Abstract
Lateral retroperitoneoscopic adrenalectomy (LRA) is performed mostly by urologists. It is gaining popularity among general surgeons because of the direct access to the adrenal gland. However, the management of large tumors remains controversial. We report our experience and discuss the advantages and the drawbacks of this approach. Between December 2011 and April 2015, 89 consecutive patients underwent LRA for adrenal tumors. Conversion to open surgery, operative time, blood loss, hospital stay, intra-operative complications, early and late postoperative complications, and mortality were analyzed. The entire group was divided into patients with large tumors (> 5 cm) and patients with small tumors (≤ 5 cm), which were further compared. The conversion rate was 1.1%. The mean operative time was 107.4 ± 27.95 min, the mean blood loss 33.15 ± 25.45 ml. The mean hospital stay was 4.7 ± 2.05 days. Most of the complications were minor. There was zero mortality. Concerning the size of the tumor, we found statistically significant difference in operative time (p = 0.001), hospital stay (p = 0.020), incidence of early postoperative complications (p = 0.049), and conversion rate to open surgery (p = 0.037). LRA is a feasible, effective and safe procedure that offers additional advantages over the standard transabdominal approach because of its direct access to the adrenal gland. However, malignancy, large tumor size, bilateral pathology, and concomitant intra-abdominal pathology may represent a potential setback for this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several different approaches currently exist for performing laparoscopic adrenalectomy: lateral transabdominal, anterior transabdominal, posterior retroperitoneal and lateral retroperitoneal. Lateral retroperitoneoscopic adrenalectomy (LRA) has gained popularity mostly among the urology surgeons due to the familiarity with the anatomy of laparoscopic nephrectomy [1]. Recently, it is being performed more often by general surgeons with consistently good results when compared to the conventional transabdominal approach [2]. The main advantage of the LRA is the direct access to the adrenal gland, which avoids the need of mobilization of the abdominal organs [3]. This makes it appealing in the settings of prior abdominal surgery and intra-abdominal adhesions [4]. The drawbacks are the relatively restricted retroperitoneal space and the need to reposition the patient in case of bilateral tumors [5]. The management of tumors larger than 5 cm remains controversial [6]. For better results, patients with adrenal tumors should be referred to high-volume centers with extensive experience in the surgery of adrenals, irrespective of the surgeon specialty—general or urologic [7, 8].
The aim of this study was to report our experience and to discuss the outcomes and the controversies of LRA.
Methods
Patient selection
Our surgical department is the referral university center for the treatment of adrenal pathology in our country. In the era of laparoscopy, LRA was introduced in 1996 and was the initial laparoscopic technique accepted for adrenalectomy in our institution. Since then, it is the operative method of choice for all adrenal tumors except for primary adrenal malignancy and the presence of concomitant intra-abdominal pathology. Between December 2011 and April 2015, 90 patients underwent endoscopic adrenalectomy. After an informed consent, all the operations were performed by a mentor general surgeon. The indications for adrenalectomy were hormonally active adrenal tumors, tumors with size ≥ 4 cm, tumors with size < 4 cm with rapid growth of at least 0.5 cm and/or with newly appeared hormonal activity during the follow-up, and all tumors suspected for isolated metastases. When a bilateral adrenalectomy was indicated, a staged approach was chosen with LRA performed on the side with the larger tumor, and after receiving the histological result, the patient was scheduled for LRA on the contralateral side. All patients under the age of 18 and patients with high suspicion for primary adrenal malignancy were excluded from this study.
Preoperative preparation
The preoperative workup included complete hormonal activity evaluation carried out by endocrinologists, cardiac and anesthesia evaluation, computed tomography, or if necessary, magnetic resonance imaging and positron emission computed tomography. Patients with pheochromocytoma were prepared with α-1 blocker—doxazosin—for at least 10 days before surgery and with β-blocker, if needed. Patients with aldosterone-producing adenoma were prepared with spironolactone and potassium to reach a normal range of potassium levels. All patients received antibiotic prophylaxis and enoxaparin 40 mg/24 h until the discharge.
