Abstract
The control of walking in insects is to a substantial amount a function of neuronal networks in the thoracic ganglia. While descending signals from head ganglia provide general commands such as for walking direction and velocity, it is the thoracic central nervous system that controls movements of individual joints and legs. The coordination pattern of legs is velocity dependent. However, a clear stereotypic coordination pattern appears only at high velocities. In accordance with the unit burst oscillator concept, oscillatory networks (central pattern generators (CPGs)) interlocked with movement and load sensors control the timing and amplitude of joint movements. For a leg’s movements different joint CPGs of a leg are mainly coupled by proprioceptors. Differential processing of proprioceptive signals allows a task specific modulation of leg movements, for example, for changing movement direction. A switch between walking and searching movements of a leg is under local control. When stepping into a gap missing sensory input and the activation of a local command neuron evokes stereotypic searching movements of the leg.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Walking is arguably the most common mode of locomotion in terrestrial animals. From bipeds to millipedes (Illacmeplenipes has up to 750 legs [24]; to date we know no animal that has more) all animals have to solve the same tasks during walking, just as for other forms of locomotion (Fig. 1). Apart from some escape responses, the decision to move is made in an animal’s brain. This results in the activation of downstream command structures in the nervous system which, in turn, induce and maintain the motor activity that is specific for a particular form of locomotion. In vertebrates, these command structures are located in the brainstem; in invertebrates corresponding descending neurons are situated in the protocerebrum of the supra-esophageal ganglion. These neuronal systems activate neuronal networks in the segments of the central nervous system (CNS) that are close to extremities used for locomotion. For instance, the networks responsible for the control of hind leg extremities in mammals are located in the lumbar part of the spinal cord; leg movements in arthropods are controlled by networks in the ventral nerve cord. The activation of leg muscles responsible for leg movements is based on the rhythmic activity of these networks. An important property in the neuronal control of locomotion is that neuronal signals are not only transmitted in a descending fashion but also in reverse direction (review in [25]). In that way, signals carrying sensory information are fed back into the CNS, and activity in downstream networks influences higher centers in the brain.
Schematic of the components and the functional organization of the neural control system for locomotion in insects. Arrows indicate influences, in general, not neuronal connectivity; these influences can affect few, many, or all elements on a respective level, for instance the local controllers. For clarity, we only used parallel arrows. Numbers 1 through N identify the controllers, that is, the pattern generating circuits, of the legs as well as the legs and leg joints themselves. The seemingly hierarchical structure is broken up by various feedback loops. After a decision to walk has been made, information regarding direction and velocity are transmitted to the controllers located in the thoracic ganglia in the ventral nerve cord. These controllers consist of neuronal networks, each controlling the movements of one leg. Each leg controller consists of several joint controllers. The activity of the controllers, or pattern generating circuits, is determined by central nervous system elements, that is, oscillatory neuronal networks, also called central pattern generators, and sensory feedback. This sensory feedback is provided by proprioceptors, that is, movement and load sensors in the legs (for details see main text). Leg controllers can also be influenced by exteroceptors
Walking movements have to be coordinated on several levels of behavior. First, the muscles actuating an individual leg segment have to be coordinated. Furthermore, the movements of the segments in a single leg have to be coordinated. Finally, all legs that participate in walking have to be coordinated among each other. These tasks are controlled by neuronal networks in the spinal cord in vertebrates and the ventral nerve cord in invertebrates, respectively. A functional motor output constituting proper walking, however, emerges only by integrating activity generated centrally with proprioceptive signals from the legs. Like in all walking animals, descending signals originating in neurons in the brain or head ganglia in invertebrates initiate or terminate walking activity, control speed and direction, and provide fine tuning (Fig. 1).
