Abstract
Recently, individualized approaches for the treatment of locally advanced rectal cancers (RC) have been introduced to determine the most beneficial one for boosting the tumor response and assessing the response more accurately. However, despite each patient and tumor have different molecular features, the studies at the molecular level are very limited. In this study, examining the clinical factors which are predictive of pathologic complete response (pCR), helping to determine a treatment program for the management of patients with locally advanced RC, and evaluating the relation between regression grade and MMR-MSI were aimed. 341 RC cases who had undergone surgery were included and divided into three groups according to their response to neoadjuvant treatment. The following parameters were analyzed for all patients: age at diagnosis, sex, tumor location, tumor differentiation, TNM stage, histological subtype, CEA (mean: < 5 ng/ml) level, lymphovascular-neural invasion, presence of mucinous subtype, grade, MMR, and MSI statuses. 147 patients (43.2%) had no response (group 1), 141 patients (41.3%) had an intermediate response (group 2), and 53 patients (15.5%) had a complete response (group 3). Neoadjuvant chemoradiotherapy was used in all of the patients with the same protocol. Multivariate analysis revealed that clinical T stage (p: 0.099) and MMR (p: 0.048) were the parameters which were significantly associated with pCR. Since MMR and MSI statuses were found to affect pCR, more careful patient selection for “watch and wait” protocol and further studies on molecular structures of the tumors for individualized therapies are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rectal cancer (RC) has the second highest-increasing incidence among the digestive tract cancers according to the data of the American Cancer Society (ACS) [1]. Surgery is the gold standard treatment for early stage rectal cancers [2]. However, standard treatment for locally advanced rectal cancer includes neoadjuvant chemoradiation (CCRT), total mesorectal excision (TME), and adjuvant chemotherapy (CT) [3]. These treatment methods have improved organ preservation, the local control of the disease, and thus increased patients’ overall survival (OS) [4]. Recently, individualized approaches have been introduced to determine the most beneficial neoadjuvant treatment for boosting the tumor response, assessing the response more accurately, and amending the relation between clinical results and pathologic findings. Pathologic complete response (pCR) is observed in about 10–30% of patients who received neoadjuvant treatments [5]. Thus, patients with pCR lack any viable tumor cells in the final surgical specimen.
Previous retrospective studies reported that tumor response to preoperative CCRT is influenced by some clinical factors such as smoking, tumor size, TNM classification, and carcinoembryonic antigen (CEA) level [6]. However, despite each patient and tumor have different molecular features, the studies at the molecular level are very limited. Whereas colorectal cancer (CRC) is a heterogeneous disease that can occur with different molecular pathological pathways. The possible defined pathways in which CRC can occur are chromosomal instability (CIN), epigenetic alterations, and defects in the DNA mismatch repair (MMR) system. Microsatellite instability (MSI) is a phenotypic evidence which shows that MMR system is not functioning normally, and it exists in around 15% of all CRCs [7]. The outcomes of long-term prognosis of deficient mismatch repair (dMMR) rectal cancers who received curative therapy have not been reported yet. There are reports in the literature supporting that high microsatellite instability (MSI-H) CRCs have a better prognosis compared to low MSI (MSI-L) or microsatellite stable (MSS) RCs [8]. On the other hand, there are also studies reporting the opposite [9]. However, there is no definite information on whether germline mutations have an effect on radiation sensitivity of the colorectal tumor.
In this study, examining the clinical factors which are predictive of pCR after neoadjuvant CCRT and helping to determine a treatment program for the management of patients with locally advanced RC were aimed. Furthermore, the relation between regression grade and MMR-MSI was evaluated, and its effect on prognosis and survival was presented.
Materials and methods
The retrospective study protocol was approved by the institutional Ethics Committee (Number: 253, Date: 26.09.2018). A written informed consent was obtained from each patient. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Patients
341 RC cases who had undergone surgery in our department between January 2014 and August 2018 were included in the study. Specimens were subjected to histological examination, and histological response was defined in accordance with American Joint Committee on Cancer’s (AJCC) classification [10]. Tumor regression is graded (TRG) from 0 to 3. TRG0 means no existence of viable tumor cells which is also called pCR. TRG1, 2, and 3 represent a small group of tumor cells, residual cancer with fibrosis, and intense residual cancer, respectively.
Our patients were divided into three groups according to their response to CCRT: the patients with no response (Group 1), intermediate response (Group 2), and complete response (pCR) (Group 3).
