches had been routinely applied for over 20 years to the avocado orchards from which soils were collected for use in the current experiments. Thus nutrients and organic matter may have already been optimal and this could account for the lack of any further reduction of damage from Phytophthora through the application of manure and organic mulches to the pots This suggests that currect orchard practise is effective. Rhizosphere soil from unamended pots and those dressed with the manure and organic mulches showed little difference in the abundance of rhizosphere bacteria, but there were proportionally more Chloroflexi and Actinobacteria in the latter (Farooq et al. 2022). However, studies of the bacterial microbiome using metabarcoding do not distinguish living and dead organisms, nor indicate changes in bacterial metabolism induced by soil treatments. A transcriptomics analysis is necessary to determine if the phosphite and mineral mulch treatments are linked with expression of microbial genes that might inhibit Phytophthora.
Addition of commercial microbial soil conditioners or mineral mulch
The only microbialcommercial conditioner to improve root growth and health was Conditioner 1(T4) in experiment 2. This conditioner contained Lactobacillis, Bacillus, Saccharomyces, Acetobacter and Azotobacter, genera which have been previously shown to include species that improve the uptake and availability of nutrients, soil structure, root and plant growth, and promote beneficial organisms that suppress disease (Dipta et al. 2021; Bradáčová et al. 2019).
The beneficial effect of the mineral mulch, which improved root growth and decreased root damage in two of the three experiments could be due to its silica or calcium content. Phosphite and calcium were shown to have an additive protective effect against Phytophthora damage in Banksia (Stasikowski et al. 2014). In the current experiment there was no additive effect of the mineral mulch and phosphite so it is considered that the silica content was important rather than the calcium. Silicate improves root growth and increases the defence mechanisms of roots when plants are infected with Phytophthora (Bekker et al. 2006, 2007; Bekker 2011; Dann and Le 2017; Serrano et al. 2013; Sugimoto et al. 2010). The silicate-based mineral mulch may also act as a fertilizer and improve the availability of micronutrients and cation exchange activity, and reduce the excess uptake of Fe, Mn and Al (Etesami 2018; Etesami and Jeong 2018), and thus improve the growth of shoots and roots (Al-Garni et al. 2019). In the potted soils used in the current experiments, the silica-based mineral mulch was shown alter the composition of the rhizosphere microbiome, increasing the relative abundance of Proteobacteria and Actinobacteria, where as phosphite spray did not result in an increase of Actinobacteria (Farooq et al. 2022).
Use of an herbicide on weeds and fungicides
The reduction of growth of the avocado plants in pots in which weeds were treated with glyphosate suggests that the use of this herbicide in orchards may damage avocado trees through their roots, possibly resulting in reduced water uptake and mineral deficiencies, which in turn increases disease susceptibility (Kremer et al. 2009; Zobiole et al. 2010). It may also be detrimental to beneficial rhizosphere microbes (Zobiole et al. 2011).
When compared to the addition of manure and organic mulches, spraying plants with phosphite increased the total root dry weight and fine root dry weight in non-inoculated plants in two of the three experiments, and for inoculated plants, total root dry weight was increased in two experiments and fine root dry weight in one experiment. It is possible that phosphite was providing a source of phosphate nutrition as other studies have reported phosphite may act as a fertilizer and a fungicide (Achary et al. 2017; Manna et al. 2016). However, it is also possible that although the soils were tested and found to be free of Phytophthora, phosphite inhibited pathogen(s) other than Phytophthora that were present and thus promoted root growth. The current experiments showed that if chemical control of Phytophthora is necessary, phosphite is better than metalaxyl as it has less detrimental effects on overall plant growth. Another drawback of metalaxyl use is that Phytophthora resistant isolates were reported to develop faster using metalaxyl than in phosphite treated plants, as the former has a targeted mode of action (Browne and Viveros 2005), while phosphite does not (McDonald et al. 2001; Browne and Viveros 2005).
Combined application of mineral mulch and phosphite
Both phosphite and mineral mulch 1 improved root growth in the absence of Phytophthora, and reduced the damage caused by the presence of Phytophthora to a similar extent. However, the lack of an additive or synergistic effect when applied in combination, indicates that they operate similarly. These treatments have different effects on the soil bacterial microbiome (Farooq et al. 2022), so it is most likely that the similarity of their effect is from them both stimulating plant defence mechanisms.
