Abstract
We used molecular profiling with 454 pyrosequencing to identify ectomycorrhizal, arbuscular mycorrhizal fungi, and other fungal communities associated with containerized Eucalyptus gomphocephala seedling roots. We found a higher proportion of ectomycorrizal fungi associated with seedling roots grown in soil collected from sites with healthy trees, and of arbuscular mycorrhizal fungi from seedling roots grown in soil collected from sites with declining trees. We also found a relatively high proportion of pathogenic fungi present in roots from declining sites compared to healthy sites. This research should be extended to the field to further investigate mycorrhizal and pathogenic fungal populations associated with tree declines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The forests and woodlands of South-Western Australia are vulnerable to climate change (Evans et al. 2013; Laurence et al. 2011; Matusick et al. 2013; Ruthrof et al. 2015) and globally the Mediterranean biome is a high priority for conservation practices (Olson and Dinerstein 2002). Eucalyptus gomphocephala dominates a Mediterranean type forest that has experienced severe decline in recent decades (Scott et al. 2013). A range of factors potentially influencing the decline have been explored including soil fertility (Close et al. 2011), drought associated with climate change (Matusick et al. 2012), fire frequency and intensity (Archibald et al. 2010), soil bacteria (Cai et al. 2010), and root pathogenic fungi (Scott et al. 2012), but the primary cause of the decline remains enigmatic.
Ectomycorrizal fungi play an important role in ecosystem function and tree health (Sapsford et al. 2017) and might be related to E. gomphocephala health (Ishaq et al. 2013). Our previous bioassay studies found the type of mycorrhiza (arbuscular or ectomycorrhizal) formed in containerized seedlings was related to the canopy condition of E. gomphocephala at the sites where the soil cores were taken. Ectomycorrhizal colonization was high in seedlings grown in soil collected from under healthy canopies, and was positively related to the canopy condition of E. gomphocephala in the field, suggesting the relative importance of ECM for maintaining the health of E. gomphocephala in natural ecosystems (Ishaq et al. 2013). These conclusions, based on morphotyping and microscopic examination only, did not identify fungal taxa associated with seedling roots. In this paper, the roots were re-examined using 454 pyrosequencing to validate the previous findings. This technology has been widely used to analyse fungal diversity including mycorrhizal fungi (Öpik et al. 2009; Blaalid et al. 2012; Kauserud et al. 2012).
Materials and methods
Root samples
The E. gomphocephala roots (96 samples) used for this study were obtained from the previous study (Ishaq et al. 2013). Briefly, E. gomphocephala seedlings were grown under controlled glasshouse conditions for 7 months in soils collected from 12 sites (GPS coordinates are given in Cai et al. 2010), where the health of trees ranged from healthy, to moderately healthy and declining. At each site, four trees were randomly selected, and two intact soil cores each from the north and south were collected 5 m from the trunk of each tree. For this study, a mixture of root fragments and root tips were randomly collected from samples that had been preserved in 100% EtOH at 4 °C for 6 months. The samples were transferred into a 1.5 ml eppendorf tube, allowed to dry in a laminar flow overnight, freeze dried for 3 days (Hetosicc CD 4), and then kept at −80 °C before DNA extraction (Griffin et al. 2002).
Molecular identification
The roots were manually ground by dipping the 1.5 ml eppendorf tube containing root samples into liquid nitrogen until frozen before grinding. The DNA was extracted according to the manufacturer’s instruction manual of ZR Fungal/Bacterial DNA MiniPrep™ Catalog No. D6005. The pure DNA, both as full DNA and diluted DNA (1:10 dilution) (Williams et al. 2001), was sent to Australian Genome Research Facility Ltd. (AGRF) Perth-Western Australia (www.agrf.org.au) for 454 sequencing analysis.