The inclusion criteria were (1) patients with pathologically confirmed RC and had undergone curative surgical resection, (2) patients with stage II–III disease, and (3) patients who had received neoadjuvant CCRT. We excluded the patients with (1) two or more primary tumors, (2) carcinoma in situ (CIS) tumors, and (3) patients who had received neoadjuvant alone RT (to reduce pCR rate).
Disease staging was performed according to the fifth edition of the AJCC TNM classification. The patients’ demographics and clinicopathological characteristics were collected from a medical data platform by trained staff who used standardized data collection and quality-control procedures.
The initial evaluation included digital examination of the rectum and colonoscopy (biopsy performed). The screening and clinical staging was established using thoraco-abdominal computed tomography, endorectal ultrasonography, and magnetic resonance of the pelvis.
The following parameters were analyzed for all patients: age at diagnosis, sex, tumor location, tumor differentiation, TNM stage, histological subtype, CEA (mean: < 5 ng/ml) level, lymphovascular-neural invasion, presence of mucinous subtype, grade, and MMR and MSI statuses.
Chemoradiotherapy and surgery
Patients received preoperative RT to the primary tumor and perirectal metastatic lymph nodes in 55 Gy dose, 5 days a week for 30–35 days. Either two cycles of bolus infusions of 5-fluorouracil (5-FU) at 425 mg/m2 per day, five times weekly, every 4 weeks or capecitabine at 825 mg/m2 twice a day, and 5 days per week for 6 weeks were given concurrently.
Patients underwent low anterior resection or abdominoperineal resection approximately 9–12 weeks after neoadjuvant treatment.
All patients received FOLFOX (folinic acid, 5-flurouracyl, oxaliplatin) regimen as adjuvant chemotherapy in 3–6th weeks after the surgery.
Mismatch repair (MMR) and microsatellite instability (MSI)
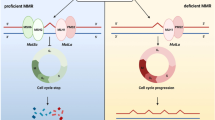
Cases who were negative for the expression of human mutL homolog 1 (hMLH1), human mutS homolog 2 (hMSH2), human mutS homolog 6 (hMSH6), and/or PMS2 genes were defined as having mismatch repair deficiency (dMMR), and all other patients were defined as having mismatch repair proficiency (pMMR). In our cases, all genes of MMR system were evaluated by immunohistochemistry (IHC). The detailed distribution of the negative expression of these genes (most common ones were MLH1 and PMS2) was not given in this study, since this study was focused on the only dMMR/pMMR statuses rather than a genetic aspect.
A change that occurs in the DNA of certain cells (such as tumor cells) in which the number of repeats of microsatellites (short, repeated sequences of DNA) is different than the number of repeats that was in the inherited DNA. These microsatellites are also used as dMMR markers. MSI is caused by dMMR which results in production of a truncated, nonfunctional protein or loss of a protein. Therefore, dMMR is frequently analyzed by testing for loss of an MMR protein or for MSI using a PCR-based assay.
It should be noted that MSI testing is not a genetic test, but rather helps to stratify the risk of having an inherited cancer predisposition syndrome and identifies patients who might benefit from subsequent genetic testing. Immunohistochemistry is available as an add-on to this test (IHC/Mismatch Repair (MMR) Protein Immunohistochemistry Only, Tumor).
MSI is determined by five markers; BAT25/26, D2S123, D5S346 and D17S250. The MSI status was classified into three groups: a high-frequency group (MSI-H) with ≥ 2 of the five markers, a low-frequency group (MSI-L) with one marker, and a stable group (MSS) with no markers all of instability. The status of MSI was determined according to these standards in all cases. MSI testing was performed on paraffin-embedded tumor tissue due to the insufficiency of pre-treatment biopsies.
Follow-up
Follow-up data were collected from the follow-up platform of our hospital. OS was defined as the time from the initial surgical resection until death for any reason. Disease-free survival was defined as the time from the initial surgical resection to recurrence or metastasis of CRC. The median duration of follow-up for all participants was 21 months (range 6.4–55.2 months).