Surgical technique
The patient was placed in a lateral position with the umbilicus at the level of the bending axis of the table and with a supporting pillow to optimize the trunk extension (Fig. 1). A 1.5 cm incision was made below the angle of 12th rib. The index finger was used to create a space in the retroperitoneum and to guide the placement of a 5-mm trocar just lateral in the anterior axillary line. A blunt 11-mm trocar was then inserted into the initial incision for a 0° optic system. After the insufflation of the CO2 to 15 mmHg pressure, more space was created bluntly, and a 5-mm trocar was placed in the angle between the paraspinal muscle and the 12th rib. If needed, one more 5-mm trocar was inserted in the area of the conventional flank incision. After opening Gerota’s fascia, the kidney was visualized. The fatty tissue containing the adrenal gland was elevated and separated from the superior pole of the kidney using an energy-based device. The adrenal vein was then identified, clipped, and divided (Fig. 2). The entire specimen was removed in a custom-made plastic bag through the initial incision, which was then closed with several figure-of-eight absorbable sutures. A 16 Fr drain was routinely placed.
Statistical analysis
Data were collected prospectively in a dedicated database and reviewed retrospectively. Baseline patient characteristics were recorded including age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) risk group, tumor size, tumor side, previous abdominal surgery and are represented in Table 1. The studied continuous variables included operative time, blood loss, and hospital stay. The studied categorical variables were conversion to open surgery, intra-operative complications, early and late postoperative complications, and mortality. Tumor size was estimated in imaging studies. Operative time was measured from skin incision to closure. The intra-operative blood loss was calculated from the amount of blood aspirated in the suction system. Patients were discharged after a regular diet, a normal ambulation, and adequate pain control. Intra-operative complications were divided into peritoneal tears, diaphragmatic injuries, kidney injuries, injuries of other adjacent structures, and hemodynamic instability. Early postoperative complications (within the first 30 days of operation) were active bleeding, retroperitoneal hematomas, wound-related (surgical site infections, hematomas, seromas, and ecchymosis), subcutaneous emphysemas, and non-surgical complications—deep vein thrombosis (DVT), pulmonary thromboembolism, and others. Late postoperative complications (after 30 days of operation) included trocar site hernias, incisional hernias at the site of specimen extraction, and non-surgical complications. A non-surgical complication was defined as a medical complication resulting from factors other than directly related to the surgical procedure. Follow-up protocol consisted of consultations with a surgeon at 10 days, endocrinologist at 30 days and 6 months, and telephone consultation after that.
The continuous data were expressed as the mean and standard deviation, while the categorical data were presented as the percentage. The patients were divided into two groups depending on the tumor size—with large tumors (> 5 cm) and with small tumors (≤ 5 cm). The continuous variables between the groups were compared with Student’s t test, and the categorical variables between groups were compared with chi-square test. All data for this study were analyzed using the IBM SPSS Statistics v20.0. Results were considered statistically significant when p value was found to be less than 0.05.
Results
A total of 89 LRA’s were performed. In one case, the transabdominal approach was used, and a simultaneous adrenalectomy and cholecystectomy was performed. The most common histological type of removed tumors was nonfunctioning adenoma—36 (40%). 18 patients (20%) had a diagnosis for Cushing’s adenoma or hyperplasia, while 13 (14.4%) had aldosterone-producing adenoma. In ten cases (11.1%), the postoperative pathologic evaluation confirmed the diagnosis of pheochromocytoma. There were two patients with isolated adrenal metastasis (one from lung and one from breast cancer), two patients with unsuspected adrenal carcinoma, and two patients with sex hormone-producing adenoma. The rest of the patients 7 (7.8%) were with other histological diagnosis—myelolipoma (two cases), haemangioma (one case), angiomyelolipoma (one case), and cysts (three cases) (Table 2).
Conversion was necessary in one case of LRA (1.1%). The reason was that an infiltration to the vessels of the kidney necessitated an en bloc nephrectomy. The mean operative time was 107.4 ± 27.95 min without statistically significant differences between the left and right LRA (p = 0.45). The mean blood loss was 33.15 ± 25.45 ml without the need for blood transfusion. The mean hospital stay was 4.7 ± 2.05 days. The percentage of intra-operative complications was 18% (16 of 89 cases). Eight small peritoneal tears that resulted in the loss of working space solved with a transabdominal Veress needle placement. One diaphragmatic injury necessitated chest drainage. One injury of the kidney necessitated a suture. One case of bleeding from m. latissimus dorsi necessitated additional hemostasis, and five cases of hemodynamic instability (in two patients with pheochromocytoma and three patients with aldosterone-producing adenoma) managed with adequate resuscitation and medications. The rate of early postoperative complications was 34.8% (31 of 89 cases). Ecchymosis at the site of specimen extraction (22.5%) and subcutaneous emphysema (6.7%) were the most common, and they were resolved spontaneously. One case of retroperitoneal hematoma, one case of DVT (in a patient with previous history of DVT), one case of pulmonary thromboembolism (in a patient with Cushing’s syndrome), and one case of pulmonary edema, treated uneventfully. Among the late postoperative complications—7.9% (7 of 89 cases)—three patients were registered with incisional hernia at the site of specimen extraction, one patient with trocar site hernia, and three had non-surgical complications more than 30 days after the operation. The operative and postoperative outcomes are represented in Table 3.