In our work on insects, we currently focus on the mechanisms that contribute to single-leg control and to the coordination of several legs during walking in different behavioral context. In principle, the cyclic movement of an individual leg during walking can be divided into two phases. During the stance phase, the leg touches the ground and contributes to the movement into the desired direction. During the swing phase, the leg is lifted off and moved to the starting position for the next stance phase. The duration of these two phases is asymmetric, especially during slow walking; stance phase duration varies strongly with walking speed while the swing phase duration is relatively constant. The coordination of swing and stance phases of different legs, that is, the gait, is mainly correlated with walking speed and load. The faster an animal moves the more legs are in concurrent swing phase. In horses, for instance, we find the familiar gaits of walk, trot, and gallop. With an increase in locomotion speed, these gaits not only allow for increasingly shorter ground contact intervals but are in addition the energetically best mode of locomotion. In six-legged insects, we can classically differentiate three coordination patterns in which either one leg (wave gait), two legs (tetrapod coordination), or three legs (tripod coordination) execute their swing phase simultaneously (Fig. 2). Wave gait is characterized by the successive swing phases in the hind, middle, and front leg of one body side followed by the same succession of swing phases on the other body side. During tetrapod coordination, there are two legs in concurrent swing at a given time; for instance, the left front leg and the right hind leg, the right front leg and the left middle leg, or the right middle leg and the left hind leg. Tetrapod coordination exists as two mirror-symmetric patterns. The tripod coordination pattern is characterized by the concurrent swing phase in three legs: the front and hind leg of one body side and the middle leg of the opposite side. Different insect species prefer particular coordination patterns. Fast runners, such as cockroaches and ants, use predominantly tripod coordination at speeds of 32 or 60 body lengths per second, respectively. In contrast, the slow-walking stick insect prefers tetrapod coordination. All investigated insects, however, can change from one coordination pattern into the other in a smooth transition. This can be shown very nicely in the fruit fly. The walking speed of this insect spans a large range from slower than 1 to up to 16 body lengths per second. It has to be noted that the ideal forms of these coordination patterns are observed only rarely; the fact that intermediate forms and continuous transitions between these has led some researchers to avoid the term gait for insects [28, 30].
Schematic of the temporal inter-leg coordination patterns of a walking fruit fly as a function of walking speed. Each panel (top to bottom indicates decreasing walking speed) shows a walking sequence consisting of the swing (black bars) and stance phases (white bars) of all six legs. With a decrease in walking speed the inter-leg coordination changes from tripod coordination (three legs are in swing phase simultaneously), to tetrapod coordination (two legs in swing phase), to wave gait (only one leg in swing phase at any time). It becomes clear that particularly the latter two examples are only approximate realization of the ideal coordination patterns. The swing phase duration is relatively constant in all three examples
Behavioral experiments by Holk Cruse, Gernot Wendler, Ullrich Bässler, et al. suggest that stance and swing phases in a leg are substantially influenced by load and position of neighboring legs [14]. For instance, the onset of a swing phase in a leg is suppressed if its ipsilateral posterior neighbor is currently in its swing phase. An additional complementary influence triggers the onset of the swing phase in a leg as soon as its ipsilateral posterior neighbor begins its stance phase. Also, the probability that a particular leg begins its swing phase increases the further its anterior neighboring leg is retracted. The neuronal basis of these so-called coordination rules is still largely unclear. It can be assumed that signals mediating information about position and load are transmitted between ganglia by intersegmental neurons. However, load information can also be transferred indirectly via the mechanical coupling of the legs by the body and ground. The load in a particular leg during its stance thereby depends on the number of legs that are in their swing phases at the same time. The load level, in turn, influences the duration of the stance phase and the level of activation of the active motor neurons. In both cases, a clear understanding of how several legs are coordinated can only be achieved if we understand the mechanisms of movement control for the single leg.