Statistical analysis
For discrete and continuous variables, descriptive statistics (mean, standard deviation, and percentile) were given. In addition, the homogeneity of the variances, which is one of the prerequisites of parametric tests, was checked through Levene’s test. The assumption of normality was tested via the Shapiro–Wilk test. To compare the differences between three and more groups, one-way analysis of variance was used when the parametric test prerequisites were fulfilled, and the Kruskal–Wallis test was used when such prerequisites were not fulfilled. The Bonferroni correction method, which is a multiple comparison test, was used to evaluate the significant results concerning three and more groups. Binary outcome variable with continuous arguments consists of both discrete variable logistic regression analysis to examine the relationship between a set can identify.
Chi-squared test was used for determining the relationships between two discrete variables. When the expected sources were less than 20%, values were determined through the Monte Carlo Simulation Method to include such sources in analysis. Survival analysis for using the Kaplan–Meier method and the comparison of the variables of the survival times of the factors between the categories was evaluated by the Log-Rank Mantel Cox test. The data were evaluated via SPPS 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). p < 0.05 and p < 0.01 were taken as significance levels.
Results
Demographics, and clinical and histopathologic characteristics
Among 341 patients with RC, 147 patients (43.2%) had no response (group 1), 141 patients (41.3%) had an intermediate response (group 2), and 53 patients (15.5%) had a complete response (group 3).
Demographics of the patients are given in Table 1. Among 341 patients, 67.7% were male. Median age at the diagnosis was 64.75 years (range 24–88 years), and the cases were mostly diagnosed at age 60 years and over (69.8%). There were one or more comorbid conditions in 20% of the patients, and hypertension, diabetes mellitus, and coronary artery disease were most frequent ones. The tumor was located in the lower rectum in 48.9% of the patients. Tumor localization and initial CEA concentration were not associated with tumor response rate.
Clinical and histopathologic characteristics of the patients are shown in Table 2. When histological types were assessed, 68% of the tumors were moderately differentiated and 17.6% were poor/undifferentiated. Poor/undifferentiated subtype was more frequent in no-response group, while well differentiation was in pCR group. Majority of the cases were diagnosed with stage III CRC (77.1%). There was a statistically significant difference between groups regarding distribution of the tumor stage. It was observed that patients with early clinical T and N stages had a better response to neoadjuvant treatment. Additionally, patients with grade 1 tumor were more common in the complete response group.
The dMMR subset accounted for 13.4% of the patients whose data of MMR status were available, and 8.3% of the cases who had available data of MSI status had high-frequency MSI (MSI-H).
Proficient MMR and MSS were found to be significantly higher in the complete response group, while dMMR and MSI-H were highest in the no-response group. This created a statistically significant difference between the groups in terms of MMR and MSI statuses.
Besides, 11 patients (3.2%) had Lynch syndrome and seven of these had dMMR and MSI-H.
In our series, lymphovascular invasion, neural invasion, and mucinous subtype were more frequent in group 1 (respectively, 54.4%, 35.4%, and 43.5%).
Mean duration of hospital stay was 11.23 days. The median duration of follow-up for all participants was 21 months (range 6.4–55.2 months). The recurrence and/or metastasis rate of 15.3% (n: 52) was observed during the follow-up, and the majority (56%) of these recurrent cases were from no-response group.
Multivariate analyses of predictive factors for pCR were performed using 13 parameters (Table 3). Multivariate logistic regression analysis revealed that clinical T stage (p: 0.099) and MMR (p: 0.048) were the parameters which were significantly associated with pCR.
Survival analyses by tumor regression grade, MSI status, and MMR status
Mean survival time was 21.7 months and median survival time was 21 months.
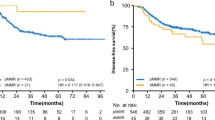
Kaplan–Meier survival curves revealed that there was no significant difference between the OS and tumor response degree (Fig. 1).
The results of OS according to MSI and OS according to MMR are shown in Figs. 2a–c and 3a–c, respectively. There was no significant difference in survival between groups in terms of both MSI and MMR statuses.
Discussion
There are many previous studies in which demographics and clinicopathologic factors affecting the pCR after neoadjuvant CCRT in locally advanced rectal cancer were assessed [11, 12]. Besides, a novel approach, “watch and wait” protocol has gained popularity in recent years. Although there are limited published data of long-term results, approximately 2.8–80% of these patients present with local regrowth [13]. It should not be forgotten that the immune and molecular structure of each patient and tumor are different.
In addition to the patients’ demographics and clinicopathologic features, if the immune and molecular structures are evaluated, both increased rates in TRG after neoadjuvant treatment and much more successful rates of DFS and OS in “watch and wait” strategy can be obtained.
