Abstract
The development of achalasia in patients with a prior Roux-en-Y gastric bypass (RYGB) is rare and it often remains unclear whether the esophageal motility disorder is a pre-existing condition in the obese patient or develops de novo after the procedure. The aim of this study was to review the available evidence regarding the management of patients with achalasia after a RYGB. Intra-sphincteric injection of botulinum toxin and pneumatic dilatation can be used to eliminate the functional obstruction at the level of the gastroesophageal junction. However, considering that achalasia patients after RYGB are often young and these treatment modalities have shown worse long-term outcomes, endoscopic or surgical myotomy is preferred. Per-oral endoscopic myotomy (POEM) is a very effective first line of treatment, and as RYGB is an excellent anti-reflux operation per se, post-POEM reflux may not be an issue in these patients. Laparoscopic Heller myotomy (LHM) is also an effective and safe therapy in achalasia patients with RYGB anatomy, and the gastric remnant can be safely used to perform a fundoplication to cover the myotomy. LHM and POEM are both acceptable primary treatment modalities in this setting. Further studies are needed to elucidate the pathophysiology and optimal management of patients with achalasia after RYGB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Achalasia is a rare esophageal motility disorder characterized by aperistalsis and partial or absent relaxation of the lower esophageal sphincter (LES) in response to swallowing [1]. Dysphagia is the most common symptom, experienced by about 95% of patients. Patients might also complain of regurgitation, heartburn, chest pain, and weight loss [1]. Patients’ quality of life is often significantly affected by the disease [2]. Treatment is palliative, and it is directed toward relief of symptoms by decreasing the outflow resistance caused by the dysfunctional LES. There are different treatment modalities including botulinum toxin injection, pneumatic dilatation, Heller myotomy, and per-oral endoscopic myotomy (POEM).
Obesity has severe deleterious health effects, and is associated with different comorbid conditions such as diabetes, hypertension, liver disease, chronic kidney disease, and gastroesophageal reflux disease among other disorders. In addition, several esophageal motility disorders have been linked to obesity. For instance, achalasia may coexist in obese patients with a prevalence of 0.5–1% [3, 4]. Unfortunately, as more than a half of the obese population with esophageal motility disorders is asymptomatic, the likelihood of misdiagnosis is high [4].
Bariatric surgery is the most effective treatment for morbid obesity. Currently, Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy are the most frequently performed bariatric operations. The lack of esophageal testing before bariatric procedures makes difficult to know the real prevalence and linkage between morbid obesity and achalasia.
Currently, there are limited data regarding the management of achalasia after RYGB.
The aim of this study was to review the available evidence regarding the management of patients with achalasia after a RYGB.
Methods
A systematic Medline literature search of articles on achalasia after RYGB was performed. Key terms used were: “achalasia,” “myotomy,” “Heller myotomy after bariatric surgery,” “achalasia after bariatric surgery”. The keywords were used in all possible combinations to obtain the maximal number of articles. The search was limited to the English language. Only patients with prior RYGB surgery were included. Patients who underwent sleeve gastrectomy were excluded. A total of 58 articles were thoroughly revised, and 27 were finally included in the review.
Clinical case
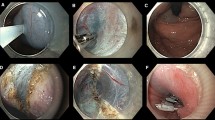
A 40-year old obese woman with body mass index (BMI) of 44 kg/m2 underwent laparoscopic RYGB at our institution. One year after the operation, her BMI decreased to 31 kg/m2 and she started complaining of nausea, cough, and regurgitation. She denied any changes in her appetite and bowel movements. An upper endoscopy was performed and showed retained food in the esophagus, resistance at the gastroesophageal junction, a gastric pouch without lesions, and no evidence of anastomotic stenosis. The barium swallow showed narrowing at the level of the gastroesophageal junction and slow emptying of contrast. A high-resolution manometry was performed, which confirmed the diagnosis of achalasia with absent peristalsis and panesophageal pressurization in 100% of the swallows (Chicago Classification Type II). We decided to perform a laparoscopic Heller myotomy. The myotomy was extended 5 cm up to the distal esophagus and 2 cm along the gastric pouch (Fig. 1). An intraoperative endoscopy was done showing no perforation and adequate extent of the myotomy. Considering the young age of the patient, an anterior fundoplication was performed using the gastric remnant to further prevent abnormal reflux (Figs. 2, 3). At 6-month follow-up, the patient remains asymptomatic.