The genomic DNA samples were amplified separately in a two step PCR using the fungal primer pair ITS1F (5’-CTT GGT CAT TTA GAG GAA GTA A-3′) and ITS2 (5’-GCT GCG TTC TTC ATC GAT GC-3′) to generate PCR ITS rRNA fragments of c. 400 base pairs. In the second round of PCR, the primers were modified to include adaptors for pyrosequencing with ITS1F primer individually barcoded for each sample. The sequence was run on a Genome Sequencer FLX 454 System. After the sequencing run, results were run through Roche’s standard amplicon analysis pipeline. This is a very stringent pipeline which results in only the long high quality reads remaining. Demultiplexing of the results based on the barcode assigned to a sample was performed. Each sample underwent the bioinformatic processing for the diversity profiling report, and ITS samples were processed under the UNITE database (http://greengenes.secondgenome.com/). The composition of species diversity in high-throughput amplicon sequencing data was carried out using the Quantitative Insights Into Microbial Ecology (QIIME) software package version (http://qiime.org).
For the calculation of fungal distribution, only identified operational taxonomic units (OTU’s) were considered. The calculation was made at phyla and genus levels, except when only mycorrhizal fungi were considered; for these the calculation was at species level and other fungi were not included. Proportional distribution of fungal OTU’s of each sample was calculated, and then averaged. At the genus level, all the fungi which might be ecologically important and related to the health of E. gomphocephala were included in the calculation. The fungi were grouped into pathogens (leaf, stem, and root pathogens), saprophytes, endophytes, ECM and AMF). Some considerations were taken into account when grouping the fungi. For instance, putative pathogens such as Botryosphaeria, Mycosphaerella and Teratosphaeria, and genera of major fungal plant pathogens such as Alternaria, Cytospora, and Phaeosphaeriopsis were grouped as pathogens. Fungal genera known to cause root rot were also grouped as pathogens. Basidiomycota which were neither mycorrhizal nor root rot fungi were grouped as saprophytes. The endophyte group was considered as neutral endophytic fungi. Pathogenic endophytic fungi, such as the genus Diaporthe, were grouped with pathogens.
Statistical analyses
Calculation of proportional distribution of fungal OTU’s was performed using Excel Program. To determine the correlation between the richness of ECM/AM fungi OTU’s and crown health, Pearson’s correlation coefficient was employed. In the calculation, richness is defined as the OTU’s at the species level found at the 12 sites. The analyses were conducted using SPSS for Windows version 17.0 (SPSS).
Results
Fungal community
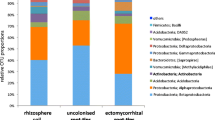
The DNA sequence analysis detected six fungal Phyla associated with the seedling roots, namely Ascomycota, Basidiomycota, Glomeromycota, Chytridiomycota, Blastocladiomycota and Zygomycota. The Ascomycota contributed 85.9% of the total DNA sequences detected in the roots from 12 sites, whilst Basidiomycota and Glomeromycota contributed 11 and 2.1%, respectively. The Chytridiomycota contributed 0.9% whereas the Blastocladiomycota and Zygomycota contributed less than 0.1% each.
Related to the canopy condition of E. gomphocephala in the field, the highest proportion of the Ascomycota was found for seedling roots grown in soil collected from the moderately healthy sites (90.6%) compared with seedling roots grown in soil collected from the healthy (85.4%) and declining sites (84.4%). The phylum Basidiomycota was mostly detected from seedling roots in soil collected from the healthy sites (13.5%), and a smaller proportion was found for seedling roots grown in soil collected from the moderately healthy (9.23%) and declining sites (8.21%). A higher proportion of the Glomeromycota was identified for roots in soil from the declining sites (5.3%). On the other hand, only 0.65% and 0.11% of this phylum were detected for the seedling roots grown in soil collected from the healthy and moderately healthy sites, respectively.
