Abstract
Pancreaticoduodenectomy (PD) is associated with high postoperative morbidity. The management of postoperative complications is paramount for reducing the mortality rate. The aim of this study was to evaluate the importance of surgical and hospital experience on outcomes by comparing postoperative results in three different hospitals with increasing resources for supporting the same surgical team. Patients data and surgical outcome of 300 consecutive patients undergoing PD were collected prospectively in the department database and divided into three periods (A = 1990–2000, B = 2001–March 2007, C = April 2007–2015). Pancreatico-jejunostomy was the procedure of choice between 1995 and 2004, and pancreatico-gastrostomy was performed afterwards. In the periods A, B and C, a total of 78, 85 and 137 PD were performed, respectively, and the number of PDs per year increased from 5 to 25. Between the three periods, the death rate (10.4 vs. 6 vs. 1.6%, p = 0.01) and intraoperative RBC transfusion rate (84.9 vs. 42.4 vs. 6.5%, p = 0.01) decreased significantly, whereas the vascular resection rate increased significantly (1.2 vs. 7 vs. 14.5, p < 0.002). Morbidity and reoperation rates did not change significantly between the three periods as well as operative time and median length of stay. Infectious complications and sepsis represented the most frequent major complication. Massive bleeding associated with uncontrolled pancreatic leak represented the major cause of morbidity and reoperation in the three periods, however, the relative mortality rate decreased significantly with no deaths in the last period. PD remains a challenging procedure with high morbidity and mortality rate. A multidisciplinary pancreatic team represents the “safety net” of pancreatic surgeon because it improves the results beyond the surgeon skills and experience.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreaticoduodenectomy (PD) is a complex surgical procedure with substantial risks that requires long learning curve and clinical experience. This operation was popularized by Whipple in 1935, and at the end of his career in 1963 he had performed only 37 such procedures [1, 2]. Since then, this challenging procedure has remained infrequently performed with disappointing results. In the 80s and 90s, large nationwide registries analysis from USA and Europe showed 60–80% of the procedures were performed in low volume hospitals with mortality rates up to 20% [3,4,5,6]. However, high volume hospitals (more than 10 PDs per year) reported mortality rates ranging between 1 and 6%. Surgeon experience and hospital volume were shown to represent major outcome prognostic factors, and suggested that such complex surgical procedure should be performed in centers with sufficient experience and resources for support. After three decades, this scenario is unchanged. A recent observational study of the statewide inpatient database of California showed that in this region, 143 hospitals performed their first PD between 1996 and 2010 and that only 5 among them performed more than 50 PD during the study period [7]. In this study, 77% of the hospitals performed ten or less PD and the overall mortality rate has remained as high as 9.7%. This report shows that at the end of the surgeon learning process the curve reach the “plateau” at which point there are no further significant changes in success parameter. Postoperative outcomes may remain disappointing in term of high mortality rates despite the increasing surgeon experience and skills. Other factors contribute significantly to the surgical outcome after PD such as the coalescence of a surgical Unit, an established postoperative recovery pathway, hospital experience, and ancillary support system. The reduction of the mortality rate is mainly due to the management of postoperative complications by a multidisciplinary support system that represent the “safety net” of the pancreatic surgeon. High volume hospitals have developed such multidisciplinary care, and despite the morbidity rate has remained relatively consistent over the time at 40%, the associated mortality rate has shown a dramatic reduction to less than 4% [8, 9]. We evaluated results and outcomes of 300 consecutive PDs of a single surgical unit that moved sequentially in three different hospitals with increasing support supplies over the past 25 years. In undertaking this study, we hypothesized that once the surgeon learning curve has reached the “plateau”, the improvements of surgical outcomes rely on the “safety net” that surrounds the surgical unit.
Methods
Patients
We retrospectively reviewed a prospectively updated database of 300 patients (178 males and 122 females; age 12–84 years, median 65 years) who underwent PD for suspected malignant diseases of the pancreatic head region and were operated by the same surgical team, in three different hospitals sequentially from 1990 to 2015 in Rome, Italy, (1990–2000: Department of Oncologic Surgery of the Regina Elena Cancer Institute-old location; 2001–2007: Department of Digestive Surgery and Liver Transplantation of the Regina Elena Cancer Institute-new location; 2007–2015: Department of Surgery and Liver Transplantation of the San Camillo General Hospital). The three hospitals had increasing ancillary support system. Patients data and surgical outcomes were collected prospectively in the department database. To evaluate the effect of the surgical and hospital experience, patient outcomes in each period were compared. The following factors were collected: age, sex, history of jaundice, preoperative biliary drainage, blood tests, intraoperative findings as type of resection and pancreatic anastomosis, intraoperative red blood cell (RBC) transfusion, duration of surgery, and postoperative complications, reoperation, pathologic findings and hospitalization time. Estimated intraoperative blood loss (EBL) were accurately recorded only in the last period. All patients underwent preoperative spiral computed tomography. The use of preoperative magnetic resonance imaging increased after 2000.