Physiopathology of achalasia after bariatric surgery
The increased association of achalasia and bariatric surgery may be in part secondary to a higher awareness of the disease. Considering that bariatric patients rarely undergo esophageal testing, it is difficult to determinate whether achalasia develops de novo after the procedure, or was present before the operation.
The risk of developing achalasia following bariatric surgery differs with the type of surgery, being more common after laparoscopic adjustable gastric banding (LAGB) [6,7,8]. Robert M et al. [7] described a 1.9% prevalence of achalasia like disorders after LAGB. From 359 patients undergoing LAGB between 2000 and 2008, 20 patients suffered from esophageal motility disorders confirmed by high-resolution manometry. It has been postulated that the band creates an obstacle at the level of the gastroesophageal junction, creating a high-pressure area which can induce esophageal aperistalsis. In fact, some patients recover the normal esophageal peristalsis after the band is removed.
The pathophysiology of achalasia after RYGB remains unknown. Due to the similar clinical presentation, it is important to rule out stenosis of the gastro-jejunostomy in these patients. A retrospective study presented by Shah et al. [5] demonstrated that 25% of patients with achalasia had history of trauma (operative or non-operative trauma) to the upper gastrointestinal tract before the onset of the symptoms: six patients underwent an operation involving the gastroesophageal junction and two of them underwent RYGB. These data suggest that some patients may have esophageal motility disorders related to surgical trauma and vagal nerve damage, leading to nerve dysfunction and subsequent development of achalasia. Schrumpf et al. [9] postulated that the vagotomy in the gastric remnant leads to decreased postprandial gastrin levels, which provoke an increasing resting tone of the LES.
Diagnosis of achalasia after RYGB
The main clinical manifestations of achalasia after bariatric surgery are the classic symptoms: dysphagia, heartburn, regurgitation, and chest pain [10, 11]. Weight loss is often attributed to the bariatric procedure. When a patient with a previous RYGB presents with any of these symptoms, surgical complications such as anastomotic stenosis or small pouch size should be ruled out.
The upper endoscopy usually reveals a dilated esophagus, retained food in the esophagus, and resistance at the level of the gastroesophageal junction [11]. The esophageal mucosa can be normal or show signs of esophagitis usually secondary to food stasis or candida infection. A barium swallow shows narrowing at the level of the gastroesophageal junction (so called “bird beak”), esophageal dilation, and poor esophageal emptying [12].
As for non-obese patients with classic achalasia, high-resolution manometry is the gold standard for the diagnosis of the disease. Lack of peristalsis and partial or absent relaxation of the LES in response to swallowing is the key criteria for the diagnosis. The Chicago classification divides achalasia in three subtypes: type I: incomplete LES relaxation, aperistalsis and absence of esophageal pressurization; type II: incomplete LES relaxation, aperistalsis and panesophageal pressurization in at least 20% of swallows; and type III: incomplete LES relaxation and premature “spastic” contractions (distal latency < 4.5 s) in at least 20% of swallows.
Management of achalasia after RYGB
Due to the difficult diagnosis and lack of established guidelines, the management of achalasia after RYGB remains controversial.
Intra-sphincteric injection of botulinum toxin can be used to block the release of acetylcholine at the level of the LES, thereby restoring the balance between excitatory and inhibitory neurotransmitters. Pneumatic dilatation may also eliminate the functional obstruction at the level of the gastroesophageal junction by disrupting the circular muscle fibers of the LES. Since achalasia patients after RYGB are often young and these treatment modalities have shown worse long-term outcomes, endoscopic or surgical myotomy is preferred.
Per-oral endoscopic myotomy (POEM)
In 2010, Haruhiro Inoue published the results of a new endoscopic technique called per-oral endoscopic myotomy (POEM) in 17 patients with esophageal achalasia [13]. The procedure consists in an endoscopic creation of a submucosal tunnel, which allows performing a myotomy by transection of the circular fibers of the distal esophagus. POEM is a very effective first line of treatment, but it is associated with a high incidence of post-procedure gastroesophageal reflux (POEM ablates the LES without any form of anti-reflux procedure) [14]. However, considering that the RYGB is an excellent anti-reflux operation per se, reflux may not be an issue in these patients. Unfortunately, we do not have robust data regarding the incidence of gastroesophageal reflux in patients treated with POEM after RYGB.