At the genus level, there were some interesting trends in fungal distribution particularly regarding saprophytic, pathogenic and mycorrhizal fungi between the sites (Supplementary Table 1). The proportion of saprophytes was higher for the seedling roots grown in soil collected from the moderately healthy and the healthy sites, contributing 83.2 and 62.9%, respectively, compared with those seedling roots grown in soil collected from the declining sites (44.8%). On the other hand, the pathogenic fungi were more prevalent with seedlings roots grown in soil collected from the declining sites contributing 36.7% compared to those associated with seedling roots grown in soil collected from the moderately healthy sites (12.7%) and the healthy sites (25.5%). The proportion of ECM to AM fungi was high for seedling roots grown in soil collected from the healthy sites whilst the proportion of AM to ECM fungi was high for seedling roots grown in soil collected from the declining sites. The proportion of endophytes was relatively similar in the seedlings roots grown in soil collected from the healthy and moderately healthy sites, but higher in the seedling roots grown in soil collected from the declining sites (Fig. 1a, b and c).
ECM/AM communities
The DNA sequence analysis identified six ECM families associated with seedlings roots, namely Cortinariaceae, Rhizopogonaceae, Sclerodermataceae, Sebacinaceae, Thelephoraceae, and Tuberaceae. All six families were found for the seedlings roots in soil collected from the healthy sites, whereas only two families (Cortinariaceae and Tuberaceae) were detected from roots in soil from the moderately healthy sites, and four families (Sclerodermataceae, Sebacinaceae, Thelephoraceae, and Tuberaceae) from declining sites. At the species level, not many ECM fungal communities associated with seedling roots were detected by molecular analysis, and only fourteen ECM fungi OTU’s in total were found (Table 1). Pearson’s correlation analysis revealed that the number of ECM fungi OTU’s present at the species level was not significantly correlated with canopy condition of E. gomphocephala in the field; however, there was a trend for more ECM fungi OTU’s for seedlings roots grown in soil collected from the healthy sites than in the declining sites. Twelve of the 14 ECM fungi OTU’s were associated with seedling roots grown in soil collected from the healthy sites, whilst only two and eight ECM fungi OTU’s were found for seedling roots grown in soil collected from the moderately healthy and declining sites, respectively.
There were four AM families associated with the roots. These were Diversisporaceae, Claroideoglomeraceae, Glomeraceae and unidentified Glomeraceae. All four AM families were found for seedling roots grown in soil collected from the declining sites, whereas only two AM families (Diversisporaceae and Glomeraceae) were identified from seedlings grown in soil collected from the healthy and one family (Diversisporaceae) from moderately healthy sites. At the species level, a total of 13 AM fungi OTU’s were found in the roots grown in soil collected from the 12 sites (Table 1). Glomus was the dominant AM genus present in roots followed by Rhizophagus. Pearson’s correlation analysis revealed that the number of AM fungal OTU’s was significantly (r = −0.41) negatively correlated to crown health of E. gomphocephala. Ten AM fungi OTU’s out of 13 were found for seedling roots grown in soil collected from the declining sites compared with three OTU’s for roots in soil collected from moderately healthy and five OTU’s from healthy sites.
Proportion of ECM/AM fungi within the roots
For the calculation of the proportional distribution of mycorrhizal fungi associated with the seedling roots, only the ECM and AM fungi were included. Ectomycorrhizal fungi contributed 75% of the total proportion of mycorrhizal fungi colonizing the seedling roots grown in soil collected from the healthy sites, whilst the AM fungi only contributed 25%. On the other hand, seedling roots grown in soil collected from the declining sites were highly colonized by AM fungi. The AM fungi contributed 52.1% of the total proportion, whilst the ECM fungi were 47.9%. A high proportion of ECM fungi (66.7%) was found for mycorrhizal fungi colonizing the seedling roots grown in soil collected from the moderately healthy sites, whilst the AM proportion was low (33.3%) (Fig. 2).
Discussion
Our molecular findings indicate the dominance of ECM fungi for the seedling roots grown in soil collected from the healthy sites, and vice versa for the AM fungi for the seedling roots grown in soil collected from the declining sites. This finding validated our previous study where the mycorrhizal assessment was based on microscopic examination (Ishaq et al. 2013). That study suggested that the gross type of mycorrhiza formed in the seedlings could predict E. gomphocephala health in the field where the soil cores were taken. In particular, ECM colonization was high in seedling roots grown in soil collected from healthy sites whilst AM colonization was high in seedling roots grown in soil collected from declining sites.