Conclusion
Although these pot experiments attempted to simulate the conditions of an orchard, it is difficult to completely replicate orchard conditions in a container trial in a glasshouse and ensure that the soil additives will have similar effects in the field. Temperature regimes, water retention, aeration, and surface evaporation are factors that differ in pots and the field and impact on the growth of plants and soil microbes, and the survival of microbes applied in the soil conditioners (Poorter et al. 2016). In addition, soil structure and the larger soil organisms are lost when soil is collected from the field for use in pots, and it is necessary to add another substrate to provide adequate aeration and drainage of the mix in pots. These additives may also affect plant and microbial responses. An additional drawback of glasshouse trials is that when large plants are utilised, the vigour of plant growth means that they need to be harvested in a relatively short time frame to avoid root binding, and this may not be sufficient time for the root damage to be accurately reflected in root and shoot mass. It is encouraging that field trials using mineral mulch have also shown improvement in the health of trees declining with Phytophthora root rot (Dann and Le 2017).
Disease control methods that reduce the use of chemicals or avoid the possible build-up of resistance in pathogens are highly desirable for commercial horticulture. These experiments indicated that a silicate-based mineral mulch was beneficial in reducing Phytophthora root damage and resulted in a level of control equivalent to the use of phosphite. Silicate based mulch could be a viable alternative to phosphite, especially where there might be evidence of resistant isolates developing to phosphite. There was evidence that a microbial soil conditioner containing Lactobacillis, Bacillus, Saccharomyces, Acetobacter and Azotobacter also improved disease resistance and plant growth. These results for the control of Phytophthora root rot were obtained in glasshouse experiments and need to be confirmed in the field, together with experiments to test effectiveness and costs of alternative sources of silicate.
Availability of data and material
The data are available if required from corresponding author.
Code Availability
R statistical software was used for data and analysis and code is available if required.
Change history
14 January 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13313-023-00904-0
References
Achary VMM, Ram B, Manna M, Datta D, Bhatt A, Reddy MK, Agrawal PK (2017) Phosphite: a novel P fertilizer for weed management and pathogen control. Plant Biotechnol J 15:1493–1508. https://doi.org/10.1111/pbi.12803
Aghighi S, Burgess TI, Scott JK, Calver M, Hardy GESJ (2016) Isolation and pathogenicity of Phytophthora species from declining Rubus anglocandicans. Plant Pathol 65:451–461. https://doi.org/10.1111/ppa.12436
Al-Garni SMS, Khan MMA, Bahieldin A (2019) Plant growth-promoting bacteria and silicon fertilizer enhance plant growth and salinity tolerance in Coriandrum sativum. J Plant Interact 14:386–396. https://doi.org/10.1080/17429145.2019.1641635
Bekker TF (2011) Efficacy of water soluble silicon for control of Phytophthora cinnamomi root rot of avocado. Doctoral Dessertation, University of Pretoria
Bekker TF, Kaiser C, Labuschagne N (2006) Efficacy of water soluble silicon against Phytophthora cinnamomi root rot of avocado: A progress report. South Afr Avocado Growers Association Yearbook 29:58–62
Bekker TF, Labuschagne N, Aveling T, Kaiser C (2007) Efficacy of water soluble potassium silicate against Phytophthora root rot of avocado under field conditions. South Afr Avocado Growers Association Yearbook 30:39–48
Belanger RR, Benhamou N, Menzies JG (2003) Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f. sp. tritici). Phytopathology 93:402–412. https://doi.org/10.1094/PHYTO.2003.93.4.402
Berg G (2009) Plant–microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol 84:11–18. https://doi.org/10.1007/s00253-009-2092-7
Bhattacharjee R, Dey U (2014) An overview of fungal and bacterial biopesticides to control plant pathogens/diseases. Afr J Microbiol Res 8:1749–1762. https://doi.org/10.5897/AJMR2013.6356
