Abstract
We examined how short-term (19 days) nutrient enrichment influences stream fungal and diatom communities, and rates of leaf decomposition and algal biomass accrual. We conducted a field experiment using slow-releasing nutrient pellets to increase nitrate (NO3-N) and phosphate (PO4-P) concentrations in a riffle section of six naturally acidic (naturally low pH due to catchment geology) and six circumneutral streams. Nutrient enrichment increased microbial decomposition rate on average by 14%, but the effect was significant only in naturally acidic streams. Nutrient enrichment also decreased richness and increased compositional variability of fungal communities in naturally acidic streams. Algal biomass increased in both stream types, but algal growth was overall very low. Diatom richness increased in response to nutrient addition by, but only in circumneutral streams. Our results suggest that primary producers and decomposers are differentially affected by nutrient enrichment and that their responses to excess nutrients are context dependent, with a potentially stronger response of detrital processes and fungal communities in naturally acidic streams than in less selective environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nutrient loading from organic inputs and agricultural run-off is a major threat to aquatic ecosystems. While point-source nutrient pollution is increasingly better controlled, non-point sources continue to rise and natural background concentrations have been far exceeded worldwide (Vörösmarty et al. 2010). Excessive nutrient loading has already degraded freshwater ecosystems and is likely to further impair their biodiversity and ecosystem functioning, potentially leading to the loss of key ecosystem services (Woodward et al. 2012). Ecosystem functioning is considered to be driven primarily by either primary producers (‘green food webs) or decomposers (‘brown food webs’), with often complex interactions between the two (Zou et al. 2016). Despite increasing loads of nutrients to freshwater ecosystems, little is known about the mechanisms driving the simultaneous responses of both pathways to nutrient enrichment. Furthermore, most studies thus far have focused on nutrient enrichment as a press disturbance while in many real-world situations nutrient concentrations may fluctuate rapidly, potentially causing a pulse-type disturbance to stream organisms. Such short-term changes are best captured by studies focusing on microbial organisms with short generation times.
Leaf litter is the primary source of energy and carbon in small woodland streams and microbes are key players in the decomposition of this material (Bärlocher 2005). Dissolved inorganic nutrients may provide an exogenous nutrient subsidy that stimulates microbial activity and decomposition (Gulis and Suberkropp 2003; Kominoski et al. 2015). Long-term in situ field experiments have shown that nutrient enrichment stimulates algal growth and leaf litter decomposition (Sabater et al. 2011; Rosemond et al. 2015). However, nutrient enrichment does not always result in increased decomposition (Baldy et al. 2007; Garcia et al. 2017) and in highly polluted streams enrichment may even inhibit decomposition (Pascoal and Cassio 2004; Dunck et al. 2015). Similarly, although enrichment generally increases algal biomass (Slavik et al. 2004), this effect is often overridden by other environmental factors (Schneider et al. 2013; Bondar-Kunze et al. 2016), further implying that ecosystem-level responses to nutrient enrichment are highly variable and context dependent.
Environmental conditions can influence nutrient bioavailability which may partly explain variable responses of ecosystems functioning to nutrient enrichment. For example, nutrients may not be utilized to their full potential in humic lakes because of limited bioavailability of nutrients and low light penetration due to dissolved organic matter (Jansson et al. 2001). Initial community composition may also influence how communities and ecosystem functioning respond to nutrient enrichment (Clements et al. 2016). Environmental conditions have a strong imprint on stream microorganisms and variation in their community composition often tracks closely variation in major environmental gradients (e.g., Annala et al. 2014; Heino et al. 2014). Communities are thus sorted along environmental gradients, being composed of species adapted to local conditions. Increased productivity can mask species sorting and result in opportunistic dominance by individual species. For example, Chase (2010) showed that compositional variability of communities in highly productive experimental ponds increased as a result of stochastic processes due to differential colonization history and priority effects.
We have previously found that naturally acidic streams that drain black shale deposits support acid-tolerant fungi (Tolkkinen et al. 2015) and diatoms (Annala et al. 2014) that are very abundant in, or even unique to, these streams. The influence of nutrient enrichment on communities and ecosystem functioning could thus be very different in naturally acidic streams compared to circumneutral streams where environmental conditions are likely less influential to community assembly. However, additional anthropogenic stress affected fungal communities and decomposition more in naturally acidic than in circumneutral streams, suggesting that fungal communities in naturally stressful environments may be sensitive to novel stressors (Tolkkinen et al. 2015). While context-dependent responses of biological communities have gained increasing attention in contaminant assessment (Clements et al. 2016), studies on the context-dependency of biological responses to nutrient enrichment are rare (Burkepile and Hay 2006).