Surgical approach
All resections were approached with curative intent. Diagnostic laparoscopy has been routinely performed since 1995 to rule out the presence of hepatic metastases or peritoneal carcinomatosis. Standard Whipple procedure was performed in all 300 cases by proceeding in a typical fashion, while eight more patients underwent total pancreatectomy (TP) during the same period. All procedures were performed by two senior surgeons (S. E. or E. G. M), or under their supervision. In standard PD, the distal-end of the stomach, duodenum, pancreatic head and uncinate process of pancreas, gallbladder and common bile duct were resected. Lymph node dissection included number 3, 4, 5, 6, 8, 9, 12 13 series of lymph nodes according to the Japanese Pancreas Society classification (Japan Pancreas Society. Classification of pancreatic carcinoma. 2nd English edn. Kanehara & Co. Ltd, Tokyo, 2003). Extension of resection to the mesenteric/portal vein axis and its reconstruction was also performed when necessary, whereas resection of the mesenteric artery or celiac trunk was never performed. Total pancreatectomy was performed in the first period in case of cancer invasion of the pancreatic resection margin. Pylorus preserving PD was mostly performed in the second period when feasible according to surgeon preference.
In the first 50 cases, different techniques were performed including pancreatico-jejunostomy (PJ) with or without pylorus preservation, on a single-loop or double-loop and also pancreatic duct occlusion. Pancreatico-jejunostomy by end-to-side or duct-to-mucosa reconstruction on an isolate jejunal loop was the procedure of choice between 1995 and 2004 [10]. Pancreatico-gastrostomy (PG) by end-to-side reconstruction on the posterior wall of the stomach with antro-pyloric resection was performed afterwards [11]. A short tube placed in the pancreatic duct to drain the pancreatic juice into the stomach, using the same technique for more than 150 cases to date spanning over the last two periods. In the last period, in two patients a PJ and a pancreatic duct intubation without anastomosis were performed for technical reasons, respectively. An omental flap was used to cover the vessels since 2013 to date. Reconstruction was completed by hepaticojejunostomy between common hepatic duct stump and jejunum side wall without T tube double layer and by end-to-side gastrojejunostomy or duodenojejunostomy. Three drainages were routinely placed in the peritoneal cavity: one drainage tube was put in the sub-hepatic space, and two other drainages were put close to the pancreatic anastomosis.
Postoperative treatment
All patients received intensive care for at least 12 h in Intensive Care Unit. Somatostatin or its analogs were not used. Total parenteral nutrition (TPN) by central venous catheter was started on postoperative day (POD) 1 and discontinued when oral fluid intake and feeding was adequate. Proton pump inhibitors and low molecular weight heparin (LMWH) were routinely administered. After 2000, the level of amylase in the drainage fluids was measured on POD 5 and 7, and an abdominal CT scan was routinely performed in all patients on POD 7 to detect any sign of abdominal collections or postoperative pancreatic fistula (POPF). In case of PJ, the nasogastric (NG) tube was removed after the first flatus. After PG the NJ tube was removed if any sign of POPF was not detected after POD 7. In case of POPF, patients were treated by TPN and NG intubation for 4 weeks and controlled by the same protocol weekly, regardless of the grade of POPF. The abdominal drainage was removed when the level of amylase was not more of threefolds the normal value.
Postoperative complications
Surgical complications were classified as procedure-related such as postoperative pancreatic fistula (POPF), hemorrhage, biliary leakage, intraabdominal abscesses and delayed gastric emptying, or general (pulmonary and cardiac) [12]. Since 2005, pancreatic fistula was defined according to the International Study Group of Pancreatic Fistula (ISGPF) which classified the severity of POPF into three grades (A, B, C) depending on its clinical impact [13]. Biliary fistula was defined as the presence of bile, more than 50 mL in volume per day, in the abdominal drainage beyond POD 3. After 2009, patients are routinely screened for bacterial contamination before, during and after the surgical procedure. Postoperative infection criteria were hyperleukocytosis, combined with body temperature higher than 38.5 centigrade, positive biologic fluid cultures, such as blood, abdominal fluid, sputum or bile. Delayed gastric emptying (DGE) was defined as a daily output from the nasogastric tube >500 ml that persisted beyond POD 10, the failure to maintain oral intake by POD 14, or reinsertion of a nasogastric tube after removal. Postoperative death was defined as any death during hospital stay.