In our study, patients with locally advanced RC were divided into three groups according to their response to neoadjuvant CCRT and clinicopathological features of these three groups were compared. The pCR rate in our study was 15.5%. Factors which were found to have an effect on pCR in univariate analysis were subjected to multivariate analysis to detect the most significant ones. Furthermore, we evaluated the possible predictive and prognostic roles of dMMR and MSI statuses, before and after preoperative therapy in patients who received preoperative CCRT and curative surgery for locally advanced RC. To the best of our knowledge, this is the largest clinical series of patients with dMMR RC and its findings supported several important results. First, we showed that patients with dMMR had lower rate of pCR (0.001). Second, we found that dMMR and MSI-H were poor prognostic factors for RC, and although they do not have an effect on OS, DFS rate was lower and distant metastasis rate was higher.
Grading the histological changes is an alternative method for evaluating the response to the treatment, which is called TRG. Semiquantitative grading of viable tumor density is the way of TRG for evaluating the response.
Tumor regression grading was showed to be an independent prognostic factor in esophagus, gastric, bladder, head, and neck cancers. Previous studies showed that the incidence of distant metastasis and treatment failure were higher, while the local recurrence rate was not affected in RCs. However, the long-term results showed a significant association of TRG with DFS [14].
The risk of CRC is higher in males and its incidence increases significantly in older ages. Also, in many previous studies, it was reported that age did not affect TRG in RCs, but males had a better response to CCRT [15]. Additionally, the effect of CEA level on TRG is still controversial. While some studies suggested that high CEA level represented heavy tumor burden and might require a higher dose of radiation to achieve the same tumor response and prognosis [16], there are also publications asserting that CEA level had no effect on TRG [15].
In our study, the male population was significantly higher than females, and males were found to have higher TRG rate when compared to females. The number of RC patients aged 60 and over was significantly higher, but age was not observed to be dependent to TRG. In addition, CEA level was found to have no effect on TRG.
Advanced clinical T stage (ycT) is always associated with large tumor size and introduced as a prominent prognostic factor in locally advanced rectal cancer after preoperative CCRT. Therefore, the radiobiological paradigm for destroying tumor cells which depends on the tumor size may partially explain the relation between the ycT and TRG.
Previous studies identified cN0 as an independent predictive factor of pCR. The use of TME, since the mesorectal lymph nodes which contain tumor deposits were removed, decreased the recurrence rate in RC patients [17]. However, the value of pathological lymph-node positivity (ypN +) as an indicator of oncologic outcome is still unclear. Patients with ypT3–4 or ypN1–2 disease were found to have higher rates of distant metastasis and local recurrence in a post hoc analysis of the German rectal cancer trial [14]. Pate et al. reported that pathological T stage (ypT) affected local recurrence, but there was no relation between pathologic lymph-node status (ypN) and recurrence [18].
Our data suggested that ypT, ypN, and advanced grade of tumors are associated with DFS but not with OS. We also demonstrated that low T stage was the strongest determinant of pCR by multivariate analysis, while cN0 was associated with higher rates of pCR in univariate analyses.
Presences of lymphovascular invasion, neural invasion, and mucinous subtype were reported to be poor prognostic factors and adversely affect TRG [19]. This is possibly related to the autonomic pelvic nerve and lymphovascular preservation in patients with the neural and lymphovascular invasion [19]. Additionally, previous evidence has shown that mucinous adenocarcinomas were associated with poor survival and, therefore, require a more aggressive treatment strategy, especially if TRG was inadequate [20]. Our results which are corresponded with the literature suggested that lymphovascular-neural invasion and mucinous subtype affect the response to the radiation and prognosis adversely.
Colorectal cancer is a disease deriving from genetic alterations which involve changes in the DNA of oncogenes and/or tumor suppressor genes. MSI-high tumors consist of cells with mismatch repair deficiency that accumulate DNA errors throughout the genome and are associated with a superior outcome. MSI-H tumors constitute about 15% of CRCs and 2–9.3% of RCs [21, 22].
MMR system has several roles in the response to DNA damage and conduct the alteration from regular cell cycle to cell death. Although common opinion accepts that MSI-H and dMMR display poor response to adjuvant fluoropyrimidine, their behavior with neoadjuvant fluoropyrimidine in terms of combination with radiation is controversial [23]. There are publications which reported that MSI-H and dMMR reduce pCR, as well as have no effect [11, 21, 24, 25].