We examined how nutrient enrichment influences the assembly of stream fungal and diatom communities, and algal biomass accumulation and leaf litter decomposition in distinctly different environmental settings. We increased nutrient concentrations in riffle sections of naturally acidic and circumneutral streams to test (i) if short-term (19 days) nutrient addition stimulates algal accrual and leaf decomposition rates and (ii) if this effect is context dependent, i.e., if responses of diatom and fungal communities to nutrient enrichment are different in naturally acidic streams versus circumneutral streams. We further tested (iii) if nutrient enrichment alters (either increases or decreases) compositional turnover of communities and (iv) if changes in community composition arise from deterministic or stochastic processes, i.e., if changes in compositional turnover are different from random expectation.
Materials and Methods
Study streams
The experiment was conducted in September 2014 in central Finland (between 64–66°N; 26–29°E). Twelve first-to-second order streams, six of which had naturally low pH (catchment area 0.6–2.8 km2, minimum pH 3.7–5.1) and six were circumneutral (catchment area 0.35–5.0 km2, minimum pH 5.7–6.9) were selected for the experiment. Streams with naturally low pH are located in an area where bedrock geology is dominated by black shale that causes low pH and high metal concentrations of the stream water (Loukola-Ruskeeniemi et al. 1998). Circumneutral streams are located in the Iijoki drainage basin, approximately 170 km north of the low-pH region. All streams were in near-pristine condition, i.e., without obvious signs of human impact in the stream channel or in the riparian zone. Streams in both regions drain largely similar catchments (proportion of coniferous forests 51–88% in the acidic and 42–80% in the circumneutral region; proportion of peatlands 22–56% in the acidic versus 18–60% in the circumneutral region) except that streams in the low-pH region run through a black-shale deposit. For more information about the study area, see Annala et al. (2014).
Nutrient enrichment
A randomly selected riffle in each stream was divided into 10-m-long upstream and downstream sections. The downstream section was treated with nutrient enrichment for 19 days in autumn (September–October) 2014 while the upstream section served as control. The duration of the experiment was selected so that it co-occurred with the timing of the maximal leaf-fall and was long enough to allow the detection of responses by both periphyton and decomposition in our study region (see Mustonen et al. 2016). We used slow-releasing fertilizing pellets to increase nutrient levels in downstream sections to predefined (about 2 × ambient) levels. A mixture of 1.5 kg of Multicote 2M NPK 14–6–12 (polymer coating, total nitrogen content 13.5% and total phosphorus content 5.9%) and 0.5 kg of Superphosphate P20 (clay matrix, 20% P2O5) was used. The pellets were enclosed in a 50-cm-long and 15-cm-wide mesh bag (mesh size 0.5 mm) and placed in the streambed in a narrowed section at the upstream end of the experimental reach to ensure that at least 70% of the flow passed through the bag. It has been shown that slow-releasing fertilizers with polymer coating allow a relatively constant release even during 6- to 8-week experiments (Worm et al. 2000). In a preliminary trial, we observed that most (about 80%) of the uncoated Superphosphate P20 was released in 4 days. Therefore, pellets were replaced every 5 days (three times) during the experiment. Nutrient enrichments were conducted with permission of regional environmental authorities and local land-owners.
Leaf litter breakdown
Leaf breakdown assays followed the protocol presented by Benfield (1996), with some modifications. Six grams of dried alder (Alnus incana) leaves were enclosed in 15 × 15 cm mesh bags. The leaves were collected just prior to abscission and they were air-dried for 2 weeks. We used mesh size of 0.2 mm to prevent invertebrates from entering the bags. Four individually tagged bags were placed onto the stream bed at both experimental sections (the first bag being placed 1.5 m downstream from the pellets, others in ca. 1-m intervals in downstream direction) and anchored using steel rods. Leaf bags were placed in slow-flowing positions (mean 10 cm s−1) at each site. Bags were removed at the end of the experiment, sealed in zip-lock bags and transferred to a laboratory freezer. In the laboratory, litter bags were gently cleaned to remove any fine sediments and organic material other than leaves. A small subsample (0.07 g) of frozen leaf material was taken from each bag for the extraction of fungal DNA and measurement of ergosterol content. These samples were pooled into a composite sample for each section. The weight of the subsample was taken into account when calculating leaf decomposition rate. The remaining leaf material was dried at 60 °C for 48 h and subsamples were ashed for 4 h at 550 °C to convert dry mass to ash-free dry mass (AFDM). Leaf mass loss caused by leaching and handling was measured and accounted for in the analyses. Leaf breakdown rates were determined using the negative exponential decay model (Benfield 1996). As temperature can influence decomposition, breakdown rates were adjusted for degree days. Stream water temperature in each stream was measured at 30-min intervals using temperature loggers (iButton, Thermochron, Maxim Integrated, San Jose, USA).