Statistical analysis
Continuous variables were presented as medians (ranges), and categorical variables as numbers (percentage). Statistical analysis was performed using χ 2 test and Student’s t test, when appropriate. Differences were considered significant at p value <0.05. The analysis was performed using SPSS 18 software program (Chicago, IL, USA).
Results
Patients and operative details
All demographic data and indication for surgery of 300 patients undergoing PD in the three study periods are given in Table 1. Overall median age was 65 years (range 22–84), and 59% were male. Preoperative serum albumin and serum creatinine were not significantly different between groups. The clinic-pathological data in the three periods were compared. Pancreatic duct adenocarcinoma was the most common indication for resection in all three periods (86%), with a higher percentage of patients in period 1 (1990–2000). The number of patients who underwent PD for benign disease, mainly chronic pancreatitis or benign cystic tumors, was slightly higher in periods 2 (2001–2007) and 3 (2007–2015). Other preoperative variables such as serum creatinine and albumin were not significantly different between the three study groups.
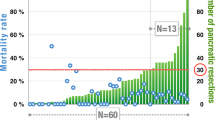
As shown in Fig. 1 and Table 2, during the three periods, the volume of pancreatic head resections increased from 8.6 procedure per year to 13 in the intermediate period to 15.5 per year in the last period. Particularly, the number of resections per year increased from 5 to 25. Pancreatico-jejunostomy was mostly performed in period 1, while pancreatico-gastrostomy was progressively introduced in period 2 and always performed in period 3 (97.8% of cases) (Fig. 2). Most of vascular resections (20 of 27 patients, 74% of cases) were performed in the last period of the study, with a significant difference between the three groups (p = 0.002). Conversely, intraoperative blood transfusion rate was significantly higher in the first period, compared to the last period (p = 0.01). Operative time did not differ between the three periods of study, ranging from 160 to 500 min (median 310 min).
Postoperative complications and deaths
Overall morbidity and reoperation rates were 36.3 and 10.6%, respectively, and remained relatively consistent in the three periods (32 vs. 42 vs. 39% and 9.3 vs. 8.3 vs 13%, respectively) (Table 3). The incidence of pancreatic fistula in the first period was low because of the retrospective analysis and because the evaluation of the amylase in the drainage fluids was not routinely performed before 2000, whereas in the second and third period was 21 and 23%, respectively. All patients with grade A POPF after PG anastomosis were successfully treated by total parenteral nutrition and maintenance of nasogastric decompression for 4 weeks and none of these patients was readmitted after discharge. The incidence of delayed massive bleeding associated with POPF was 3% and did not change significantly. After PJ, eight patients were reoperated on for this life threatening complication and five of them died of bleeding recurrence or sepsis, whereas after PG all 11 patients that were reoperated survived after emergency laparotomy, bleeding control and taking down of the PG anastomosis. Completion pancreatectomy was performed in one case only at the beginning of the experience with PJ with unfavorable outcome, and then this very challenging procedure was abandoned in such difficult situations. In the last period, patients with infectious complications were systematically screened by culture of biological specimens (blood, bile, drained fluids, and stools) and treated in collaboration with infectious disease specialists of the “Lazzaro Spallanzani” National Institute for Infectious Disease. More than 40% of the patients had positive cultures during the postoperative course, and the type of microbial species isolated from all biological specimens is depicted in Table 4. The subgroup of patients with preoperative biliary drainage (PBD) had higher incidence of postoperative infectious complications.
Overall mortality rate was 4.9%, and there was a significant decrease in the three periods (10.4 vs. 6 vs. 1.6%, respectively, p = 0.01), with no death in a consecutive series of 144 PD spanning over the last two periods (Fig. 3). Pancreatic stump-related surgical complications represented the main causes of death in the first and second periods, whereas since 2005 no patients died for such complications. The cause of death in the two patients who died since 2005 were respiratory failure following abdominal sepsis, and massive mesentericoportal vein thrombosis immediately after vascular resection and reconstruction with vein allograft, in one case each. Median hospital stay was 21 days (range 11–117) and was comparable in the three periods (21 vs. 23 vs. 21 days) as well as the 30-day readmission rate that was 4.6% (7 vs. 3.5 vs. 3.9%). The median LOS of patients with uneventful postoperative course was 18 days after PJ and 16 days after PG.