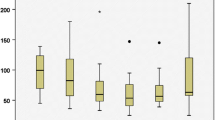
17 adrenalectomies for large tumors (> 5 cm) were performed and 72 for small tumors (≤ 5 cm). There were statistically significant differences between the two groups in terms of operative time (127.8 ± 28.3 vs. 102.9 ± 26 min; p = 0.001), hospital stay (5.75 ± 3.4 vs. 4.4 ± 1.5 days; p = 0.020), incidence of early postoperative complications (53% vs. 30.5%; p = 0.049), and conversion rate to open surgery (5.9% vs. 0%; p = 0.037). There were no statistically significant differences between the groups in terms of blood loss, intra-operative, and late postoperative complications (Table 4).
Discussion
Laparoscopic adrenalectomy is the standard of care for all non-primary adrenal cancer tumors [9]. The overall incidence of conversion to open surgery is 1.4% [10,11,12]. We showed that LRA is a feasible procedure with low conversion rate —1.1%. One of the potential advantages of the direct retroperitoneal access is that LRA avoids the need to enter the peritoneal cavity and mobilize adjacent intra-abdominal organs such as the spleen, pancreas, liver, and colon, which is more traumatic and seems illogical, considering the small size of the adrenal gland and its placement deeply in the retroperitoneal space. Furthermore, the main causes for conversion during transabdominal approach are splenic and pancreatic injuries, which are very difficult for treatment [13, 14]. The reasons for conversion after retroperitoneal approach, cited by the other authors, are failure to progress, inability to develop the retroperitoneal space, and loss of pneumoretroperitoneum, mainly associated with inexperience of the surgeon [15]. Another issue of the transabdominal approach could be the intra-abdominal adhesions from previous surgeries [16]. LRA is ideal for patients who underwent a previous laparotomy, a procedure previously performed on 33.3% of the patients in our study [17]. In case of unexpected primary adrenal malignancy and when an en bloc nephrectomy is required, we prefer to convert into open surgery, especially for large tumors. In our experience, the access to the vessels of the kidney via the lateral retroperitoneal approach is more difficult and compromises the oncological outcomes, comparing to the transabdominal approach. Though, the intraperitoneal dissemination of tumor cells is avoided in LRA. In addition, the transabdominal approach for adrenalectomy has no alternative in the cases of concomitant intra-abdominal pathology and for performing combined abdominal procedure [18].