DNA isolation, library construction, and sequencing
Fungal assemblage structure was examined through high throughput sequencing. DNA was extracted from frozen leaf material using PowerSoil DNA Isolation Kit (MOBIO Laboratories, Carlsbad, CA). rDNA coding regions were amplified using the fungal ITS primers 5′-CTTGGTCATTTAGAGGAAGTAA-3′ and 5′-TCCTCCGCTTATTGATATGC-3′ (Gardes and Bruns 1993). The amplicons were sequenced with Ion Torrent™ Personal Genome Machine® (PGM) System with 400-base read length chemistry method. All sequences were analyzed using the Quantitative Insights Into Microbial Ecology (QIIME) pipeline (Caporaso et al. 2010). The sequence library was split by samples and quality-filtered based on quality scores for each sequence. Sequences with quality scores below 25 were removed. Sequences shorter than 100 bp or longer than 1000 bp, as well as sequences with more than two mismatches in the primer, ambiguity, or maximum homopolymer run exceeding six were also removed. Sequences were clustered as OTUs (Operational Taxonomic Unit) using the Usearch algorithm (Edgar 2010) with 97% sequence similarities. Sequencing failed for the control section of one stream in Taivalkoski and we therefore excluded this stream from the analyses of fungal communities.
Fungal biomass
Fungal biomass was estimated from freeze-dried, pulverized leaf samples using a modified ergosterol assay (Nylund and Wallander 1992). Ergosterol extracts were quantified with high pressure liquid chromatography (HPLC) using a reverse-phase C18 column equipped with a pre-cartridge and methanol as the eluant (1.0 mL min−1, column temperature 30 °C). Commercial ergosterol (5,7,22-Ergostatrien-3ß-ol, Fluka AG) was used as standard. Results are expressed as ergosterol concentration in the litter (μg g−1 litter DW).
Algal biomass accrual and periphyton diatoms
Algal biomass was quantified on ceramic tiles (5.0 × 5.0 cm, n = 4 tiles per section) left for algal colonization for the duration of the experiment. The measurements were averaged to obtain a single value of algal biomass for each section. We quantified algal biomass using bbe-Moldaenke BenthoTorch (BT) (bbe Moldaenke 2013). BT is a Pulse-Amplitude Modulated (PAM) fluorometer emitting light pulses at four different wavelengths (470, 525, 610, and 700 nm). It records the response of benthic algae at 690 nm and calculates both total algal biomass (as chlorophyll a) and the biomass of green algae, blue-green algae, and diatoms. BT has been shown to reliably measure algal biomass as the amount of chl-a cm−2, although measuring the relative proportions of individual algal groups of total community biomass may be problematic (Kahlert and McKie 2014).
Diatom samples were brushed off from four cobble-sized stones at each site. The four subsamples were pooled into a composite sample for a site. Pooled samples were cleaned from organic material in the laboratory using wet combustion with acid (HNO3:H2SO4; 2:1) and mounted in Naphrax. A total of 400–500 valves per sample were identified and counted using magnification of 1000 x in light microscope equipped with differential interference condenser (DIC). Diatoms were identified to the lowest possible level, usually species.
Environmental variables
Current velocity and water depth were measured at 20 random locations along five cross-sectional transects at each section. Stream width and riparian shading (20 measurements using a cylindrical tube) were measured from the same transects. Water samples were collected four times during the experiment, and they were analyzed for nitrate (NO3-N) and phosphate (PO4-P) using national standards (National Board of Waters 1981). Water pH and conductivity were measured simultaneously with the collection of water samples using a portable meter (YSI Professional Plus meter, YSI Inc., USA).