Discussion
Our study confirms that despite the incidence of complications remained unchanged to 40% during the three periods, the refinements of the surgical technique and the advances in critical care provided significant decrease of the mortality rate from 10.4 to 1.6%, as shown in other single institution experiences [5, 9, 14]. The significant reduction of intraoperative blood product transfusion during the study period, despite the expansion of indications to more difficult cases, is an indirect parameter of the increasing surgeon experience associated with refinements of the technique. As shown in other series, the transfusion rate was inversely correlated with surgeon experience and postoperative mortality [5, 15]. The preoperative planning of the procedure with high fidelity imaging is mandatory in the modern era to recognize technical difficulties and minimize blood loss during resection. Sharp dissection and wide exposure of the portal vein without moving on to the next step until the operative field is dry has become the routine technique and is paramount. Reconstruction is not performed until immaculate hemostasis has been achieved. Vascular resection must be planned before surgery and the vascular conduit should be available or prepared before starting the procedure. In our experience, we used vein allograft from cadaveric donors in case of extended vein resection and prolonged clamping without the need if intraoperative blood product transfusions [16]. An increase of the operation time was also reported in our study, despite the increasing surgeons experience. This is probably due to the more expanded patient selection criteria, on one side, and on the other side to the adoption of the bloodless surgical technique that is time consuming. Ball et al. strongly suggested that surgeons must be facile with vascular surgical technique to avoid hemorrhage during PD, and that is important to continue to exercise caution throughout the learning curve [17]. As a matter of fact, in our experience a dramatic fall of intraoperative blood product transfusion was seen once our surgical team implemented the number of liver resections (LR) and started the liver transplant (LT) program in 2002, with more than 1700 LR and 350 LT performed since 2002.
The decrease in the mortality rate in our series, cannot be associated only with surgeon experience and capabilities, but is also associated with the improvements of surgical critical care. Identifying high risk surgical patients in the preoperative workup and the management of POPF in the postoperative course were paramount. The PREPARE score is helpful to recognize preoperatively compromised patients with increased risk of postoperative complication and the Braga score well define the intraoperative findings that are correlated with postoperative complications [18, 19]. The diameter of the pancreatic duct and the consistency of the pancreas have been shown to influence morbidity and mortality since the 90s [15, 20]. Patients with preoperative and intraoperative risks for complications have been treated differently and followed more closely during the postoperative course on individual basis. Particularly, patients with a thin Wirsung duct and the soft texture of pancreatic stump are treated with caution throughout the postoperative course because of an increased risk of POPF and other associated complications, especially in case of reconstruction with PG [21, 22]. Conversely, in patients with hard pancreatic textures, dilated pancreatic ducts, or both, the risk of POPF is low, and an enhanced recovery program is feasible [23, 24]. A very important issue that has not been taken into account in the PREPARE and Braga scores is the preoperative microbial exposition of the patient that represents a predisposing factor for complications. In fact, most of these patients are malnourished and are likely to be colonized with resistant Enterobacteriaceae because of multiple hospitalizations in the course of the preoperative evaluation, including preoperative biliary drainage (PBD). The sharp analysis of postoperative complications after pancreatic surgery at the John Hopkins Hospital provided a new classification in grading complications, and the most common complications were of infections nature [12]. However, no mention was done on the role of the infectious disease specialist in pancreatic surgery [25]. In our recent experience, more than 40% of the patients had positive culture during the postoperative course, and these patients were treated in collaboration with infectious disease specialists of the “Lazzaro Spallanzani” National Institute for Infectious Disease Infections.