A retrospective study presented by Sanaei et al. [15] analyzed the outcomes of 10 patients with prior RYGB that underwent POEM. Clinical success was achieved in all patients with a significant decrease in Eckardt score from 6.5 to 1 (p < 0.001). None of the patients complained of regurgitation. Post-procedural pH testing was obtained in six patients and was normal in all of them.
Potential advantages of POEM include: lack of abdominal incisions, faster recovery than a laparoscopic operation, and ease of performing a longer myotomy.
Overall, POEM seems to be a safe and effective therapy for patients with prior RYGB and achalasia, and thereby could be considered a primary treatment modality for such patients.
Laparoscopic Heller myotomy
Laparoscopic Heller myotomy (LHM) and partial fundoplication is the standard surgical approach for achalasia. The operation consists of a controlled section of the muscle fibers of the lower esophagus (6 cm) and proximal gastric wall (2 cm–2.5 cm), followed by a partial fundoplication to prevent postoperative gastroesophageal reflux. A total 360° Nissen fundoplication should be avoided because of the higher risk of postoperative dysphagia [16]. In patients with a prior RYGB, a LHM may be challenging and there are scarce data regarding the long-term outcomes of procedure in this rare scenario.
Fisichella and colleagues suggested a concomitant LHM and laparoscopic RYGB as an approach that can provide excellent relief of symptoms and adequate control of reflux while promoting weight loss in obese patients with achalasia [3]. In 2009, Ramos et al. [17] described one of the first cases of LHM after RYGB. The myotomy was extended 4 cm up to the distal esophagus and 2 cm along the gastric pouch. The patient was discharged on postoperative day 2, and was symptom free at 6 months of follow-up. The authors concluded that clinical investigation of typical signs and symptoms of achalasia should be performed in obese patients that will undergo bariatric surgery. Although a preoperative esophageal manometry may be advised during the workup of a bariatric patient, we do not have yet enough evidence to support such recommendation [18].
Chapman et al. [19] reported the case of a patient who developed achalasia 2 years after an open gastric bypass. A LHM was performed, extending the myotomy for 4 cm proximal to the LES and 5 cm distally. The authors decided not to do a fundoplication using the gastric remnant due to the minimal risk of reflux after bariatric surgery.
Overall, LHM is also an effective and safe therapy in patients with RYGB anatomy and achalasia. We believe that the gastric remnant can be safely used to perform a fundoplication to cover the myotomy.
Table 1 summarizes the current evidence regarding the management of achalasia after RYGB. Unfortunately, all the studies have low quality of evidence because of their retrospective nature and the few number of patients included.
Conclusions
The development of achalasia in patients with a prior RYGB is rare and it often remains unclear whether the esophageal motility disorder is a pre-existing condition in the obese patient or develops de novo after the procedure. LHM and POEM are both acceptable primary treatment modalities in this setting. Further studies are needed to elucidate the pathophysiology and optimal management of patients with achalasia after RYGB.
References
Schlottmann F, Patti MG (2018) Esophageal achalasia: current diagnosis and treatment. Expert Rev Gastroenterol Hepatol 12(7):711–721
Nenshi R, Takata J, Stegienko S, Jacob B, Kortan P, Deitel W, Urbach DR (2010) The cost of achalasia: quantifying the effect of symptomatic disease on patient cost burden, treatment time, and work productivity. Surg Innov 17(4):291–294
Fisichella PM, Orthopoulos G, Holmstrom A, Patti MG (2015) The surgical management of achalasia in the morbid obese patient. J Gastrointest Surg 19(6):1139–1143
Jaffin BW, Knoepflmacher P, Greenstein R (1999) High prevalence of asymptomatic esophageal motility disorders among morbidly obese patients. Obes Surg 9:390–395
Shah RN, Izanec JL, Friedel DM, Axelrod P, Parkman HP, Fisher RS (2004) Achalasia presenting after operative and nonoperative trauma. Dig Dis Sci 49:1818–1821
Boules M, Corcelles R, Zelisko A, Batayyah E, Froylich D, Rodriguez J, Kroh M (2016) Achalasia after bariatric surgery. J Laparoendosc Adv Surg Tech A 26(6):428–432