Variable results have been reported regarding the relationship between ECM and stand/forest health. For instance, the proportions of ECM colonization, diversity or both, were higher in healthy trees than declining trees (Causin et al. 1996; Swaty et al. 2004; Horton et al. 2013). However, it is also reported that the state of tree health may not impact on ECM fungi (Perrin and Estivalet 1989; Peter et al. 2008; Lancellotti and Francheschini 2013). Montecchio et al. (2004) found a higher number of ECM morphotypes associated with declining trees than asymptomatic trees, and hypothesized that some ECM morphotypes might be essential for maintaining the stability of the modified ecosystem processes, but less efficient in their mutualistic manner. Peter et al. (2008) observed Norway spruce decline due to air pollution was related to ECM, and reported the ECM colonization was high, but ECM species richness decreased in heavily polluted sites. Conversely, Swaty et al. (2004) reported both reduced ECM colonization and diversity in high mortality pinyon pine (Pinus edulis) sites.
Our results indicate ECM might be important for the healthy functioning of E. gomphocephala. Eucalypts are well known to form mycorrhizal associations (Lapeyrie and Chilvers 1985), and may benefit from this association in their natural environment especially where abiotic factors such as soil conditions or climatic factors are unfavorable for growth. The soils in the study region are generally of low fertility (Moore 1998), and increased temperature and reduced precipitation due to climate change has been an issue in Western Australia (Bates et al. 2008). The loss of ECM, therefore could have impacted on health of E. gomphocephala in its natural stands.
Our molecular study found a higher proportion of ECM fungi associated with seedling roots grown in soil collected from woodland with healthy canopies suggesting the possible involvement of ECM on E. gomphocephala health. Since our study was based on a glasshouse assay where environmental conditions within the pots and glasshouse differed from the natural forest, it is possible these conditions could have influenced fungal diversity. Future studies on ECM colonization and communities should be extended to the field. Furthermore, as spatial diversity in time and place could be an issue in exploring ECM communities and diversity, repeated samplings for a longer period of time would be preferable in order to get a more representative understanding of actual ECM communities and diversity in the field.
Other microbes were also evaluated in this study including saprophytes and pathogens. The diversity of saprophytic fungi was high for seedling roots grown in soil collected from the healthy and moderately healthy sites, but low for seedling roots grown in soil collected from the declining sites. Whether the lower proportion of saprophytes in the declining sites is related to E. gomphocephala health at these sites is not known.
Some saprophytes might be ecologically important for organic matter decomposition, contributing to nutrient release into the soil. Recently, it has been reported that a decrease in utilization of carbohydrates, carboxylic acid, amino acids and amines by the soil bacteria community was correlated to poor E. gomphocephala crown health indicating a strong relationship between the bacterial community and E. gomphocephala decline (Cai et al. 2010).
The proportion of pathogenic (leaf, stem and root pathogens) fungi was higher for seedling roots grown in soil collected from the declining sites compared to seedling roots grown in soil collected from the moderately healthy and healthy sites. As the soils used to grow the seedlings were collected from the field, these pathogens could either have been introduced with the soil, leaf litter in the soil or some may have been endophytes within the seed used for the trial. Furthermore, some of these organisms such as Botryosphaeria, Mycosphaerella and Teratosphaeria, may be putative pathogens in E. gomphocephala and other native woody plants such as Acacia cochlearis, A. rostellifera, Allocasuarina fraseriana, Agonis flexuosa, Banksia grandis and E. marginata that grow in association with E. gomphocephala (Taylor et al. 2009; Hunter et al. 2011). Whether the pathogen groups found in this study behave as opportunistic fungi or they are primary pathogens on E. gomphocephala remains to be determined. However, it is widely accepted that some opportunistic fungi may become pathogenic when the environmental conditions are unfavourable and trees are under stress. It is possible that there is a higher diversity and inoculum load of putative pathogens in soil from declining sites and this might be a contributing factor to the declining health of trees at these sites. Spores of these species could have been brought into these sites by wind, rain, air currents or other factors. It has been suggested that fungal spore dispersal by the wind can spread plant disease over long distances (Brown and Hovmeller 2002), and global movement/gene flow of some pathogens causing canker and leaf spot disorders in eucalypts has been detected (Burgess et al. 2008; Hunter et al. 2008). More recently, the soil-borne plant pathogen Phytophthora multivora has been found to be associated with declining E. gomphocepahala in Yalgorup near the study site (Scott et al. 2012). However, the presence of this pathogen could not be detected by DNA sequence analysis in the present study, as the primers used are designed for true fungi only, consequently it is not surprising Phytophthora species or other oomycetes were not observed.