Bradáčová K, Florea AS, Bar-Tal A, Minz D, Yermiyahu U, Shawahna R, Kraut-Cohen J, Zolti A, Erel R, Dietel K (2019) Microbial consortia versus single-strain inoculants: an advantage in PGPM-assisted tomato production? Agronomy 9:105. https://doi.org/10.3390/agronomy9020105
Browne GT, Viveros MA (2005) Effects of phosphonate and mefenoxam treatments on development of perennial cankers caused by two Phytophthora spp. on almond. Plant Dis 89:241–249. https://doi.org/10.1094/PD-89-0241
D’Souza NK, Colquhoun IJ, Sheared BL, Hardy GESJ (2005) Assessing the potential for biological control of Phytophthora cinnamomi by fifteen native Western Australian jarrah-forest legume species. Australas Plant Pathol 34:533–540. https://doi.org/10.1071/AP05067
Dann EK, Le DP (2017) Effects of Silicon Amendment on Soilborne and Fruit Diseases of Avocado. Plants (Basel) 6:51–65. https://doi.org/10.3390/plants6040051
Datnoff LE, Rodrigues FA, Seebold KW (2007) Silicon and plant disease. In: Datnoff LE, Elmer WH, Huber DM (eds) Mineral nutrition and plant disease. APS Press – The American Phytopathological Society, Minnesota, USA, pp 233–246
Debona D, Rodrigues FA, Datnoff LE (2017) Silicon’s role in abiotic and biotic plant stresses. Annu Rev Phytopathol 55:85–107. https://doi.org/10.1146/annurev-phyto-080516-035312
Dipta B, Bhardwaj S, Kaushal M (2021) Overview of Nutrient and Disease Management in Banana. In: Kaushal M, Prasad R (eds) Microbial Biotechnology in Crop Protection. Springer, Singapore, pp 55–78. https://doi.org/10.1007/978-981-16-0049-4_2
Dobrowolski MP, Shearer BL, Colquhoun IJ, O’Brien PA, Hardy GESJ (2008) Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant Pathol 57:928–936. https://doi.org/10.1111/j.1365-3059.2008.01883.x
Etesami H (2018) Can interaction between silicon and plant growth promoting rhizobacteria benefit in alleviating abiotic and biotic stresses in crop plants? Agric. Ecosyst Environ 253:98–112. https://doi.org/10.1016/j.agee.2017.11.007
Etesami H, Jeong BR (2018) Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol Environ Saf 147:881–896. https://doi.org/10.1016/j.ecoenv.2017.09.063
Faber BA, Downer AJ, Menge JA (2000) Differential effects of mulch on citrus and avocado. Acta Hort 557:303–308. https://doi.org/10.17660/ActaHortic.2001.557.39
Farooq QUA, Hardy GES, Mccomb JA, Thomson PC, Burgess TI (2022) Changes to the bacterial microbiome in the rhizosphere and root endosphere of Persea americana (avocado) treated with organic mulch and a silicate-based mulch or phosphite, and infected with Phytophthora cinnamomi. Front Microbiol 13:870900. https://doi.org/10.3389/fmicb.2022.870900
Gill HK, Garg H (2014) Pesticides: environmental impacts and management strategies. In: Solenski S, Larramenday ML (eds) Pesticides-toxic aspects. IntechOpen, Croatia, pp 187–210. https://doi.org/10.5772/57399
Guest DI, Bompeix G (1990) The complex mode of action of phosphonates. Australas Plant Pathol 19:113-115. https://doi.org/10.1071/APP9900113
Hardy GESJ, Barrett S, Shearer BL (2001) The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australas Plant Pathol 30:133–139. https://doi.org/10.1071/AP01012
Huberli D, Tommerup IC, Hardy GESJ (2000) False-negative isolations or absence of lesions may cause misdiagnosis of diseased plants infected with Phytophthora cinnamomi. Australas Plant Pathol 29:164–169. https://doi.org/10.1071/AP00029
Kasuya M, Olivier AR, Ota Y, Tojo M, Honjo H, Fukui R (2006) Induction of soil suppressiveness against Rhizoctonia solani by incorporation of dried plant residues into soil. Phytopathology 96:1372–1379. https://doi.org/10.1094/PHYTO-96-1372
King M, Reeve W, Van der Hoek MB, Williams N, McComb J, O’Brien PA, Hardy GESJ (2010) Defining the phosphite-regulated transcriptome of the plant pathogen Phytophthora cinnamomi. Mol Genet Genomics 284:425–435. https://doi.org/10.1007/s00438-010-0579-7
Klein E, Katan J, Austerweil M, Gamliel A (2007) Controlled laboratory system to study soil solarization and organic amendment effects on plant pathogens. Phytopathology 97:1476–1483. https://doi.org/10.1094/PHYTO-97-11-1476
Klein E, Katan J, Gamliel A (2016) Soil suppressiveness by organic amendment to Fusarium disease in cucumber: effect on pathogen and host. Phytoparasitica 44:239–249. https://doi.org/10.1007/s12600-016-0512-7