LRA is a safe procedure. In our study, the mortality was 0% after mean follow-up of 19.75 ± 8.10 months, which is the same like the overall incidence of mortality after laparoscopic adrenalectomy – < 0.5% [19]. One of the well-known drawbacks of the LRA is the restricted working space in the retroperitoneum. This might lead to serious additional technical difficulties and related complications. We reported relatively high incidence of intra- and postoperative complications, comparing to the postoperative morbidity rate after minimally invasive adrenalectomy that ranges from 3 to 20% [20]. On the other hand, in our study, the majority of them are negligible, e.g. small peritoneal tears and subcutaneous emphysema, and the others were treated uneventfully [21]. Only one patient in this study needed a reoperation due to postoperative bleeding and none needed blood transfusion. In this case, the routine retroperitoneal drainage helped us taking the early decision for a revision. However, we believe that it is optional to omit the drain placement after an uncomplicated adrenalectomy [22]. Some authors apply high-pressure CO2 insufflation—20–30 mmHg during retroperitoneal approaches. As a result, an increase in the deleterious effects of CO2 such as hypercarbia, acidosis, and hypertension can be observed [23, 24]. On the other side, some patients tolerate retroperitoneal CO2 insufflation better than intraperitoneal CO2 insufflation from a hemodynamic and respiratory perspective [25]. In our practice, the CO2 insufflation pressure was usually between 12 and 15 mmHg, which is a standard pressure for laparoscopic operations. We registered five cases of hemodynamic instability—two in patients with pheochromocytoma and three in patients with aldosterone-producing adenoma—which are well-known events even with proper preoperative preparation and are previously described by Gockel et al. [26, 27]. All of them were successfully managed by the anesthesiologist. For this reason, we believe that the presence of an experienced anesthesiologist in the treatment of the adrenal pathology is mandatory. Despite the use of lower insufflation pressure, venous thromboembolic events still can occur in risky patients like the two cases in this series. Therefore, we suggest prolonged anticoagulant prophylaxis in patients with Cushing’s syndrome [28]. We also registered 0% of surgical site infection, which is better compared to the results of other authors, and no case of neuromuscular pain linked to subcostal nerve injury—a complication, specific for the retroperitoneal approach [29, 30]. In this study, the mean blood loss was 33.15 ± 25.45 ml, which is lower than that of other authors who used other approaches—74 ml [7, 10]. We believe that this might be due to the direct access to the gland in LRA, which provides easy ligation of the adrenal vein.
As reported recently, the most important advantages of the retroperitoneal approaches and their direct access to the adrenal gland are shorter operative time, better postoperative pain score, shorter hospital stay [31,32,33]. It is estimated that the learning curve of laparoscopic adrenalectomy is approximately 30–40 procedures [34]. Bakkar et al. [35] described a rapidly achieved learning curve for posterior retroperitoneoscopic adrenalectomy (PRA) by experienced surgeons and noted significant reduction in operative time after the sixth procedure. Due to the profile of our department, which is one of the largest teaching units in the country, we do not expect the mean operative time to be reduced. Although training has an obvious impact on our operative time, we registered a mean operative time of 107.4 ± 27.95 min, which appears comparable to that of other authors [36]. However, some authors reported a shorter mean operative time. For example, Walz et al. [37] reported a mean operative time of 57 min after PRA. Zhang et al. [38] reported even better mean operative time— 43 min—for LRA. In our experience, the left LRA can be sometimes more challenging due to the higher position of the left kidney necessitating its retraction caudally for better exposure. However, we did not find statistically significant differences in the operative time between the left and right LRA (p = 0.45). The mean hospital stay in our study was 4.7 ± 2.05 days. Kiriakopoulos et al. [39] reported reduction of postoperative pain and faster recovery of bowel movements after retroperitoneal adrenalectomy due to the avoidance of pneumoperitoneum. 60% of the patients did not require narcotic analgesia, and were mobilized and started diet on the evening of the surgery. This led to mean hospital stay of 1–2 days. LRA demands repositioning of the patient for bilateral adrenalectomy compared to the PRA. In our experience, this is associated with increased operative time and morbidity. For these reasons, staged approach would be indicated, although the ideal approach for bilateral adrenalectomy is still debated [40]. On the other hand, LRA may be more favorable in morbidly obese patients due to technical difficulties associated with the prone position in the PRA [41]. In our study, we reported success in the LRA in ten patients with BMI > 35 kg/m2. LRA can be also more easily converted to an open surgery or flank transperitoneal approach compared to the PRA [42].
Tumor size is also an important consideration of the LRA regarding the restricted working space. Previous authors’ opinions showed that the transabdominal approach is better than the retroperitoneal in the treatment of large adrenal tumors often defined as tumors larger than 5 cm [43,44,45]. We anticipate that with the improvement of the technique and the experience, LRA can be performed for larger tumors with good results [46]. In our study, we confirmed the feasibility of the LRA for adrenal tumors larger than 5 cm with only one case of conversion to open surgery and with comparable results to the other approaches. We found that LRA for larger tumors has statistically significant longer operative time and hospital stay compared to the group of patients with small tumors (≤ 5 cm). We also found a statistically significant increased rate of early postoperative complications and two cases of incisional hernia, which in our opinion is due to the difficulties in specimen extraction, sometimes necessitating a major enlargement of the incision. To minimize such complications in the future, a successful alternative in some cases of large adrenal tumors could be a partial adrenalectomy or morcellation of the tumor [47]. Recently, Chen et al. [6] also compared LRA for large tumors (> 5 cm) and small tumors (≤ 5 cm). They confirmed statistically significant longer operative time and longer hospital stay after LRA for larger tumors. However, no significant differences in the number of conversions and the number of postoperative complications were observed.