We substantially improved with regards to successfully treating and temporizing most complications, and as shown in other larger experiences, the multidisciplinary care was proven to be extremely important [5, 9, 14, 15]. The education and coalescence of the surgical team, including the nursing staff represent the first step to improve the results beyond the surgeon experience. The second step is the participation of the other health care professionals and colleagues of the hospital to the care of these patients. During the study period, the surgical team moved from a small Cancer Institute with limited resources to a huge General Hospital with all kind of support and the team has been progressively surrounded by the other specialists of the hospital who started an intensive and fruitful collaboration. The rate of grade III or IV Clavien-Dindo complications has remained high at 20% and the ancillary support system that was growing with interest around the surgical team became crucial for successfully treating complicated patients. In the last period, radiologists and interventional radiologists, gastroenterologists and specialists in internal medicine and infectious disease, have been routinely involved in the management of severe postoperative complications in collaboration with the intensivists and surgeons with impressive results. The third step is the standardization of the postoperative recovery pathways. In all the main high volume pancreatic units, PD is usually performed by senior surgeons, or under their supervision, and reconstruction is performed according to the standard restorative technique chosen on single hospital basis. The pancreatic anastomosis is performed in the same fashion for hundreds of cases, unless randomized or controlled studies are carried on, and the postoperative recovery pathways are well designed. Since 2000, our intraoperative and postoperative recovery pathways have been standardized and remained unchanged independent of the type of pancreatic anastomosis. The early detection and treatment of POPF and prevention of pulmonary complications represented the main targets of these pathways, and provided significant results. Massive bleeding and abdominal collections as well as respiratory failure represent the most life threatening complications and need prompt multidisciplinary treatment according to standardized procedures. The different outcome of patients with grade III or IV complications during the study period represents a demonstration of the increased clinical experience and improvements in critical care by all the health care professionals of the hospital during the years. Toomey et al. showed that when a high volume surgeon relocate to a low-volume hospital, performing educational programs allow to perform PD with low mortality rate also in peripheral hospital with reduced support supplies [26].
From the technical point of view, we routinely performed PJ for a more than a decade. In 2004, we decided to perform PG because POPF with massive delayed hemorrhage still represented the most life threatening surgical complication with high mortality rate, despite the increasing experience with PJ. This decision was taken after some surgeons of the team trained in other high volume centers that routinely performed PG at that time, reporting encouraging evidence in terms of reduced incidence of POPF and associated mortality compared to PJ [11, 27,28,29]. In our experience, reoperation for delayed massive arterial bleeding associated with POPF was required after both PJ and PG, and the incidence of this life threatening complication remained unchanged. However, reoperation after PG was easier and the postoperative management provided favorable outcome. The reoperation rate increased in the last period because we decided to be more aggressive in case of uncontrolled abdominal complication. Particularly, relaparotomy has become the treatment of choice in case of massive bleeding associated with POPF, and the mortality rate associated with grade C POPF had a dramatic fall to 0% [30, 31]. Recent multicentre randomized trials and meta-analysis showed that despite PG do not reduce the overall morbidity and mortality rates in high volume centers, it reduces the severity of POFP [32, 33]. Thus, low or medium volume hospital may benefit more from PG than PJ in clinical daily practice. In light of our results we are reluctant to abandon PG in favor of PJ.
The enhanced recovery pathway after surgery (ERAS) represents a new frontier in pancreatic surgery. In Europe and Far Eastern countries, the duration of hospitalization varies greatly among centers and is often influenced by local historical practice and by the national health system organization of each country [5, 9, 15, 21, 24, 27, 28]. The length of stay (LOS) remains significantly higher compared to those reported by high volume centers in the USA [14, 34, 35] where patients with uneventful postoperative course are discharged without postoperative imaging control. However, the readmissions rate for postoperative complications is as high as 20% [36, 37] that is significantly higher compare to that reported in Europe [8, 24, 32]. In addition, readmission to a secondary hospital without an adequate safety net may be underestimated and not recommended. The LOS has been shown to be inversely correlated with hospital volume, however, there is an inverse relationship between LOS and readmission rate, too [38]. In our experience, the postoperative median LOS did not show a significant reduction during the study period being 21 days, and we look with interest at the ERAS protocol [23, 24]. However, the readmission rate in our series was as low as 5%. In our opinion, cost containment in pancreatic surgery is not an interesting issue because the risk of grade III and IV postoperative complications and death remains high even after an apparently early uneventful course, and patients discharge policy should be considered only on clinical basis without any form of economic conditioning. Savings from early discharge after PD is a mere debate since it remains such a rare procedure that only one single surgeon in the world performed more than 2000 procedures over a 43-year-period spanning six different decades [35]. Cost containment from eliminating waste in other field of health care spending, i.e., failure of care coordination or administrative complexity or fraud and abuse, is enormous compared to reducing LOS after PD [39, 40]. Early discharge according to the ERAS protocol is recommended and feasible in selected patients, with the aim to improve the patients’ quality of life exclusively. Our policy has always been to discharge the patients when the predictors and causes of early readmissions are excluded regardless of the length of stay.
In conclusions, PD remains a challenging procedure with high morbidity rate, and pancreatic surgeon should continue to use the technique with which they are most familiar. However, all the health professionals of the hospital must be involved in the management of these patients, and the multidisciplinary team represents an essential “safety net” to improve results beyond the surgeon skill and experience.