The weak relationship between ECM fungal communities and crown health of E. gomphocephala might be due to a relatively small number of ECM fungal communities detected by DNA sequence analysis. A key ECM family, the Russulaceae, and some key genera such as Pisolithus, Russula, Cortinarius, Laccaria and Cenococcum are missing from the molecular analysis. Indeed, some of the mat-forming ECM fungi like Hysterangium observed in the pot trial were not detected in the molecular analysis. Recently, Horton et al. (2013) investigated the relationship between ECM fungi communities, soil chemistry and E. delegatensis decline in Tasmania. Using ECM root tips coupled with sporocarps collected from the field to extract DNA for molecular analysis, the authors were able to detect many important families and key genera, and successfully showed a significant relationship between reduced ECM richness and declining tree health. The smaller number of ECM taxa detected in the current study compared to findings by Horton et al. (2013) could be due to differences in ecosystems between the two regions such as climate and soil. Eucalyptus delegatensis is a high-altitude, high-rainfall, lower temperature species with a closed canopy and rich organic soil, whereas E. gomphocephala forest is much lower in altitude, hotter and drier with sandy soil. However, there are other possibilities that might be relevant in explaining the smaller number of ECM taxa detected by molecular analysis in the current study. For instance, it is not known whether the plants only trapped a limited number of ECM fungi in the soil, or whether some fungi did not persist under glasshouse conditions. Besides, it might be also possible that the absence of key ECM in the sequences is due to the age of the seedlings being sampled. Generally, “late stage fungi” such as Russulaceae members tend to associate with more mature trees in undisturbed sites, thus it is less likely that seedlings or young trees could d pick up these types of fungi. Alternatively, the absence of key genera such as Russula and Cortinarius in the sequences might be also due to the sensitivity of the fungi to disturbance. For instance, in a Phytophthora die back-induced tree decline, Anderson et al. (2010) showed that fungal diversity and “the late stage fungi” were generally firstly impacted.
As mentioned earlier, not all morphotypes observed were detected in the molecular study. For example, Cenococcum with its distinctive black, hairy ECMs was observed microscopically, but was not detected by molecular analysis. Similarly, distinctive spores of the AM genera including Gigaspora and Acaulospora were observed, but were missing from the molecular analysis. Kauserud et al. (2012) compared 454 sequencing and the Sanger sequencing approach for their ability to characterize fungal communities of the ECM plant Bistorta vivipora, and reported that 454 performed poorly in detecting Cenococcum and Thelephoraceae, although the method had a higher affinity to detect Glomales than the cloning/Sanger approach. A similar study by Tedersoo et al. (2010) reported that several taxa were not captured by Cloning/Sanger and some taxa were poorly detected using 454. Sakakibara et al. (2002) on the other hand suggested that morphological techniques in conjunction with the use of molecular techniques may provide more powerful information on mycorrhizal communities.
Surprisingly, Tuber indicum was detected by molecular analysis. Apart from introduced Tuber species into commercial tuber farms, as close as 100 km from the study site, as yet there is no record of a true Tuber species in Australia. Tuber indicum is known to be native to Asia but it is widely dispersed as food around the world including Melbourne (Australia) (Wang et al. 2006; Bonito et al. 2011). The fact that Rhizopogon was detected by molecular analysis was quite interesting. Rhizopogon is usually associated with conifers such as Pinus species, and only formed surface colonization with a loose mantle in associations with eucalypts (Malajczuk et al. 1994). Introduced Rhizopogon is one of the ECM fungi commonly associated with Pinus in Western Australia (Dunstan et al. 1998) and is used commercially in pine nurseries in the region. Since there are Pinus plantations near the Yalgorup National Park, it is possible that the Rhizopogon spores could have been blown into this area.