In conclusion, LRA is a feasible, effective, and safe procedure that offers additional advantages over the standard transabdominal approach because of its direct access to the adrenal gland. However, malignancy, large tumor size, bilateral pathology, and concomitant intra-abdominal pathology may represent a potential setback for this approach.
References
Gasman D, Droupy S, Koutani A, Salomon L, Antiphon P, Chassagnon J, Chopin DK, Abbou CC (1998) Laparoscopic adrenalectomy: the retroperitoneal approach. J Urol 159:1816–1820
Ramacciato G, Nigri GR, Petrucciani N, Di Santo V, Piccoli M, Buniva P, Valabrega S, D’Angelo F, Aurello P, Mercantini P, Del Gaudio M, Melotti G (2011) Minimally invasive adrenalectomy: a multicenter comparison of transperitoneal and retroperitoneal approaches. Am Surg 77:409–416
Suzuki K, Kageyama S, Hirano Y, Ushiyama T, Rajamahanty S, Fujita K (2001) Comparison of 3 surgical approaches to laparoscopic adrenalectomy: a nonrandomized, background matched analysis. J Urol 166:437–443
Lin Y, Li L, Zhu J, Qiang W, Makiyama K, Kubota Y (2007) Experience of retroperitoneoscopic adrenalectomy in 195 patients with primary aldosteronism. Int J Urol 14:910–913
Dickson PV, Jimenez C, Chisholm GB, Kennamer DL, Ng C, Grubbs EG, Evans DB, Lee JE, Perrier ND (2011) Posterior retroperitoneoscopic adrenalectomy: a contemporary American experience. J Am Coll Surg 212:659–665
Chen W, Liang Y, Lin W, Fu GQ, Ma ZW (2018) Surgical management of large adrenal tumors: impact of different laparoscopic approaches and resection methods on perioperative and long-term outcomes. BMC Urol 18:31
Pędziwiatr M, Wierdak M, Ostachowski M, Natkaniec M, Białas M, Hubalewska-Dydejczyk A, Matłok M, Major P, Budzyński P, Migaczewski M, Budzyński A (2015) Single center outcomes of laparoscopic transperitoneal lateral adrenalectomy-Lessons learned after 500 cases: a retrospective cohort study. Int J Surg 20:88–94
Park HS, Roman SA, Sosa JA (2009) Outcomes from 3144 adrenalectomies in the United States: which matters more, surgeon volume or specialty? Arch Surg 144:1060–1067
Gumbs AA, Gagner M (2006) Laparoscopic adrenalectomy. Best Pract Res Clin Endocrinol Metab 20:483–499
Assalia A, Gagner M (2004) Laparoscopic adrenalectomy. Br J Surg 91:1259–1274
Berber E, Tellioglu G, Harvey A, Mitchell J, Milas M, Siperstein A (2009) Comparison of laparoscopic transabdominal lateral versus posterior retroperitoneal adrenalectomy. Surgery 146:621–625
Lezoche E, Guerrieri M, Feliciotti F, Paganini AM, Perretta S, Baldarelli M, Bonjer J, Miccoli P (2002) Anterior, lateral, and posterior retroperitoneal approaches in endoscopic adrenalectomy. Surg Endosc 16:96–99
Bonjer HJ, Lange JF, Kazemier G, de Herder WW, Steyerberg EW, Bruining HA (1997) Comparison of three techniques for adrenalectomy. Br J Surg 84:679–682
Terachi T, Yoshida O, Matsuda T, Orikasa S, Chiba Y, Takahashi K, Takeda M, Higashihara E, Murai M, Baba S, Fujita K, Suzuki K, Ohshima S, Ono Y, Kumazawa J, Naito S (2000) Complications of laparoscopic and retroperitoneoscopic adrenalectomies in 370 cases in Japan: a multi-institutional study. Biomed Pharmacother 54:211s–214s
Naya Y, Nagata M, Ichikawa T, Amakasu M, Omura M, Nishikawa T, Yamaguchi K, Ito H (2002) Laparoscopic adrenalectomy: comparison of transperitoneal and retroperitoneal approaches. BJU Int 90:199–204