References
Whipple A, Parsons WB, Mullins CR (1935) Treatment of carcinoma of the ampulla of Vater. Ann Surg 102:763–779 (PMID: 17856666)
Whipple A (1963) A reminiscence: pancreaticoduodenectomy. Rev Surg 20:221–255 (PMID: 14000261)
Lieberman MD, Kilburn H, Lindsey M, Brennan MF (1995) Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg 222:638–645 (PMID: 7487211)
Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES (1999) Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery 125:250–256 (PMID: 10076608)
Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, Obertop H (2000) Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg 232:786–795 (PMID: 11088073)
Neoptolemos JP, Russell RC, Bramhall S, Theis B (1997) Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg 84:1370–1376 (PMID: 9361591)
Coe TM, Fong ZV, Wilson SE, Talamini MA, Lillemoe KD, Chang DC (2015) Outcomes improvement is not continuous along the learning curve for pancreaticoduodenectomy at the hospital level. J Gastrointest Surg 19:2132–2137. doi:10.1007/s11605-015-2967-0 (PMID: 26438484)
Addeo P, Delpero JR, Paye F, Oussoultzoglou E, Fuchshuber PR, Sauvanet A, Sa Cunha A, Le Treut YP, Adham M, Mabrut JY, Chiche L, Bachellier P, French Surgical Association (AFC) (2014) Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB (Oxford) 16:46–55. doi:10.1111/hpb.12063 (PMID: 23461663)
Pecorelli N, Balzano G, Capretti G, Zerbi A, Di Carlo V, Braga M (2012) Effect of surgeon volume on outcome following pancreaticoduodenectomy in a high-volume hospital. J Gastrointest Surg 16:518–523. doi:10.1007/s11605-011-1777-2 (PMID: 2208 3531)
Lygidakis NJ (1996) Reconstruction of alimentary continuity after subtotal duodenopancreatectomy. Hepatogastroenterology 43:971–979 (PMID: 8884323)
Munoz-Bongrand N, Sauvanet A, Denys A, Sibert A, Vilgrain V, Belghiti J (2004) Conservative management of pancreatic fistula after pancreaticoduodenectomy with pancreaticogastrostomy. J Am Coll Surg 199:198–203. doi:10.1016/j.jamcollsurg.2004.03.015 (PMID: 15275873)
DeOliveira ML, Winter JM, Schafer M, Cunningham SC, Cameron JL, Yeo CJ, Clavien PA (2006) Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 244:931–937. doi:10.1097/01.sla.0000246856.03918.9 (PMID: 17122618)
Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M, International Study Group on Pancreatic Fistula Definition (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138:8–13. doi:10.1016/j.surg.2005.05.001 (PMID: 16003309)
Schmidt CM, Turrini O, Parikh P, House MG, Zyromski NJ, Nakeeb A, Howard TJ, Pitt HA, Lillemoe KD (2010) Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg 145:634–640. doi:10.1001/archsurg.2010.118 (PMID: 20644125)
Böttger TC, Junginger T (1999) Factors influencing morbidity and mortality after pancreaticoduodenectomy: critical analysis of 221 resections. World J Surg 23:164–171 (PMID: 9880426)
Meniconi RL, Ettorre GM, Vennarecci G, Lepiane P, Colasanti M, Laurenzi A, Colace L, Santoro R (2013) Use of cold-stored vein allografts for venous reconstruction during pancreaticoduodenectomy. J Gastrointest Surg 17:1233–1239. doi:10.1007/s11605-013-2201-x (PMID: 23615805)
Ball CG, Dixon E, Vollmer CM, Howard TJ (2015) The view from 10,000 procedures: technical tips and wisdom from master pancreatic surgeons to avoid hemorrhage during pancreaticoduodenectomy. BMC Surg 15:122. doi:10.1186/s12893-015-0109-y (PMID: 26608343)
Uzunoglu FG, Reeh M, Vettorazzi E, Ruschke T, Hannah P, Nentwich MF, Vashist YK, Bogoevski D, König A, Janot M, Gavazzi F, Zerbi A, Todaro V, Malleo G, Uhl W, Montorsi M, Bassi C, Izbicki JR, Bockhorn M (2014) Preoperative Pancreatic Resection (PREPARE) score: a prospective multicenter-based morbidity risk score. Ann Surg 260:857–863. doi:10.1097/SLA.0000000000000946 (PMID: 25243549)
Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R, Di Carlo V (2011) A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg 254:702–707. doi:10.1097/SLA.0b013e31823598fb (PMID: 22042466)