Statistical analyses
Differences in water chemistry variables (pH, conductivity, NO3, PO4), diversity (richness and evenness) of biological assemblages, leaf litter decomposition rates, leaf ergosterol content, and algal biomass were examined using linear mixed effect (LME) models. We used LME models (function lme in the R package nmle; Pinheiro et al. 2014) incorporating stream type (circumneutral, naturally acidic), nutrient enrichment (enriched and control section), and their interaction as fixed factors and stream as a random factor. LME was selected because of the correlated structure (upstream versus downstream) of the experiment and changing covariates (i.e., site-specific value for each upstream and downstream section) used in the analysis. To take into account the possible co-limitation of nutrients with other factors (e.g., Warren et al. 2017), we used mean current velocity of a section as a covariate for leaf decomposition rate and riparian shading for algal biomass. Water temperature was also tested as a covariate in both models, but due to non-significant effects it was removed from the final analyses.
We developed null models for both fungi and diatoms to examine whether stochastic or niche-based processes drive their community assembly and β-diversity (Chase and Myers 2011). If neutral processes predominate, composition of communities in different stream sections should be no different from random. If niche-based processes predominate, communities should be either more similar or less similar than expected by chance. Null models were generated using the quasiswap algorithm (Miklós and Podani 2004) which maintains both original species frequencies and number of species observed for each site. Jaccard dissimilarities were calculated based on 999 randomly generated communities and observed mean dissimilarities were then compared to 95% confidence intervals derived from the randomly generated mean dissimilarity values for each stream section. We also calculated departure of the observed dissimilarity from null expectation, expressed as standardized effect size (SES = (βobs − βexp)/SD βexp). We assumed that any species observed in any sample could potentially colonize all streams in the study area and therefore used the total set of fungal OTUs and diatom taxa across all samples as the estimate of species pool in null model analyses.
Variation in community composition was first visualized for both taxonomic groups using NMDS ordination based on deviations of pairwise dissimilarities (β-deviations) from the null model. We used the SES values as our measure of β-deviation. Two-dimensional ordination solutions were used because stress values did not change appreciably with further dimensions. Differences in β-diversity between stream types and experimental sections were examined using Permutational Analysis of Multivariate Dispersions (PERMDISP; Anderson 2006). PERMDISP uses ANOVA F-statistic to compare among-group differences in the distance from individual observations to their group centroid (median). Significance of among-group differences is tested through permutation of least-squares residuals. Using β-deviations and 999 permutations, we tested the null hypothesis of no differences in the dispersion (i.e., β-diversity) between stream types, and between control and nutrient-enhanced sections. Finally, we employed indicator species analysis (IndVal; Dufrêne and Legendre 1997) to identify significant indicator taxa for our treatments (nutrient enrichment; geological stream type). IndVal combines information of species abundances and occurrences in each group; maximally strong indicators are present at each site within a group and never in other groups. Indicator values were tested using Monte Carlo permutation tests with 999 permutations. Null models, NMDS, and PERMDISP were run on presence/absence data using functions ‘oecosimu,’ ‘metaMDS,’ and ‘betadisper,’ respectively, in the package vegan (Oksanen et al. 2015) of program R. IndVal was calculated using function ‘multipatt’ in the package indicspecies (De Cáceres and Jansen 2015).
Results
Background environmental conditions and variation in water chemistry
Environmental characteristics measured at the end of the experiment were very similar among the control and nutrient-enriched sections in both stream groups. Conductivity increased in nutrient-enriched sections, particularly in naturally acidic streams, whereas water pH did not change (Table 1). Circumneutral streams were slightly wider, deeper, and warmer than naturally acidic streams. Water pH was clearly lower in naturally acidic streams although relatively high values were recorded also for some of these streams (Table 1). Minimum pH was, however, below 5.0 in all naturally acidic streams.
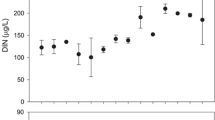
Nitrate concentrations varied from 5.0 to 23.5 µg L−1 in circumneutral streams and from 5.0 to 46.7 µg L−1 in naturally acidic streams. Concentrations were higher in downstream than in control sections in all study streams (F1, 10 = 15.77, P = 0.002), although they increased more in naturally acidic streams (Fig. 1). Phosphate concentrations varied from 3.0 to 50.5 µg L−1 in circumneutral streams and from 8.0 to 58.7 µg L−1 in naturally acidic streams, being significantly higher (F1, 10 = 25.21, P < 0.001) in enriched than in control sections (Fig. 1). Polymer-coated pellets lost roughly 26% of their weight in 5 days, with no differences in weight loss between the stream types (t test, P = 0.48). Virtually all (over 90%) phosphate in the clay matrix was lost within 5 days.