Greco F, Hoda MR, Rassweiler J, Fahlenkamp D, Neisius DA, Kutta A, Thüroff JW, Krause A, Strohmaier WL, Bachmann A, Hertle L, Popken G, Deger S, Doehn C, Jocham D, Loch T, Lahme S, Janitzky V, Gilfrich CP, Klotz T, Kopper B, Rebmann U, Kälbe T, Wetterauer U, Leitenberger A, Rassler J, Kawan F, Inferrera A, Wagner S, Fornara P (2011) Laparoscopic adrenalectomy in urological centres—the experience of the German Laparoscopic Working Group. BJU Int 108:1646–1651
Castillo O, Cortés O, Kerkebe M, Pinto I, Arellano L, Contreras M (2006) Laparoscopic surgery in the treatment of adrenal pathology: experience with 200 cases. Actas Urol Esp 30:926–932
Kim G, Lomanto D, Lawenko MM, Lopez-Gutierrez J, Lee-Ong A, Iyer SG, Cheah WK, So JB, Tsang CB, Fong YF (2013) Single-port endo-laparoscopic surgery in combined abdominal procedures. Asian J Endosc Surg 6:209–213
Constantinides VA, Christakis I, Touska P, Palazzo FF (2012) Systematic review and meta-analysis of retroperitoneoscopic versus laparoscopic adrenalectomy. Br J Surg 99:1639–1648
Tiberio GA, Solaini L, Arru L, Merigo G, Baiocchi GL, Giulini SM (2013) Factors influencing outcomes in laparoscopic adrenal surgery. Langenbecks Arch Surg 398:735–743
Conzo G, Tartaglia E, Gambardella C, Esposito D, Sciascia V, Mauriello C, Nunziata A, Siciliano G, Izzo G, Cavallo F, Thomas G, Musella M, Santini L (2016) Minimally invasive approach for adrenal lesions: systematic review of laparoscopic versus retroperitoneoscopic adrenalectomy and assessment of risk factors for complications. Int J Surg Suppl 1:S118–S123
Major P, Matłok M, Pędziwiatr M, Budzyński A (2012) Do we really need routine drainage after laparoscopic adrenalectomy and splenectomy? Wideochir Inne Tech Maloinwazyjne 7:33–39
Lombardi CP, Raffaelli M, De Crea C, Sollazzi L, Perilli V, Cazzato MT, Bellantone R (2008) Endoscopic adrenalectomy: is there an optimal operative approach? Results of a single-center case-control study. Surgery 144:1008–1014
de La Chapelle A, Deghmani M, Dureuil B (1998) Peritoneal insufflation can be a critical moment in the laparoscopic surgery of pheochromocytoma. Ann Fr Anesth Reanim 17:1184–1185
Fernández-Cruz L, Saenz A, Benarroch G, Astudillo E, Taura P, Sabater L (1996) Laparoscopic unilateral and bilateral adrenalectomy for Cushing’s syndrome. Transperitoneal and retroperitoneal approaches. Ann Surg 224:727–734
Gockel I, Vetter G, Heintz A, Junginger T (2005) Endoscopic adrenalectomy for pheochromocytoma: difference between the transperitoneal and retroperitoneal approaches in terms of the operative course. Surg Endosc 19:1086–1092
Gockel I, Heintz A, Kentner R, Werner C, Junginger T (2005) Changing pattern of the intraoperative blood pressure during endoscopic adrenalectomy in patients with Conn’s syndrome. Surg Endosc 19:1491–1497
Boscaro M, Sonino N, Scarda A, Barzon L, Fallo F, Sartori MT, Patrassi GM, Girolami A (2002) Anticoagulant prophylaxis markedly reduces thromboembolic complications in Cushing’s syndrome. J Clin Endocrinol Metab 87:3662–3666
Nocca D, Aggarwal R, Mathieu A, Blanc PM, Deneve E, Salsano V, Figueira G, Sanders G, Domergue J, Millat B, Fabre PR (2007) Laparoscopic surgery and corticoadrenalomas. Surg Endosc 21:1373–1376
Siperstein AE, Berber E, Engle KL, Duh QY, Clark OH (2000) Laparoscopic posterior adrenalectomy: technical considerations. Arch Surg 135:967–971
Rubinstein M, Gill IS, Aron M, Kilciler M, Meraney AM, Finelli A, Moinzadeh A, Ukimura O, Desai MM, Kaouk J, Bravo E (2005) Prospective, randomized comparison of transperitoneal versus retroperitoneal laparoscopic adrenalectomy. J Urol 174:442–445