When only ECM and AM fungi were considered in the calculation of proportional fungal distribution in the roots, the ECM fungi were quite high in the seedling roots grown in soil collected from healthy and moderately healthy sites, but low in the seedlings grown in soil collected from declining sites. Our results were consistent with the study of Scott et al. (2013), who investigated the relationship between crown health, fine roots and ECM density of declining and healthy E. gomphocephala in the Yalgorup region, and found that trees with crown decline symptoms had fewer fine roots and ECM density than healthy trees, suggesting decline of E. gomphocepahala is associated with absence of ECM. Similarly, it was previously reported that diversity and abundance of ECM in the jarrah (Eucalyptus marginata) forest in southwestern Australia were greatly reduced in die-back affected areas (Anderson et al. 2010).
We cannot determine whether the reduced ECM colonization and diversity are the drivers of the state of E. gomphocephala health, or vice versa, however, given the variety of services individual ECM fungi may provide to the host plants (Smith and Read 2008), and the variety of their response to environmental variables (Cairney 1999), a decrease in ECM colonization and diversity may lead to a corresponding loss of some ecosystem functions. Since this finding was based on glasshouse conditions, future studies need to be extended to the field by investigating colonization patterns in seedlings and also the molecular analysis of mycorrhizal fungi populations in sites with a range of canopy conditions. Furthermore, other soil fungal communities associated with E. gomphocephala such as saprophytes, pathogens and endophytes need to be considered. Indeed, the presence of Phytophthora multivora has been recently related to E. gomphocephala decline (Scott et al. 2012). An approach for managing the health of E. gomphocephala can be gained from these studies.
References
Anderson P, Brundrett M, Grierson M, Robinson R (2010) Impact of severe forest dieback caused by Phytophthora cinnamomi in macrofungal diversity in the northern jarrah forest of Western Australia. For Ecol Manag 259:1033–1040
Archibald R, Bradshaw J, Bowen B, Close D, McCaw L, Drake P, Hardy G (2010) Understorey thinning and burning trials are needed in conservation reserves: the case of tuart (Eucalyptus gomphocephala Dc). Ecol. Manage Restore 11:108–112
Bates BC, Hope P, Ryan B, Smith I, Charles S (2008) Key findings from the Indian ocean climate initiative and their impact on policy development in Australia. Climate Change 89:339–354
Blaalid R, Carlesen T, Surendra K, Halvorsen R, Ugland K, Fontana G, Kauserud H (2012) Changes in the root-associated fungal communities along a primary succession gradient analysed by 454 pyrosequencing. Mol Ecol 21:1897–1908
Bonito G, Trappe JM, Donovan S, Vilgalys R (2011) The Asian black truffle Tuber indicum can form ectomycorrhizas with North American host plants and complete its life cycle in non-native soils. Fungal Ecol 4:83–93
Brown JKM, Hovmeller MS (2002) Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297:537–541
Burgess TI, Sakalidis ML, Hardy GESJ (2008) Gene flow of the canker pathogen Botryosphaeria australis between Eucalyptus globulus plantations and native eucalypt forests in Western Australia. Austral Ecol 31:559–566
Cai YF, Barber P, Dell B, O'Brien P, Williams N, Bowen B, Hardy G (2010) Soil bacterial functional diversity is associated with the decline of Eucalyptus gomphocephala. For Ecol Manag 260:1047–1057
Cairney JWG (1999) Intraspecific physiological variation: implications for understanding functional diversity in ectomycorrhizal fungi. Mycorrhiza 9:125–135