Kremer RJ, Yamada T, de Camargo e Castro PR, Wood BW (2009) Glyphosate interactions with physiology, nutrition, and diseases of plants: Threat to agricultural sustainability? Eur J Agron 31:111–176. https://doi.org/10.1016/j.eja.2009.07.004
Manna M, Achary VMM, Islam T, Agrawal PK, Reddy MK (2016) The development of a phosphite-mediated fertilization and weed control system for rice. Sci Rep 6:1–12. https://doi.org/10.1038/srep24941
Masikane SL, Novela P, Mohale P, McLeod A (2020) Effect of phosphonate application timing and-strategy on phosphite fruit and root residues of avocado. Crop Protect 128:105008. https://doi.org/10.1016/j.cropro.2019.105008
McDonald AE, Grant BR, Plaxton WC (2001) Phosphite (phosphorous acid): its relevance in the environment and agriculture and influence on plant phosphate starvation response. J Plant Nutr 24:1505–1519. https://doi.org/10.1081/PLN-100106017
Menzies J, Bowen P, Ehret D, Glass AD (1992) Foliar applications of potassium silicate reduce severity of powdery mildew on cucumber, muskmelon, and zucchini squash. J Am Soc Hort Sci 117:902–905. https://doi.org/10.21273/JASHS.117.6.902
Pegg K, Whiley A, Langdon P, Saranah J (1987) Comparison of phosetyl-Al, phosphorous acid and metalaxyl for the long-term control of Phytophthora root rot of avocado. Aust J Exp Agric 27:471–474. https://doi.org/10.1071/EA9870471
Pegg KG (2010) Pathology challenges in avocado: Phytophthora management. Paper presented at the Southern Produce Avocado Conference, New Zealand, June 2010
Pegg KG, Whiley AW (1987) Phytophthora control in Australia. South Afr Avocado Growers Association Yearbook 10:94–96
Poorter H, Fiorani F, Pieruschka R, Wojciechowski T, van der Putten WH, Kleyer M, Schurr U, Postma J (2016) Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol 212:838–855. https://doi.org/10.1111/nph.14243
Pozza EA, Pozza AAA, Botelho DMDS (2015) Silicon in plant disease control. Rev Ceres 62:323–331. https://doi.org/10.1590/0034-737X201562030013
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 321:341–361. https://doi.org/10.1007/s11104-008-9568-6
Rajput VD, Minkina T, Feizi M, Kumari A, Khan M, Mandzhieva S, Sushkova S, El-Ramady H, Verma KK, Singh A (2021) Effects of silicon and silicon-based nanoparticles on rhizosphere microbiome, plant stress and growth. Biology 10:791. https://doi.org/10.3390/biology10080791
Ramirez-Gil JG, Castaneda-Sanchez DA, Morales-Osorio JG (2017) Production of avocado trees infected with Phytophthora cinnamomi under different management regimes. Plant Pathol 66:623–632. https://doi.org/10.1111/ppa.12620
Rasoolizadeh A, Labbe C, Sonah H, Deshmukh RK, Belzile F, Menzies JG, Bélanger RR (2018) Silicon protects soybean plants against Phytophthora sojae by interfering with effector-receptor expression. BMC Plant Biol 18:1–13. https://doi.org/10.1186/s12870-018-1312-7
Rasoolizadeh A, Santhanam P, Labbé C, Shivaraj SM, Germain H, Bélanger RR (2020) Silicon influences the localization and expression of Phytophthora sojae effectors in interaction with soybean. J Exp Bot 71:6844–6855. https://doi.org/10.1093/jxb/eraa101
Reeksting BJ, Olivier NA, van den Berg N (2016) Transcriptome responses of an ungrafted Phytophthora root rot tolerant avocado (Persea americana) rootstock to flooding and Phytophthora cinnamomi. BMC Plant Biol 16:205. https://doi.org/10.1186/s12870-016-0893-2
Rodrigues FA, Dallagnol LJ, Duarte HSS, Datnoff LE (2015) Silicon control of foliar diseases in monocots and dicots. In: Rodrigues F, Datnoff L (eds) Silicon and plant diseases. Springer, pp 67–108. https://doi.org/10.1007/978-3-319-22930-0_4
Rodriguez-Molina MC, Torres‐Vila LM, Blanco‐Santos A, Nunez EP, Torres‐Álvarez E (2002) Viability of holm and cork oak seedlings from acorns sown in soils naturally infected with Phytophthora cinnamomi. For Pathol 32:365–372. https://doi.org/10.1046/j.1439-0329.2002.00297.x
Samuels AL, Glass ADM, Ehret DL, Menzies JG (1991) Mobility and deposition of silicon in cucumber plants. Plant Cell Environ 14:485–492. https://doi.org/10.1111/j.1365-3040.1991.tb01518.x
Scott PM, Barber PA, Hardy GESJ (2015) Novel phosphite and nutrient application to control Phytophthora cinnamomi disease. Australas Plant Pathol 44:431–436. https://doi.org/10.1007/s13313-015-0365-4