Central mechanisms for leg joint control during walking
Cyclic movements of leg joints are the basis for the movements of a leg. Starting with the experiments of T.G. Brown, who investigated the walking system in cats more than 100 years ago, the last 50 years particularly have seen many studies on rhythmogenesis for locomotor activity (swimming, flying, and walking) in vertebrates and invertebrates. These studies have led to the concept of central pattern generators (CPG). In its most simple form a CPG can consist of a single oscillatory neuron; mostly, however, a CPG is made up of a network of neurons located in the spinal or ventral nerve cord. In the absence of phasically structured sensory or other phasic inputs, a CPG is able to produce rhythmic activity in motor neurons. In this sense, a CPG can also be seen as a central neuronal oscillator. The prerequisites for oscillatory activity in a CPG are on the one hand specific membrane properties facilitating voltage oscillations in the CPG neurons themselves; these properties are based on, for instance, voltage-dependent calcium or sodium channels which, when opened, lead to so-called plateau potentials, that is, a long-lasting depolarization of the neuron. On the other hand, alternating activity in antagonistic motor neurons can be induced by the coupling of two oscillatory neurons or CPGs, e.g. by that are mutual inhibition, which is a common motif in oscillatory networks [17]. Conversely, different CPGs responsible for synergistic muscles can be connected such that they are active in the same phase. The coupling between CPGs, which are also called unit burst oscillators, can change in a task-dependent manner, for instance for forward as opposed to backward walking. When several CPGs are coupled a higher-level CPG is formed able to produce and control the complex movements of a leg [9]. The flexibility based on this modular structure is a compelling advantage of the unit burst oscillator concept; it allows for the rapid adaptation of behavior to a changing environment. In vertebrates, the CPG activity responsible for leg control can be initiated, modulated, and terminated by descending signals from the brainstem. For invertebrates (crustaceans and insects), our knowledge about the descending control of CPGs is much more limited; the available data, however, suggest similar mechanisms; in insects, for instance, descending neurons in the protocerebrum of the supra-esophageal ganglion can elicit walking [6, 7].
Effects of descending control can be induced pharmacologically in spinalized vertebrates. The motor activity that is elicited in this way is called fictive locomotion. In arthropods, it is also possible to pharmacologically induce this type of CPG-derived locomotor output. Locomotion, however, has to be adaptive and no animal can rely on purely centrally generated, that is, CPG-controlled, leg movements. CPGs as well as motor neurons are additionally influenced by proprioceptive signals originating in the legs. These signals can modulate the amplitude as well as the temporal structure of the rhythmic activity generated by CPGs (Fig. 3). The fact that not only central but also peripheral neurons constitute the functional oscillatory networks has prompted some authors to stop using the term CPG in this context but rather to refer to a pattern generating circuit (e.g., [23]) or as in Fig. 1 to the more technical term controller, thus including central as well as peripheral aspects.
Schematic of the movement control of a single leg joint. A joint central pattern generator (CPG) generates rhythmic alternating activity in its associated antagonistic motor neurons and the respective leg muscles. Feedback about the forces and movements produced during stepping are mediated via signals provided by proprioceptors in the leg. These signals control the motor-neuronal level of activity as well as the timing of the CPG
Our lab is interested in the neuronal mechanisms that produce the cyclic activity of motor neurons in insect legs during walking and we investigate this issue in the stick insect. Similar to other animals, in the stick insect oscillatory activity in locomotor networks in the CNS can be induced pharmacologically (we will refer to this as CPG activity). When we apply the muscarinic acetylcholine agonist pilocarpine to deafferented thoracic ganglia we can induce long-lasting periods of oscillatory activity in the leg motor neurons [10]. Antagonistic motor neurons thereby generate alternating bursts of action potentials [11, 22]. These patterns of activity are based on tonic depolarization interspersed with rhythmic inhibition. Since the motor neurons themselves do not have oscillatory membrane properties, we must assume that the tonic depolarization and the phasic inhibition are caused by pre-motor interneurons; the phasic inhibition is caused by CPG activity.
The tonic depolarization as well as the prominent rhythmic inhibition can also be found in intracellular recordings from leg motor neurons during actual stepping movements in semi-intact preparations of the stick insect. The tonic depolarization, with its reversal potential of − 38 mV, is probably carried by a mixed inward/outward conductance [29]; its cause, however, is still unclear. It is conceivable that acetylcholine as an excitatory transmitter is involved at the level of motor neurons. Also, local premotor nonspiking interneurons might play a role (these neurons do not produce action potentials), Fig. 4.