Causin R, Montecchio L, Accordi SM (1996) Probability of ectomycorrhizal infection in a declining stand of common oak. Ann For Sci 53:743–752
Close DC, Davidson NJ, Swanborough PW (2011) Fire history and understorey vegetation: water and nutrient relations of Eucalyptus gomphocephala and E. delegatensis overstorey trees. For Ecol Manag 262:208–214
Dunstan WA, Dell B, Malajczuk N (1998) The diversity of ectomycorrhizal fungi associated with introduced Pinus spp. in the Southern Hemisphere, with particular reference to Western Australia. Mycorrhiza 8:71–79
Evans B, Stone C, Barber P (2013) Linking a decade of forest decline in the southwest of Western Australia to bioclimatic change. Aust For 76:164–172
Glen M, Tommerup IC, Bougher NC, O’Brien PA (2002) Are Sebacinaceae common and widespread ectomycorrhizal associates of Eucalyptus species in Australian Forest? Mycorrhiza 12:243–247
Griffin DW, Kellogg CA, Peak KK, Shinn EA (2002) A rapid and efficient assay for extracting DNA from fungi. Lett Appl Microbiol 34:210–214
Horton BM, Glen M, Davidson NJ, Ratkowsky D, Close DC, Wardlaw TJ, Mohammed C (2013) Temperate eucalypt forest decline is linked to altered ectomycorrhizal communities mediated by soil chemistry. For Ecol Manag 302:329–337
Hunter GC, van der Merwe NA, Burgess TI, Carnegie AJ, Wingfield BD, Crous PW, Wingfield MJ (2008) Global movement and population biology of Mycosphaerella nubilosa infecting leaves of cold-tolerant Eucalyptus globulus and E. nitens. Plant Pathol 57:235–242
Hunter GC, Crous PW, Carnegie AJ, Burgess TI, Wingfield MJ (2011) Mycosphaerella and Teratosphaeria diseases of Eucalyptus; easily confused and with serious consequences. Fungal Divers 50:145–166
Ishaq L, Barber PA, Hardy GESJ, Calver M, Dell B (2013) Seedling mycorrhizal type and soil chemistry are related to canopy condition of Eucalyptus gomphocephala. Mycorrhiza 23:359–371
Kariman K, Barker SJ, Finnegan PM, Tibet M (2012) Dual mycorrhizal associations of jarrah (Eucalyptus marginata) in a nature-pot system. Aust J Bot 60:661–668
Kauserud H, Kumar S, Brysting AK, Nordén J, Carlsen T (2012) High consistency between replicate 454 pyrosequencing analyses of ectomycorrhizal plant root samples. Mycorrhiza 22:309–315
Lancellotti E, Francheschini A (2013) Studies on the ectomycorrhizal community in a declining Quercus suber L. stand. Mycorrhiza 23:533–542. https://doi.org/10.1007/s00572-013-0493-z
Lapeyrie FF, Chilvers GA (1985) An endomycorrhiza-ectomycorrhiza succession associated with enhanced growth of Eucalyptus dumosa seedlings planted in a calcareous soil. New Phytol 100:93–104
Laurence WA, Dell B, Turton SM, Lawes MJ, Hutley LB, McCallum HM, Dale P, Hardy G, Prideaux G, Gawne B, McMahon CR, Yu R, Hero JM, Schwarzkopf L, Krockenberger A, Doughlas M, Silvester E, Mahony M, Vellam K, Saikia U, Wahren CH, Zhihong Xu Z, Smith B, Cocklin C (2011) The 10 Australian ecosystems most vulnarable to tipping points. Biol Conserv 144:1472–1480
Malajczuk N, Molina R, Trappe JM (1994) Ectomycorrhiza formation in Eucalyptus. II. The ultrastructure of compatible and incompatible mycorrhizal fungi and associated roots. New Phytol 96:43–53
Matusick G, Ruthrof KX, Hardy GSJ (2012) Drought and heat triggers sudden and severe dieback in a dominant mediterranean-type woodland species. J For 2:183–186
Matusick G, Ruthrof K, Brouwers N, Dell B, Hardy GJ (2013) Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia. Eur J Forest Res 132:497–510
Montecchio L, Causin R, Rossi S, Accordi SM (2004) Changes in ectomycorrhizal diversity in a declining Quercus ilex coastal forest. Phytopathol Mediterr 43:26–34