Robert M, Golse N, Espalieu P, Poncet G, Mion F, Roman S, Gouillat C (2012) Achalasia-like disorder after laparoscopic adjustable gastric banding: a reversible side effect? Obes Surg 22(5):704–711
Khan A, Ren-Fielding C, Traube M (2011) Potentially reversible pseudoachalasia after laparoscopic adjustable gastric banding. J Clin Gastroenterol 45(9):775–779
Schrumpf E, Giercksky KE, Nygaard K, Fausa O (1981) Gastrin secretion before and after gastric bypass surgery for morbid obesity. Scand J Gastroenterol 16(6):721–725
Ghanimeh MA, Qasrawi A, Abughanimeh O, Albadarin S (2017) Clarkston W Achalasia after bariatric Roux-en-Y gastric bypass surgery reversal. World J Gastroenterol 23(37):6902–6906
Pandolfino JE, Gawron AJ (2015) Achalasia: a systematic review. JAMA 313(18):1841–1852
Ates F, Vaezi MF (2015) The pathogenesis and management of achalasia: current status and future directions. Gut Liver 9(4):449–463
Inoue H, Minami H, Kobayashi Y et al (2010) Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 42:265–271
Schlottmann F, Luckett DJ, Fine J, Shaheen NJ, Patti MG (2018) Laparoscopic Heller myotomy versus peroral endoscopic myotomy (POEM) for achalasia: a systematic review and meta-analysis. Ann Surg 267(3):451–460
Sanaei O, Draganov P, Kunda R, Yang D, Khashab M (2018) Peroral endoscopic myotomy for the treatment of achalasia patients with Roux-en-Y gastric bypass anatomy. Endoscopy 51:342–345
Rebecchi F, Giaccone C, Farinella E, Campaci R, Morino M (2008) Randomized controlled trial of laparoscopic Heller myotomy plus Dor fundoplication versus Nissen fundoplication for achalasia: long-term results. Ann Surg 248(6):1023–1030
Ramos AC, Murakami A, Lanzarini EG, Neto MG, Galvão M (2009) Achalasia and laparoscopic gastric bypass. Surg Obes Relat Dis 5:132–134
Klaus A, Weiss H (2008) Is preoperative manometry in restrictive bariatric procedures necessary? Obes Surg 18:1039–1042
Chapman R, Rotundo A, Carter N, George J, Jenkinson A, Adamo M (2013) Laparoscopic Heller’s myotomy for achalasia after gastric bypass: a case report. Int J Surg Case Rep 4(4):396–398
Torghabeh MH, Afaneh C, Saif T, Dakin GF (2015) Achalasia 5 years following Roux-en-Y gastric bypass. J Min Access Surg 11:203–204
Bashir U, El Abiad R, Gerke H, Keech J, Parekh K, Nau P (2019) Peroral endoscopic myotomy is feasible and safe in a gastric bypass population. Surg Obes. https://doi.org/10.1007/s11695-019-04026-9
Luo RB, Montalvo D, Horgan S (2017) Peroral endoscopic myotomy after gastric bypass: an effective solution for de novo achalasia. Surg Obes Relat Dis 13(2):1–3
Masrur M, Gonzalez-Ciccarelli LF, Giulianotti PC (2016) Robotic Heller myotomy for achalasia after laparoscopic Roux-en-Y gastric by-pass: a case report and literature review. Surg Obes Relat Dis 12(9):1755–1757
Birriel TJ, Claros L, Chaar ME (2017) Laparoscopic Heller myotomy after previous Roux-en-Y gastric by-pass. Surg Obes Relat Dis 13(11):1927–1928
Nguyen D, Dip F, Lo Menzo E et al (2016) Heller oesophagomyotomy as treatment for achalasia after gastric bypass for morbid obesity. Ann R Coll Surg Engl 98(1):e3–e5
Johnson WD, Marshall MB (2016) Surgical management of achalasia in a patient with previous gastric bypass. Innovations (Phila) 11(3):214–216
Yang D, Draganov PV (2014) Peroral endoscopic myotomy (POEM) for achalasia after Roux-en-Y gastric bypass. Endoscopy 46(Suppl 1):E11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
María A. Casas declares that she has no conflict of interest. Francisco Schlottmann declares that he has no conflict of interest. Fernando A.M Herbella declares that he has no conflict of interest. Rudolf Buxhoeveden declares that he has no conflict of interest. Marco G. Patti declares that he has no conflict of interest.
Human and animal rights
This study does not contain any studies with human participants performed by any of the authors.
Informed consent
No informed consent was needed for this review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Casas, M.A., Schlottmann, F., Herbella, F.A.M. et al. Esophageal achalasia after Roux-en-Y gastric bypass for morbid obesity. Updates Surg 71, 631–635 (2019). https://doi.org/10.1007/s13304-019-00688-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-019-00688-3