Activity profile of a motor neuron during stepping. The upper part of the figure shows an intracellular recording of the activity of a flexor motor neuron during a sequence of steps of the associated middle leg on a treadmill. The motor neurons produce action potentials during the stance phase (black). This activity is partly caused by sensory inputs (red). In addition, the motor neurons are tonically depolarized during the complete walking sequence (orange). The exact origin of this depolarization is unknown. Between action potential bursts the motor neuron is hyperpolarized phasically (pink). This inhibition is controlled by the joint central pattern generator CPG, but additional sensory influences probably exist
In contrast to pharmacologically induced activity in deafferented preparations the tonic depolarization of motor neurons driving a stepping leg is not the only reason why these become active. The bursts a motor neuron produces are additionally facilitated by excitatory inputs from proprioceptors in the associated leg; sometimes this is actually implemented as positive feedback. For instance, the femoral chordotonal organ (fCO), a sensory structure measuring movement of the femur-tibia joint, and campaniform sensilla, responsible for the detection of load and located in the exoskeleton, support the excitation of tibial flexor motor neurons during the stance phase [1, 2]. Sensory input can be mediated via local interneurons but can also directly affect motor neurons [8]. Sensory influences of the CPG-induced inhibition of motor neurons might also exist but have not been conclusively shown to date.
The exact topology of the CPGs involved in walking is currently unknown; this is true not only for the stick insect but also all other insects and walking vertebrates. We do know, however, several identified nonspiking interneurons in the thoracic ganglia of the stick insect that are part of joint CPGs (Büschges1995). According to our current knowledge, the effects of these interneurons as well as their morphology are restricted to a specific hemi- ganglion. Nonspiking interneurons can either excite or inhibit motor neurons and are therefore mainly responsible for the rhythmic activity found in these neurons. In the stick insect, for instance, there are two indentified nonspiking interneurons, I4 and E4, that are able to re-start, that is, reset, rhythmic activity in depressor motor neurons. This suggests that both I4 and E4 are part of the depressor CPG. However, E4 and several other nonspiking interneurons influence motor neurons associated with multiple joints and it stands to reason that these interneurons are not only part of single CPGs but also they can couple these and thus combine them to functional units.
Inter-joint coordination of a single leg during walking
Despite the aforementioned interneuron-mediated coupling of CPGs the pilocarpine-induced rhythmic activity in motor neurons associated with different joints is not coupled on a cycle-to-cycle basis; only the strict alternating activity of antagonists can be regarded as a constant. This finding and further evidence have resulted in the notion that the movement of each leg joint is governed by its own CPG located in the thoracic nervous system of the stick insect. In a similar form, this organizational principle has been shown for the spinal cord in mammals [18]. For the stick insect we can assume that the CPGs responsible for the three main leg joints are independent; this organization is consistent with the concept of unit burst oscillators.
The control of joint movements during a step is quite a complex task. First, this is due to the number of muscles that has to be controlled. Mammals have to coordinate the contractions of approximately 36 muscles to produce proper swing and stance phases during walking. In a typical insect leg, this number is still higher than a dozen (Fig. 5a). Second, the temporal activation pattern of a muscle can often not directly be related to only the swing or only the stance phase. In mammals, for instance, we find muscles that span two joints and these are activated twice during a particular step phase. In insects, some muscles are active during the swing phase but their activation extends considerably into the stance phase (Fig. 5b). Furthermore, during the stance phase the leg muscles not only have to propel the body forward but also in addition have to stabilize body posture. For this, the level of muscle activation has to be adapted to the properties of the walking substrate. When we take all of the above into account it is evident that the temporal activation patterns of leg muscles cannot be orchestrated without sensory feedback.