Cabalag MS, Mann GB, Gorelik A, Miller JA (2014) Comparison of outcomes after laparoscopic versus posterior retroperitoneoscopic adrenalectomy: a pilot study. Surg Laparosc Endosc Percutan Tech 24:62–66
Callender GG, Kennamer DL, Grubbs EG, Lee JE, Evans DB, Perrier ND (2009) Posterior retroperitoneoscopic adrenalectomy. Adv Surg 43:147–157
Guerrieri M, Campagnacci R, De Sanctis A, Baldarelli M, Coletta M, Perretta S (2008) The learning curve in laparoscopic adrenalectomy. J Endocrinol Invest 31:531–536
Bakkar S, Materazzi G, Fregoli L, Papini P, Miccoli P (2017) Posterior retroperitonoscopic adrenalectomy; a back door access with an unusually rapid learning curve. Updates Surg 69:235–239
Kwan TL, Lam CM, Yuen AW, Lo CY (2007) Adrenalectomy in Hong Kong: a critical review of adoption of laparoscopic approach. Am J Surg 194:153–158
Walz MK, Alesina PF, Wenger FA, Deligiannis A, Szuczik E, Petersenn S, Ommer A, Groeben H, Peitgen K, Janssen OE, Philipp T, Neumann HP, Schmid KW, Mann K (2006) Posterior retroperitoneoscopic adrenalectomy–results of 560 procedures in 520 patients. Surgery 140:943–948
Zhang X, Fu B, Lang B, Zhang J, Xu K, Li HZ, Ma X, Zheng T (2007) Technique of anatomical retroperitoneoscopic adrenalectomy with report of 800 cases. J Urol 177:1254–1257
Kiriakopoulos A, Economopoulos KP, Poulios E, Linos D (2011) Impact of posterior retroperitoneoscopic adrenalectomy in a tertiary care center: a paradigm shift. Surg Endosc 25:3584–3589
Lan BY, Taskin HE, Aksoy E, Birsen O, Dural C, Mitchell J, Siperstein A, Berber E (2015) Factors affecting the surgical approach and timing of bilateral adrenalectomy. Surg Endosc 29:1741–1745
Agha A, von Breitenbuch P, Gahli N, Piso P, Schlitt HJ (2008) Retroperitoneoscopic adrenalectomy: lateral versus dorsal approach. J Surg Oncol 97:90–93
Karanikola E, Tsigris C, Kontzoglou K, Nikiteas N (2010) Laparoscopic adrenalectomy: where do we stand now? Tohoku J Exp Med 220:259–265
Castillo OA, Vitagliano G, Secin FP, Kerkebe M, Arellano L (2008) Laparoscopic adrenalectomy for adrenal masses: does size matter? Urology 71:1138–1141
Sharma R, Ganpule A, Veeramani M, Sabnis RB, Desai M (2009) Laparoscopic management of adrenal lesions larger than 5 cm in diameter. Urol J 6:254–259
Agha A, Iesalnieks I, Hornung M, Phillip W, Schreyer A, Jung M, Schlitt HJ (2014) Laparoscopic trans- and retroperitoneal adrenal surgery for large tumors. J Minim Access Surg 10:57–61
Chen W, Li F, Chen D, Zhu Y, He C, Du Y, Tan W (2013) Retroperitoneal versus transperitoneal laparoscopic adrenalectomy in adrenal tumor: a meta-analysis. Surg Laparosc Endosc Percutan Tech 23:121–127
Xu T, Xia L, Wang X, Zhang X, Zhong S, Qin L, Zhang X, Zhu Y, Shen Z (2015) Effectiveness of partial adrenalectomy for concomitant hypertension in patients with nonfunctional adrenal adenoma. Int Urol Nephrol 47:59–67
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study (retrospective analysis), formal consent is not required. The study was approved by the Institutional Review Board of Alexandrovska University Hospital, Sofia.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grozdev, K., Khayat, N., Shumarova, S. et al. Lateral retroperitoneoscopic adrenalectomy: advantages and drawbacks. Updates Surg 72, 1151–1157 (2020). https://doi.org/10.1007/s13304-020-00741-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-020-00741-6