Serrano MS, Fernández-Rebollo P, De Vita P, Sánchez ME (2013) Calcium mineral nutrition increases the tolerance of Quercus ilex to Phytophthora root disease affecting oak rangeland ecosystems in Spain. Agrofor Syst 87:173–179. https://doi.org/10.1007/s10457-012-9533-5
Shearer BL, Fairman RG (2007) A stem injection of phosphite protects Banksia species and Eucalyptus marginata from Phytophthora cinnamomi for at least four years. Australas Plant Pathol 36:78–86. https://doi.org/10.1071/AP06085
Simamora AV, Paap T, Howard K, Stukely MJC, Hardy GESJ, Burgess TI (2018) Phytophthora contamination in a nursery and its potential dispersal into the natural environment. Plant Dis 102:132–139. https://doi.org/10.1094/PDIS-05-17-0689-RE
Song D, Ibrahim S, Hayek S (2012) Recent application of probiotics in food and agricultural science. Probiotics 10:1–34. https://doi.org/10.5772/50121
Stasikowski PM, McComb JA, Scott P, Paap T, O’Brien PA, Hardy GESJ (2014) Calcium sulphate soil treatments augment the survival of phosphite-sprayed Banksia leptophylla infected with Phytophthora cinnamomi. Australas Plant Pathol 43:369–379. https://doi.org/10.1007/s13313-014-0303-x
Sugimoto T, Watanabe K, Yoshida S, Aino M, Furiki M, Shiono M, Matoh T, Biggs AR (2010) Field application of calcium to reduce Phytophthora stem rot of soybean, and calcium distribution in plants. Plant Dis 94:812–819. https://doi.org/10.1094/PDIS-94-7-0812
Tubana BS, Babu T, Datnoff LE (2016) A review of silicon in soils and plants and its role in US agriculture: history and future perspectives. Soil Sci 181:393–411. https://doi.org/10.1097/SS.0000000000000179
Wolstenholme BN, Sheard A (2010) Integrated management of Phytophthora root rot the “Pegg Wheel” updated. South Afr Avocado Growers Association Avoinfo Newsl 175:11–15
Xi Y, Han X, Zhang Z, Joshi J, Borza T, Aqa MM, Zhang B, Yuan H, Wang-Pruski G (2020) Exogenous phosphite application alleviates the adverse effects of heat stress and improves thermotolerance of potato (Solanum tuberosum L.) seedlings. Ecotoxicol Environ Saf 190:110048. https://doi.org/10.1016/j.ecoenv.2019.110048
Zobiole LHS, de Oliveira RS, Huber DM, Constantin J, de Castro C, de Oliveira FA, de Oliveira A (2010) Glyphosate reduces shoot concentrations of mineral nutrients in glyphosate-resistant soybeans. Plant Soil 328:57–69. https://doi.org/10.1007/s11104-009-0081-3
Zobiole LHS, Kremer RJ, Oliveira RS Jr, Constantin J (2011) Glyphosate affects microorganisms in rhizospheres of glyphosate‐resistant soybeans. J Appl Microbiol 110:118–127. https://doi.org/10.1111/j.1365-2672.2010.04864.x
Acknowledgements
We thank Russel Delroy from Delroy farms for providing mulch from his orchard, Kay Howard for help with the preparation of the manuscript, Ziad Mekkawi, Mohammad Bhaidhani, Ian McKernan and Bill Dunstan for assistance in the glasshouse, and Rajah Belhaj for laboratory work, Chris Shaw and Peter Thomson for guidance in statistical analysis. We also thank Geoleaks Solutions, Mineral Mulch and Eco Growth for providing commercial soil additives.
Funding
This research was supported by Murdoch University Scholarship and HIA (Horticulture Innovation Australia) project AV10067.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest for submitted paper.
Ethical statement
This manuscript did not involve any work/study with human participants/animals performed by any of the authors.
Consent to participate
All Authors agreed to the submission of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Shoot dry weight. a) Experiment 1: Soil treatments (T1-T12, see 1). b) Experiment 2: Soil treatments (T1, T3, T4, T7, T8, T10).c) Experiment 3: Soil treatments (T1, T3, T7, T10 and T12). Non-inoculated avocado plants (grey bars) and those inoculated with Phytophthora cinnamomi (black bars). Vertical lines indicate SE and letters that are different indicate significant (p < 0.05) differences between treatments. * indicates a significant (p < 0.05) difference between non-inoculated and inoculated plants in the same treatment.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farooq, Q.U.A., McComb, J., Hardy, G.E.S.J. et al. Soil amendments and suppression of Phytophthora root rot in avocado (Persea americana). Australasian Plant Pathol. 52, 1–11 (2023). https://doi.org/10.1007/s13313-022-00889-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-022-00889-2