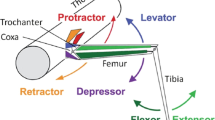
a Schematic of the stick insect middle leg with a particular focus on the three main leg joints and their associated muscles. Thorax–coxa joint (ThC joint), coxa–trochanter joint (CTr joint) (the trochanter and the femur are fused), femur–tibia joint (FTi joint). b Activity pattern of the antagonistic muscles responsible for movement in the three main leg joints of the middle leg during stepping as a function of step cycle phase. c The upper part shows a schematic of the movements of a single leg during swing and stance phase. The sequence has been projected to a plane that is orthogonal to the longitudinal axis of the animal. (1) The leg is lifted off at the end of the stance phase. (2) Termination of the swing phase and touchdown of the leg. (3) Onset of the stance phase; the leg is on the ground. (4) Termination of the stance phase and lift-off of the leg. The lower part illustrates the influences of various signals generated by sensory organs in the leg which contribute to phase and amplitude control in motor neurons during stepping. These influences are either excitatory (open arrows) or inhibitory (black circles). The individual joint CPGs, here depicted as interneurons mutually influencing each other, are colored according to the leg segments they control. Filled circles indicate activation, open circles indicate inactivation. As an example: during stage 4, that is, during the stance phase, load and ground contact signals facilitate activity in the retractor, depressor, and flexor activity; at the same time flexion signals also facilitate flexor activity in the femur–tibia joint
For the three main leg joints in the stick insect, we have a quite comprehensive picture with regard to the sensory control of the involved CPGs [12]. This control crucially depends on signals that mediate a joint’s position, velocity, and acceleration as well as on signals that carry information about the effective load and muscle forces, respectively, that are applied to the leg. Joint movements in an insect leg are measured by hairs on the outside of the cuticle. These hairs are arranged in fields and are located close to the joint membrane; they are stimulated mechanically when the leg moves. In addition, joint positions can also be measured by internal stretch receptors, for example chordotonal organs. In the stick insect, we have a very good understanding of the role of four hair fields and one chordotonal organ in the control of leg joint CPGs. Information concerning forces and load, which can be a result of muscle contractions or ground contact, are mediated by campaniform sensilla. These are sensory organs that are, either separately or clustered in groups, located in the cuticle close to joints. These sensors measure load applied to a leg segment in a highly differentiated way, either parallel or perpendicular to the leg plane. They affect the activity of those muscles that move leg segments in the respective spatial plane [31, 32].
Selective stimulation and ablation of proprioceptors in legs and the examination of active stepping movements in single legs, either in semi-intact but also very reduced preparations of the stick insect, show that the aforementioned sensory organs are determinants for the activation and inactivation of motor neurons during walking. Thus, they control the oscillatory activity of joint CPGs and the level of activation in motor neurons. To exemplify this we will take a closer look at the role of sensory information during the transition from swing to stance phase in a step cycle.
Figure 5c shows a schematic illustrating the relative positions of the segments in a stepping middle leg. The complete step cycle has been divided into four operationally distinct stages. Stage 1 designates the early swing phase, the transition between swing and stance phase occurs at the border between stages 2 and 3, the stance phase ends with stage 4. Figure 5c shows an abstract representation of the CPGs associated with the main leg joints; arrows and circles indicate excitatory or inhibitory sensory influences, respectively. In stage 2, the leg moves through the air to the starting point of the next stance phase; the tibia is being extended. This extension is detected by the joint angle sensor of the femur–tibia joint, the fCO. This information, in turn, is sent to interneurons in the CNS which affect a switch in the thorax–coxa CPG. The consequence is a switch from levator to depressor activation and the leg is moved down onto the ground (stage 3). This touchdown activates load sensors resulting not only in further facilitation of the ongoing depressor activity but also in activation of retractor muscles, that is, a switch in the thorax–coxa joint CPG. The same influence initiates the activation of flexor muscles, that is, a switch in the femur–tibia joint CPG. At this point, a new stance phase begins and the leg contributes to propulsion relative to the ground. During the stance phase load information determines the level of activity in depressor motor neurons. In addition, movement signals originating in the fCO act as positive feedback and support the flexion in the femur–tibia joint during the stance phase. A slightly simplified version of the sensorimotor coupling outlined here is not only sufficient for the construction of a functional computer model of the stepping leg [21, 27] but also can serve as control principle for event-based phase switching, a principle that can significantly improve the electronic control of walking robots, such as the bipedal robot Lola developed at the TU Munich [13]. It is important to note, that the sensory influences outlined here are sufficient only for the control of simple forward walking. Changes in walking direction, however, require the modification of these processes.