Richness and evenness of fungal and diatom communities
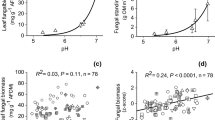
The cumulative richness of fungal OTUs was 437 in the control sections and 457 in the enriched sections of the circumneutral streams, and 562 and 548 in the control and enriched sections, respectively, of the naturally acidic streams. The number of fungal OTUs per section varied from 60 to 273 in circumneutral and from 169 to 310 in naturally acidic streams. There was a significant interaction (F1, 9 = 6.686, P = 0.029), with a lower number of OTUs in the enriched than in the control sections but only in the naturally acidic streams (t9 = − 3.057, P = 0.014) (Fig. 2). Fungal OTU evenness varied from 0.568 to 0.829 in circumneutral and from 0.583 to 0.826 in naturally acidic streams, with no significant differences among the stream types or sections (Fig. 2).
Richness and evenness of fungal OTUs and diatoms in the control and enriched sections of circumneutral and naturally acidic streams. Other explanations as in Fig. 1
Cumulative richness of diatoms was 68 in the control sections and 71 in the enriched sections of circumneutral streams and 50 and 48 for the control and enriched sections, respectively, of naturally acidic streams. The number of diatom taxa per section varied from 19 to 40 in circumneutral and from 10 to 38 in naturally acidic streams. There was a significant interaction (F1, 10 = 7.716, P = 0.020) with a higher number of taxa in the enriched than in the control sections but only in the circumneutral streams (t = 3.657, P = 0.004) (Fig. 2). Diatom evenness varied from 0.664 to 0.841 in circumneutral streams and from 0.448 to 0.831 in naturally acidic streams, but it did not differ significantly among the stream types or sections (Fig. 2).
Leaf decomposition rates and fungal and algal biomass
Leaf decomposition rates (k) varied from 0.0039 to 0.0087 in circumneutral streams and from 0.0026 to 0.0046 in naturally acidic streams. There was a significant interaction (F1, 9 = 5.770, P = 0.040) with decomposition rates being higher in the nutrient-enriched than in the control sections of naturally acidic streams (t = 3.627, P = 0.005), and also in the control sections of the circumneutral than of naturally acidic streams (t = 2.250, P = 0.048) (Fig. 3). Ergosterol content varied from 52 to 100 µg g−1 in circumneutral streams and from 49 to 118 µg g−1 in naturally acidic streams, with no significant differences among the stream types or sections (Fig. 3).
Leaf decomposition rates, fungal biomass (ergosterol content), and algal biomass in the control and enriched sections of circumneutral and naturally acidic streams. Other explanations as in Fig. 1
Algal biomass measured from tiles varied from 0.02 to 0.19 µg cm−2 in circumneutral streams and from 0.01 to 0.30 µg cm−2 in naturally acidic streams. Algal biomass was higher in the enriched sections of both stream types (F1, 9 = 5.549, P = 0.043). Interaction between sections and areas was non-significant (F1, 9 = 0.247, P = 0.63) (Fig. 3). When individual algal groups were analyzed separately, the relative proportion of cyanobacteria of total algal biomass on tiles was higher in the control than in enriched sections (F1, 10 = 9.809, P = 0.012).
Assembly mechanisms of fungal and diatom communities
Fungal communities were less dissimilar than expected by chance in both sections in circumneutral streams and in the control sections of naturally acidic streams, and more dissimilar than expected by chance in the enriched sections of naturally acidic streams (Table 2). Diatom communities were less dissimilar than expected by chance in the enriched sections of naturally acidic streams, whereas they did not differ from random expectation in circumneutral streams and control sections of naturally acidic streams (Table 2).
Compositional variability of fungal and diatom communities
Communities in circumneutral and naturally acidic streams were clearly separated in the β-deviation ordination space for both fungal (Fig. 4a) and diatom (Fig. 4b) communities, while differences between control and enriched sections were not evident. PERMDISP found significant differences only in β-diversity of fungal communities between the control sections of circumneutral streams and enriched sections of naturally acidic streams (P = 0.031) (Fig. 4b).