In addition to radiation sensitivity, the results of studies on the prognostic role of MMR or MSI statuses in RC are also contradictory and inconclusive. Some studies reported that patients with MSI-H tumors had a better prognosis than those with MSI-L or MSS tumors [26, 27], while others reported that MSI in CRC was not an independent prognostic factor [28]. From these studies, Korean group study presented MSI-H as a strong positive prognostic factor in colon cancer, whereas it had no prognostic value in rectum cancers [29]. According to the study of Samowitz et al.’s MSI-H RCs, T4 tumors, higher grade, poorly differentiated histology, and locoregional lymph-node metastasis were more common and negative prognostic factors [9].
Pathologic complete response is a strong indicator of oncologic outcomes of RC and it is known that patients with pCR have better oncologic outcomes [30]. In a single-center study, 5-year OS for complete, intermediate, and poor response were 93.4%, 87.0%, and 77.3%, respectively [31]. In another RC subanalysis, 10-year DFS rates for complete, intermediate, and poor TRG were of 89.5%, 73.6%, and 63.0%, respectively. Our results were also compatible with the literature in terms of DFS. However, in our series, pCR was found not to have an effect on OS.
According to many publications, low-lying rectal cancer is associated with poor prognosis, which could explain the poor histological response associated with this localization [32]. Our results did not support this, since distance from the anal verge was not related with pCR.
Another predictive factor for pCR is the delayed interval of neoadjuvant treatment to surgery. Usually, surgery is performed 6–8 weeks after neoadjuvant CRRT with a rate of 11–20% pCR. However, previous studies revealed that extending the waiting time did not change the pCR rate, but might only be associated with higher morbidity and more challenging surgical resection [11, 33]. Also, dose-escalated RT (over 54 Gy) and double-agent CT did not have an effect on increasing the pCR rate. In our study, the interval of neoadjuvant treatment to surgical resection and radiation dose were not predictive of pCR.
Besides its distinguished aspects, there are also few limitations of this study. First; the study population was identified using a linked administrative database that do not include information regarding various known prognostic factors in colon cancer, such as family history, smoking status, diet, or race/ethnicity. Second, KRAS, BRAF mutation, and p53 expression were unknown due to the working protocols of our hospital. Third; we could not access the MMR and MSI statuses of all patients and all patients were from single center. Additionally, salvage therapies and specific chemotherapeutic agents were not included in the data.
Conclusion
To the best of our knowledge, our series of 145 patients with dMMR and 26 patients with MSI-H RC who underwent surgery after neoadjuvant CRRT is one of the largest series in this subject. Tumors with MMR were related with a reduced pCR rate on multivariable analysis. Patients with MSI-H and dMMR had poor prognosis. Also, as non-operative management trend has increased, emphasizing the importance of MMR may lead clinicians to evaluate these statuses and practice “watch and wait” protocol more carefully in dMMR cases. We believe that with the further future studies on molecular structures of the tumors, more appropriate and individualized therapies will be selected for patients.
References
American Cancer Society (2016) Cancer facts and figures 2016. American Cancer Society, Atlanta
van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, Beets-Tan RG, van den Broek CB, Brown G, Van Cutsem E, Espin E, Haustermans K, Glimelius B, Iversen LH, van Krieken JH, Marijnen CA, Henning G, Gore-Booth J, Meldolesi E, Mroczkowski P, Nagtegaal I, Naredi P, Ortiz H, Påhlman L, Quirke P, Rödel C, Roth A, Rutten H, Schmoll HJ, Smith JJ, Tanis PJ, Taylor C, Wibe A, Wiggers T, Gambacorta MA, Aristei C, Valentini V (2014) EURECCA colorectal: multidisciplinary management: european consensus conference colon& rectum. Eur J Cancer 50:1.e1–1.e34. https://doi.org/10.1016/j.ejca.2013.06.048
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of A 11 years. J Clin Oncol 30:1926–1933. https://doi.org/10.1200/jco.2011.40.1836
Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J (2016) Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open label, randomized three-arm phase III trial. J Clin Oncol 34:3300–3307. https://doi.org/10.1200/JCO.2016.66.6198
Chawla S, Katz AW, Rauh SM, Monson JR (2015) Can surgery be avoided after preoperative chemoradiation for rectal cancer in the era of organ preservation? Current review of literature. Am J Clin Oncol 38:534–540. https://doi.org/10.1097/COC.0000000000000122
Bozkaya Y, Ozdemir NY, Erdem GU, Güner EK, Ürün Y, Demirci NS, Yazıcı O, Köstek O, Zengin N (2018) Clinical predictive factors associated with pathologic complete response in locally advanced rectal cancer. Journal of Oncological Sciences 4:5–10. https://doi.org/10.1016/j.jons.2017.12.004
de la Chapelle A, Hampel H (2010) Clinical relevance of microsatellite instability in colorectal cancer. J Clin Oncol 28:3380–3387. https://doi.org/10.1200/JCO.2009.27.0652
Kim HR, Kim HC, Yun HR, Kim SH, Park CK, Cho YB, Yun SH, Lee WY, Chun HK (2013) An alternative pathway in colorectal carcinogenesis based on the mismatch repair system and p53 expression in Korean patients with sporadic colorectal cancer. Ann Surg Oncol 20:4031–4040. https://doi.org/10.1245/s10434-012-2455-7
Samowitz WS, Curtin K, Wolff RK, Tripp SR, Caan BJ, Slattery ML (2009) Microsatellite instability and survival in rectal cancer. Cancer Causes Control 20:1763–1768. https://doi.org/10.1007/s10552-009-9410-3
Mace AG, Pai RK, Stocchi L, Kalady MF (2015) American Joint Committee on Cancer and College of American Pathologists regression grade: a new prognostic factor in rectal cancer. Dis Colon Rectum 58:32–44. https://doi.org/10.1097/DCR.0000000000000266
Figueiredo N, Panteleimonitis S, Popeskou S, Cunha JF, Qureshi T, Beets GL, Heald RJ, Parvaiz A (2018) Delaying surgery after neoadjuvant chemoradiotherapy in rectal cancer has no influence in surgical approach or short-term clinical outcomes. Eur J SurgOncol 44:484–489. https://doi.org/10.1016/j.ejso.2018.01.088
Sada YH, Tran Cao HS, Chang GJ, Artinyan A, Musher BL, Smaglo BG, Massarweh NN (2018) Prognostic value of neoadjuvant treatment response in locally advanced rectal cancer. J Surg Res 226:15–23. https://doi.org/10.1016/j.jss.2018.01.025
Mullaney TG, Lightner AL, Johnston M, Keck J, Wattchow D (2018) ‘Watch and wait’ after chemoradiotherapy for rectal cancer. ANZ J Surg 88:836–841. https://doi.org/10.1111/ans
Rodel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, Liersch T, Hohenberger W, Raab R, Sauer R, Wittekind C (2005) Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 23:8688–8696. https://doi.org/10.1200/JCO.2005.02.1329
Bohlok A, Hendlisz A, Bouazza F, Galdon MG, Van de Stadt J, Moretti L, El Nakadi I, Liberale G (2018) The potential benefit of adjuvant chemotherapy in locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy is not predicted by tumor regression grade. Int J Colorectal Dis 33:1383–1391. https://doi.org/10.1007/s00384-018-3115-6
Gash KJ, Baser O, Kiran RP (2017) Factors associated with degree of tumour response to neo-adjuvant radiotherapy in rectal cancer and subsequent corresponding outcomes. Eur J Surg Oncol 43:2052–2059. https://doi.org/10.1016/j.ejso.2017.07.024
Choi CH, Kim WD, Lee SJ, Park WY (2012) Clinical predictive factors of pathologic tumor response after preoperative chemoradiotherapy in rectal cancer. Radiat Oncol J 30:99–107. https://doi.org/10.3857/roj.2012.30.3.99
Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, Quirke P, Sebag-Montefiore D, Moran B, Heald R, Guthrie A, Bees N, Swift I, Pennert K, Brown G (2011) Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J ClinOncol 29:3753–3760. https://doi.org/10.1200/JCO.2011.34.9068
Durante AP, Bromberg SH, Barreto E, Cappellano G, de Godoy AC (2004) Prognostic value of lymphatic vessel and neural invasion in colorectal carcinoma. Rev Assoc Med Bras 50:21–26
Vallam KC, Desouza A, Bal M, Patil P, Engineer R, Saklani A (2016) Adenocarcinoma of the rectum composite of three different subtypes with varying outcomes? Clin Colorectal Cancer 15:47–52. https://doi.org/10.1016/j.clcc.2015.12.004
Oh CR, Kim JE, Kang J, Kim SY, Kim KP, Hong YS, Lim SB, Yu CS, Kim JC, Kim J, Jang SJ, Kim TW (2018) Prognostic value of the microsatellite instability status in patients with stage II/III rectal cancer following upfront surgery. Clin Colorectal Cancer 1533–0028(18):30228-7. https://doi.org/10.1016/j.clcc.2018.07.003