Differential processing of proprioceptive signals as a basis for task-specific modification of leg movements
The neuronal control of walking, like any behavior, has to be highly adaptive. For instance, walking movements have to be modified in response to sudden and unpredictable disturbances, for example, when a leg hits an obstacle or in the absence of ground contact, to ensure continued locomotion. In addition, walking has to be modifiable with regard to changing task requirements, for example, when the animal wants to change its walking speed or direction or during curve walking. To date only little is known about the neuronal control of these processes. It is unclear which of the modifications observed during adaptive behavior are controlled directly by descending signals from the brain and which are evoked by local neural networks in the ventral nerve cord or the spinal cord. In our work, we address these questions in experiments investigating the neuronal control of curve walking.
During curve walking the stance phase directions of all six legs change in such a way that the legs located on the outside of the curve push the animal’s body into the direction of the curve, while the legs on the inside of the curve pull the animal into the curve. During very tight curves, the hind leg on the inside of the curve often even ceases stepping and its contact point on the ground defines the pivot point of the movement. Compared with straight walking, the movements of all six legs change markedly during curve walking. We investigate the control of these changes in an experimental setup where all six legs are mechanically decoupled. To do this, the stick insect is tethered and held in place while the six legs perform walking movements on a slippery surface. When we now elicit curve walking in this situation, for instance by visual stimulation, each leg produces its own motor pattern corresponding to its role during curve walking. Lesion experiments have shown that the stepping movements generated for curve walking are produced for each leg individually, that is, they are independent of the movements of the other five legs.
Which changes in the neuronal control of stepping are necessary for this? Our investigations have shown that task-specific processing of proprioceptive signals from sensory organs in the leg are of great importance. Here, we discuss our findings for the middle leg. On the inside of the curve, the stance phase is mainly characterized by a flexion in the femur–tibia joint, while the leg’s protraction and retraction amplitude is only small. In contrast, on the outside of the curve, the pronounced movement in the femur–tibia joint is absent and the leg is very strongly retracted. We were able to show that the processing of flexion signals originating in the fCO differs strongly on the two body sides [20]. On the inside of the curve, the activity of flexor motor neurons is positively reinforced by these flexion signals, on the outside this influence is not present and we find a negative feedback that stabilizes the femur–tibia joint’s angular position. In addition, on the outside of the curve we observe a systematic facilitation of retractor activity by load signals originating in the leg; this facilitation is absent on the inside of the curve (Gruhn et al. in prep) (Fig. 5). These findings highlight the important role of differential processing of proprioceptive signals for the task-specific modification of leg movements. Currently, we investigate how these modifications are produced in the local premotor networks and we investigate the contribution of descending signals from the brain.
Neuronal mechanisms for the selection of different leg movements
The modular CPG structure that makes up the neuronal control of the walking system in the stick insect and its individual control instances for individual legs and joints is an ideal basis for the generation of versatile and adaptive behavior. This organization can couple the various CPGs in a task-dependent fashion and can thus contribute to the generation of different leg movements. This is captured by the concept of the unit burst oscillator [17] which explains how one particular locomotory extremity and its segments can be used in different context such as walking, running, swimming, or scratching.