Moore G (1998) Soil group of South-Western Australia. In Soil guide. A handbook for understanding and managing agricultural soils. Ed. G. Moore. Agriculture Western Australia bulletin no. 4343. Perth, Western Australia. pp 19–30
Olson DM, Dinerstein E (2002) The global 200: priority ecoregions for global conservation. Ann Mo Bot Gard 89:199–224
Öpik M, Metsis M, Daniell TJ, Zobel M, Moora M (2009) Large-scale parallel 454 sequencing reveals host ecological group specificity of arbuscular mycorrhizal fungi in a boreonemoral forest. New Phytol 184:424–437
Perrin R, Estivalet (1989) Mycorrhizal association and forest decline. Agric Ecosyst Environ 28:381–387
Peter M, Ayer F, Cudlin P, Egli S (2008) Below ground ectomycorrhizal communities in three Norway spruce stands with different degrees of decline in the Czech Republic. Mycorrhiza 18:157–169
Rees BJ, Midgley DJ, Marchant A, Perkins A, Orlovich DA (2013) Morphological and molecular data for Australian Hebeloma species do not support the generic status of Anamika. Mycologia 105:1043–1058
Ruthrof K, Matusick G, Hardy G (2015) Early differential responses of co-dominant canopy species to sudden and severe drought in a Mediterranean-climate type forest. Forests 6:2082–2091
Sakakibara SM, Jones MD, Gillespie M, Hagerman SM, Forrest ME, Simard SW, Durall DM (2002) A comparison of ectomycorrhiza identification based on morphotyping and PCR-RFLP analysis. Mycol Res 106:868–878
Sapsford SJ, Paap T, Hardy GESJ, Burgess TI (2017) The ‘chicken or the egg’: which comes first, forest tree decline or loss of mycorrhizae? Plant Ecol 218:1093–1106
Scott PM, Jung T, Shearer BL, Barber P, Calver M, Hardy GESJ (2012) Pathogenicity of Phytophthora multivora to Eucalyptus gomphocephala and Eucalyptus marginata. For Path 42:289–298
Scott PM, Barber PA, Shearer BL, Hardy GESJ (2013) Relationships between the crown health, fine root and ectomycorrhizae density of declining Eucalyptus gomphocephala. Australas Plant Pathol 42:121–131
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic/Elsevier, New York
Swaty RL, Decker RJ, Whitham TG, Gehring CA (2004) Ectomycorrhizal abundance and community composition shifts with drought: prediction from tree rings. Ecology 85:1072–1084
Taylor K, Barber PA, Hardy GESJ, Burgess TI (2009) Botryosphaeriaceae from tuart (Eucalyptus gomphocephala) woodland, including descriptions of four new species. Mycol Res 113:337–353
Tedersoo L, Nilsson RH, Abarenkov K, Jairus T, Sadam A, Saar I, Mohammad B, Bechem E, Chuyong G, Köljalg U (2010) 454 pyrosequencing and sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol 188:291–301
Wang YJ, Tan ZM, Zhang DC, Murat C, Jeandroz S, Le Tacon F (2006) Phylogenetic and populational study of the Tuber indicum complex. Mycol Res 110:1034–1045
Williams RH, Ward E, McCartney HA (2001) Methods for integrated air sampling and DNA analysis for detection of airborne fungal spores. Appl Env Micro 67:2453–2459
Acknowledgements
This work was supported by grants from the Australian Research Council and Murdoch University. We are grateful to Diana White for DNA preparation and to Assoc. Professor Treena Burgess for advice on molecular analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 123 kb)
Rights and permissions
About this article
Cite this article
Ishaq, L., Barber, P.A., Hardy, G.E.S.J. et al. Diversity of fungi associated with roots of Eucalyptus gomphocephala seedlings grown in soil from healthy and declining sites. Australasian Plant Pathol. 47, 155–162 (2018). https://doi.org/10.1007/s13313-018-0548-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-018-0548-x