Shin JS, Tut TG, Yang T, Lee CS (2013) Radiotherapy response in microsatellite instability related rectal cancer. Korean J Pathol 47:1–8. https://doi.org/10.4132/KoreanJPathol.2013
Lim SH, Chua W, Henderson C, Ng W, Shin JS, Chantrill L, Asghari R, Lee CS, Spring KJ, de Souza P (2015) Predictive and prognostic biomarkers for neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Crit Rev Oncol Hematol 96:67–80. https://doi.org/10.1016/j.critrevonc.2015.05.003
Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, French AJ, Kabat B, Foster NR, Torri V, Ribic C, Grothey A, Moore M, Zaniboni A, Seitz JF, Sinicrope F, Gallinger S (2010) Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 28:3219–3226. https://doi.org/10.1200/JCO.2009.27
Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, McCormick J, Kirichenko A (2018) Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer: a National Cancer Database (NCDB) Analysis. Ann Surg. https://doi.org/10.1097/SLA.0000000000003051
Klarskov L, Holck S, Bernstein I, Okkels H, Rambech E, Baldetorp B, Nilbert M (2011) Challenges in the identification of MSH6-associated colorectal cancer: rectal location, less typical histology, and a subset with retained mismatch repair function. Am J Surg Pathol 35:1391–1399. https://doi.org/10.1097/PAS.0b013e318225c3f0
Nazemalhosseini Mojarad E, Kashfi SM, Mirtalebi H, Taleghani MY, Azimzadeh P, Savabkar S, Pourhoseingholi MA, Jalaeikhoo H, Asadzadeh Aghdaei H, Kuppen PJ, Zali MR (2016) L ow level of microsatellite instability correlates with poor clinical prognosis in stage ii colorectal cancer patients. J Oncol 2016:2196703. https://doi.org/10.1155/2016/2196703
Lamberti C, Lundin S, Bogdanow M, Pagenstecher C, Friedrichs N, Büttner R, Sauerbruch T (2007) Microsatellite instability did not predict individual survival of unselected patients with colorectal cancer. Int J Colorectal Dis 22:145–152. https://doi.org/10.1007/s00384-006-0131-8
Hong SP, Min BS, Kim TI, Cheon JH, Kim NK, Kim H, Kim WH (2012) The differential impact of microsatellite instability as a marker of prognosis and tumour response between colon cancer and rectal cancer. Eur J Cancer 48:1235–1243. https://doi.org/10.1016/j.ejca.2011
Sinukumar S, Patil P, Engineer R, Desouza A, Saklani A (2014) Clinical Outcome of patients with complete pathological response to neoadjuvantchemoradiotherapy for locally advanced rectal cancers: the Indian scenario. Gastroenterol Res Pract 2014:1–6. https://doi.org/10.1155/2014/867841
Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, Hu CY, Chang GJ (2012) Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J ClinOncol 30:1770–1776. https://doi.org/10.1200/JCO.2011.39.7901
Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Wolff RA, Eng C, Krishnan S, Janjan NA, Crane CH (2007) Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer 109:1750–1755. https://doi.org/10.1002/cncr.22625
Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, McCormick J, Kirichenko A (2018) Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer: a National Cancer Database (NCDB) Analysis. Ann Surg. https://doi.org/10.1097/SLA.0000000000003051
Acknowledgments
The authors thank all the general surgery staff for their cooperation. All the authors read and approved the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest and no financial issues to disclose.
Research involving human participants and/or animals
This study, involving only human participants, has been conducted in accordance with the ethical standards of the Declaration of Helsinki.
Informed consent
Although our paper did not contain any of the stated (by the journal editorial board) individual data, written informed consent which is a legal obligation in our country were obtained from all the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Acar, T., Acar, N., Kamer, E. et al. Do microsatellite instability (MSI) and deficient mismatch repair (dMMR) affect the pathologic complete response (pCR) in patients with rectal cancer who received neoadjuvant treatment?. Updates Surg 72, 73–82 (2020). https://doi.org/10.1007/s13304-019-00697-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-019-00697-2