Insects use their legs in various ways, often in a rhythmic fashion, for example, for searching and grooming movements or for stridulation. During stridulation grasshoppers rub their hind legs against a cuticular vein on the forewings to produce sound; these rhythmic movements result in stereotypical songs that are used for intra-species communication [16]. The temporal structure and amplitudes of the associated leg movements are song-specific. The work of Berthold Hedwig et al. has shown that different songs are elicited and maintained by specific command neurons located in the grasshopper’s supra-esophageal ganglion [19]. However, the structure of the associated thoracic song CPGs as well as their interaction with the upstream command neurons is unknown. It has been unclear for a long time whether command neurons located in the head control and select stereotypical leg movements in general or whether command functions can be adopted by local interneurons, similar to reflexes that are realized by local networks.
The first evidence for the importance of local mechanisms implementing command functions for the generation of leg movements were found in the locust. These animals use their hind legs to groom their bodies and wings. The leg movements for grooming are CPG-controlled and independent of descending signals; they can be produced in the absence of sensory feedback coming from the hind legs and are still functional after severing anterior connectives [5]. Grooming and scratching movements in vertebrates are organized in a similar fashion [26].
A recent study from our lab has substantiated that local thoracic neurons can assume command function controlling the initiation and maintenance of rhythmic leg movements. Stick insect legs produce stereotypical searching movements when they cannot find foothold after a swing movement [3, 15]. In the middle leg, these searching movements are initiated and maintained by the depolarization of a single local nonspiking neuron, interneuron I4, in the mesothoracic ganglion. Depolarization of I4 is sufficient and necessary for the initiation of searching movements; it is therefore a local command neuron. However, searching movements can only be elicited as long as the leg is not on the ground. In this way, the decision to search is only made in the appropriate context [4]. It is not surprising that I4 and other nonspiking neurons involved in the control of searching movements are also important for the control of joint movements during walking; in this context, however, I4 does not act as a command neuron [4].
Our status report on the current knowledge of the neuronal basis of movement control in insects focuses on conceptional aspects. For a broad readership, we reasoned it might be more interesting to learn about the complexity of a seemingly automatic behavior and focus on a part of the CNS that is typically not the general center of attention, rather than delve into details on the level of neuronal networks, individual neurons, or muscles. Nevertheless, we would be glad to answer any question regarding these levels of description. Our report illustrates that the largest part of our work is still ahead. This will involve elucidating how the nervous system controls the diversity and adaptivity of extremity movements in this fascinatingly effective manner. This means that we have to know much more about the structure and functional mechanisms of the involved networks and neurons. In addition, we have to find out which functions are carried out by the brain and which functions are implemented decentrally further downstream.
References
Akay T, Bässler U, Gerharz P, Büschges A (2001) The role of sensory signals from the insect coxa-trochanteral joint in controlling motor activity of the femur-tibia joint. J Neurophysiol 85:594–604
Bässler U (1988) Functional principles of pattern generation for walking movements of stick insect forelegs: the role of the femoral chordotonal organ afferences. J Exp Biol 136:125–147
Berg E, Büschges A, Schmidt J (2013) Single perturbations cause sustained changes in searching behavior in stick insects. J Exp Biol 216:1064–1074
Berg EM, Hooper SL, Schmidt J, Büschges A (2015) A leg-local neural mechanism mediates the decision to search in stick insect. Curr Biol 25:2012–2017
Berkowitz A, Laurent G (1996) Local control of leg movements and motor patterns during grooming in locusts. J Neurosci 16:8067–8078