van Berge Henegouwen MI, De Wit LT, Van Gulik TM, Obertop H, Gouma DJ (1997) Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg 185:18–24 (PMID: 9208956)
Joliat GR, Petermann D, Demartines N, Schäfer M (2015) Prediction of complications after pancreaticoduodenectomy: validation of a postoperative complication score. Pancreas 44:1323–1328. doi:10.1097/MPA.0000000000000399 (PMID: 26465955)
Pillai SA, Palaniappan R, Pichaimuthu A, Rajendran KK, Sathyanesan J, Govindhan M (2014) Feasibility of implementing fast-track surgery in pancreaticoduodenectomy with pancreaticogastrostomy for reconstruction—a prospective cohort study with historical control. Int J Surg 12:1005–1009. doi:10.1016/j.ijsu.2014.07.002 (PMID: 25014648)
Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, Parks RW, Fearon KC, Lobo DN, Demartines N, Braga M, Ljungqvist O, Dejong CH, ERAS® Society, European Society for Clinical Nutrition and Metabolism, International Association for Surgical Metabolism and Nutrition (2012) Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 31:817–830. doi:10.1016/j.clnu.2012.08.011 (PMID: 23079762)
Braga M, Pecorelli N, Ariotti R, Capretti G, Greco M, Balzano G, Castoldi R, Beretta L (2014) Enhanced recovery after surgery pathway in patients undergoing pancreaticoduodenectomy. World J Surg 38:2960–2966. doi:10.1007/s00268-014-2653-5 (PMID: 24870390)
Rush TJ (2001) The role of the infectious disease specialist in pancreatic surgery. Surg Clin North Am 81:647–650 (PMID: 11459278)
Toomey PG, Teta AF, Patel KD, Ross SB, Rosemurgy AS (2016) High-volume surgeons vs high-volume hospitals: are best outcomes more due to who or where? Am J Surg 211:59–63. doi:10.1016/j.amjsurg.2015.08.021 (PMID: 26542187)
Bassi C, Falconi M, Molinari E, Salvia R, Butturini G, Sartori N, Mantovani W, Pederzoli P (2005) Reconstruction by pancreaticojejunostomy versus pancreaticogastrostomy following pancreatectomy: results of a comparative study. Ann Surg 242:767–771 (PMID: 16327486)
Oussoultzoglou E, Bachellier P, Bigourdan JM, Weber JC, Nakano H, Jaeck D (2004) Pancreaticogastrostomy decreased relaparotomy caused by pancreatic fistula after pancreaticoduodenectomy compared with pancreaticojejunostomy. Arch Surg 139:327–335. doi:10.1001/archsurg.139.3.327 (PMID: 15006893)
McKay A, Mackenzie S, Sutherland FR, Bathe OF, Doig C, Dort J, Vollmer CM Jr, Dixon E (2006) Meta-analysis of pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy. Br J Surg 93:929–936. doi:10.1002/bjs.5407 (PMID: 16845693)
Santoro R, Carlini M, Carboni F, Nicolas C, Santoro E (2003) Delayed massive arterial hemorrhage after pancreaticoduodenectomy for cancer. Management of a life-threatening complication. Hepatogastroenterology 50:2199–2204 (PMID: 14696498)
Chen JF, Xu SF, Zhao W, Tian YH, Gong L, Yuan WS, Dong JH (2015) Diagnostic and therapeutic strategies to manage post-pancreaticoduodenectomy hemorrhage. World J Surg 39:509–515. doi:10.1007/s00268-014-2809-3 (PMID: 25287917)
Topal B, Fieuws S, Aerts R, Weerts J, Feryn T, Roeyen G, Bertrand C, Hubert C, Janssens M, Closset J, Belgian Section of Hepatobiliary and Pancreatic Surgery (2013) Pancreaticojejunostomy versus pancreaticogastrostomy reconstruction after pancreaticoduodenectomy for pancreatic or periampullary tumours: a multicentre randomised trial. Lancet Oncol 14:655–662. doi:10.1016/S1470-2045(13)70126-8 (PMID: 23643139)
Menahem B, Guittet L, Mulliri A, Alves A, Lubrano J (2015) Pancreaticogastrostomy is superior to pancreaticojejunostomy for prevention of pancreatic fistula after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Ann Surg 261:882–887. doi:10.1097/SLA.0000000000000806 (PMID: 24979604)