Bidaye SS, Machacek C, Wu Y, Dickson BJ (2014) Neuronal control of Drosophila walking direction. Science 344:97–101
Böhm H, Schildberger K (1992) Brain neurons involved in the control of walking in the cricket Gryllus bimaculatus. J Exp Biol 166:113–130
Burrows M (1996) The neurobiology of an insect brain. Oxford University Press, Oxford
Büschges A (2005) Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J Neurophysiol 93:1127–1135
Büschges A, Schmitz J, Bässler U (1995) Rhythmic patterns in the thoracic nerve cord of the stick insect induced by pilocarpine. J Exp Biol 198:435–456
Büschges A, Ludwar BCh, Bucher D, Schmidt J, DiCaprio RA (2004) Synaptic drive contributing to rhythmic activation of motoneurons in the deafferented stick insect walking system. Eur J Neurosci 19:1856–1862
Büschges A, Akay T, Gabriel JP, Schmidt J (2008) Organizing network action for locomotion: insights from studying insect walking. Brain Res Rev 57:162–171
Buschmann T, Ewald A, von Twickel A, Büschges A (2015) Controlling legs for locomotion—insights from robotics and neurobiology. Bioinspir Biomim 10:041001
Cruse H (1990) What mechanisms coordinate leg movement in walking arthropods? Trends Neurosci 13:15–21
Dürr V (2001) Stereotypic leg searching movements in the stick insect: kinematic analysis, behavioural context and simulation. J Exp Biol 204:1589–1604
Elsner N (1974) Neuroethology of sound production in gomphocerine grasshoppers (Orthoptera: acrididae). I. Song patterns and stridulatory movements. J Comp Physiol 88:67–102
Grillner S (2006) Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52:751–766
Hägglund M, Dougherty KJ, Borgius L, Itohara S, Iwasato T, Kiehn O (2013) Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc Natl Acad Sci 110:11589–11594
Hedwig B, Heinrich R (1997) Identified descending brain neurons control different stridulatory motor patterns in an acridid grasshopper. J Comp Physiol 180:285–294
Hellekes K, Blincow E, Hoffmann J, Büschges A (2012) Control of reflex reversal in stick insect walking: effects of intersegmental signals, changes in direction, and optomotor-induced turning. J Neurophysiol 107:239–249
Knops S, Tóth TI, Guschlbauer C, Gruhn M, Daun-Gruhn S (2013) A neuromechanical model for the neuronal basis of curve walking in the stick insect. J Neurophysiol 109:679–691
Ludwar BC, Westmark S, Büschges A, Schmidt J (2005) Modulation of membrane potential in mesothoracic moto- and interneurons during stick insect front-leg walking. J Neurophysiol 94:2772–2784
Marder E, Calabrese RL (1996) Principles of rhythmic pattern generation. Physiol Rev 76:687–717
Marek PE, Bond JE (2006) Biodiversity hotspots: rediscovery of the world’s leggiest animal. Nature 441:707
Orlovsky G, Deliagina T, Grillner S (1999) Neuronal control of locomotion. Oxford University Press, Oxford
Stein PS (2008) Motor pattern deletions and modular organization of turtle spinal cord. Brain Res Rev 57:118–124
Tóth TI, Knops S, Daun-Gruhn S (2012) A neuro-mechanical model explaining forward and backward stepping in the stick insect. J Neurophysiol 107:3267–3280
Wendler G (1965) The co-ordination of walking movements in arthropods. Symp Soc Exp Biol 20:229–249
Westmark S, Oliveira EE, Schmidt J (2009) Pharmacological analysis of tonic activity in motoneurons during stick insect walking. J Neurophysiol 102:1049–1061
Wosnitza A, Bockemühl T, Dübbert M, Scholz H, Büschges A (2013) Inter-leg coordination on the control of walking speed in Drosophila. J Exp Biol 216:480–491
Zill SN, Schmitz J, Chaudhry S, Büschges A (2012) Force encoding in stick insect legs delineates a reference frame for motor control. J Neurophysiol 108:1453–1472
Zill SN, Chaudhry S, Exter A, Büschges A, Schmitz J (2014) Positive force feedback in development of substrate grip in the stick insect tarsus. Arthropod Struct Dev 43:441–455
Acknowledgments
We thank Dr. Till Bockemühl for reviewing and translating the text into English and Sherylane Seeliger for help with the figures. The work of the authors is supported by DFG grants Bu 857 and Schm 1084.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Büschges, A., Schmidt, J. Neuronal control of walking: studies on insects. e-Neuroforum 6, 105–112 (2015). https://doi.org/10.1007/s13295-015-0017-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13295-015-0017-8