Lee GC, Fong ZV, Ferrone CR, Thayer SP, Warshaw AL, Lillemoe KD, Fernández-del Castillo C (2014) High performing Whipple patients: factors associated with short length of stay after open pancreaticoduodenectomy. J Gastrointest Surg 18:1760–1769. doi:10.1007/s11605-014-2604-3 (PMID: 25091843)
Cameron JL, He J (2015) Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 220:530–536. doi:10.1016/j.jamcollsurg.2014.12.031 (PMID: 25724606)
Sadot E, Brennan MF, Lee SY, Allen PJ, Gönen M, Groeger JS, Peter Kingham T, D’Angelica MI, DeMatteo RP, Jarnagin WR, Fong Y (2014) Readmission after pancreatic resection: causes and causality pattern. Ann Surg Oncol 21:4342–4350. doi:10.1245/s10434-014-3841-0 (PMID: 25047467)
Sutton JM, Wilson GC, Wima K, Hoehn RS, Cutler Quillin R 3rd, Hanseman DJ, Paquette IM, Sussman JJ, Ahmad SA, Shah SA, Abbott DE (2015) Readmission after pancreaticoduodenectomy: the influence of the volume effect beyond mortality. Ann Surg Oncol 22:3785–3792. doi:10.1245/s10434-015-4451-1 (PMID: 25840560)
Goodney PP, Stukel TA, Lucas FL, Finlayson EV, Birkmeyer JD (2003) Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann Surg 238:161–167. doi:10.1097/01.SLA.0000081094.66659.c3 (PMID: 12894006)
Berwick DM, Hackbarth AD (2012) Eliminating waste in US health care. JAMA 307:1513–1516. doi:10.1001/jama.2012.362 (PMID: 22419800)
Joliat GR, Labgaa I, Petermann D, Hübner M, Griesser AC, Demartines N, Schäfer M (2015) Cost-benefit analysis of an enhanced recovery protocol for pancreaticoduodenectomy. Br J Surg 102:1676–1683. doi:10.1002/bjs.9957 (PMID: 26492489)
Acknowledgements
Other members of the Polo Ospedaliero Interaziendale Trapianti (P.O.I.T.) ‘‘Silvio Natoli’’ Multidisciplinary Group of Rome are M. Burocchi, A. Campanelli, A. Scotti, A. Laurenzi, S. Di Filippo, R. Lorusso, P. Mancini, A. Di Castro and E. Santoro, Department of General Surgery and Transplantation, A.O San Camillo-Forlanini General Hospital (SCF); M. Antonini, D. Busso, I. Caravella, C. Dantimi, R. Di Lorenzo, M. Fusetti, G. Garotto, F. Giansante, L. Marchioni, M. Maritti, S. Scarcia, G.V. Stazi, Anesthesiology and Intensive Care Unit, National Institute for Infectious Disease (INMI) ‘‘L. Spallanzani,’’; N. Petrosillo, A. Capone, F. Taglietti, G. Noto. Division of Systemic Infectious Disease and in Immunocompromised pazients, INMI; G. D’Offizi, R. Lionetti, L. Loiacono, M. Montalbano, A. Abdeaim, A. Rianda, L. Vincenzi, and U. Visco; Division of Hepatology and Infectious Disease, INMI; G. Cerasari, C. D’Ambrosio, L. Fondacaro, P. Guarascio, A. Pellicelli, R. Villani, Division of Hepatology, SCF; A. Tesi, C. Marrollo, P. Meddi, R. Mangiarotti, L. Giglio, C. Camastra, Division of Gastroenterology, SCF; E. Busi Rizzi, M. Cristofaro, F. Di Stefano, N. Fusco, F. Albarello and V. Schinina`, Imaging Division, INMI; M. Atzori, A. Cortese, P. Ialongo, G. Regine, and S. Pascoli, Imaging Division, SCF; Agresti, S. Giuliani, S. Minucci, M. Morucci, S. Pieri and P. Riu, Interventional Radiology, SCF; L.R. Grillo, Pathology Unit, SCF; A. Baiocchini and F. Del Nonno, Pathology Unit, INMI. We would like to thank F. Carboni, M. Carlini, M. Sacchi, M. D’Annibale, S. Canitano, F.L. Carpanese, M. Caterino, E. Forastiere, R. Kayal, P. Moricca, G. Pizzi, and G. Vallati for their valuable help at the Regina Elena Cancer Institute, Rome.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
The present study is a retrospective review of medical data and the human research ethics committee of our institution stated to exempt it from formal ethical review according to the ethical principles laid forth by the Helsinki Declaration.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Santoro, R., Meniconi, R.L., Lepiane, P. et al. Lessons learned from 300 consecutive pancreaticoduodenectomies over a 25-year experience: the “safety net” improves the outcomes beyond surgeon skills. Updates Surg 69, 451–460 (2017). https://doi.org/10.1007/s13304-017-0490-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-017-0490-4