NMDS ordinations of β-deviations of fungal (a) and diatom (b) communities, representing 95% concentration ellipses for each experimental section. Gray symbols = circumneutral streams, black symbols = naturally acidic streams. Circles, dashed line = control sections; triangles, solid line = enriched sections
Despite the weak differences in community composition, indicator species analysis found several indicators for both control and nutrient-enriched sections for fungi (Electronic Supplementary Material, Table S1). OTUs belonging to Flagellospora sp. and Helotiales sp. were strong indicators of nutrient enrichment in both stream types, while the control sections were best indicated by Alatospora sp. (circumneutral streams) or a more variable group of Ascomycota and Basidiomycota (naturally acidic streams), which are leaf endophytes and thus likely originated from riparian forests (Table S1). We also detected 21 indicator OTUs for circumneutral streams and 15 for naturally acidic streams. Almost all indicators identified at species level belonged to Basidiomycota and were already present in the leaves before the onset of the experiment or originated from soils (Table S1).
For diatoms, significant indicators for nutrient enrichment were found only for circumneutral streams where Aulacoseira subarctica was a strong indicator of the control sections while Eolimna subminuscula indicated nutrient enrichment (Table S2). Karayevia oblongella, Eolimna minima, Eunotia faba, and Fragilaria gracilis were strong indicators of circumneutral streams while Eunotia mucophila and Eunotia rhomboidea were indicators of naturally acidic streams (Table S2).
Discussion
Although nutrient enrichment often increases rates of decomposition and primary production, responses of biological communities to nutrients are variable and may depend on differences in initial environmental conditions and community composition (Clements et al. 2016; Reisinger et al. 2016). Consistent with our hypothesis, nutrient enrichment increased leaf decomposition rate and primary production, but the effect was partly context dependent as decomposition rates increased only in naturally acidic streams. Fungal OTU richness was reduced but compositional variability of fungal communities was increased by nutrient enrichment in naturally acidic streams. In circumneutral streams, compositional variability of fungal communities decreased in response to nutrients, further indicating deterministic responses of fungal communities to nutrient enrichment. Diatom richness responded positively to nutrient enrichment in circumneutral streams, but the overall effects of enrichment on diatoms were weak. Our results thus suggest that primary producers and detritivores may be differentially affected by nutrient enrichment and that biological responses to nutrients are context dependent, with detrital processes and fungal communities responding more in naturally acidic streams than in more benign environments.
Nutrient enrichment had an overall weak influence on community variability, but it did affect fungal community assembly. Fungal communities were less dissimilar than expected by chance in the control sections of naturally acidic streams, implying that low pH acted as a habitat filter that selected species tolerant of acidity. In response to increasing nutrients, fungal communities in naturally acidic streams became more variable, likely reflecting species sorting in novel environmental conditions. At the same time, nutrient enrichment decreased fungal OTU richness, suggesting that conditions became unsuitable for some OTUs. This finding concurs with our previous observation that fungal communities in naturally acidic streams may be more sensitive to novel stressors than are those in more benign environments (Tolkkinen et al. 2015). Interestingly, the control sections of acidic streams were best indicated by terrestrial taxa that may have been present in the leaves before the onset of the experiment. Nutrient enrichment may thus enable faster establishment of true aquatic taxa which may explain the absence of terrestrial taxa from the leaves in the enriched sections.
Naturally acidic streams harbored species-poor diatom communities, consistent with earlier findings of a negative influence of low pH on diatom richness (Ledger and Hildrew 2005). Nutrient enrichment modified diatom communities in naturally acidic streams by decreasing their compositional variability relative to random expectation. Enrichment did not influence diatom richness, however, suggesting that some rare species may have been replaced by common species that benefited from nutrient addition. In fact, the only indicator of the enriched sections (Eolimna subminuscula) is a known indicator of eutrophication (Gevrey et al. 2004). In a previous study in the same region, diatom communities were tolerant of stressors (increased metal concentration of the stream water) from forestry-related land use (Annala et al. 2014), suggesting that diatoms characteristic of naturally acidic streams may be generally tolerant of environmental changes.
Communities of microorganisms are extremely diverse and therefore possess a high degree of functional redundancy, i.e., tendency of different taxa to contribute to the same ecosystem function (Langenheder et al. 2005, 2006). Our results are consistent with this assumption as decomposition rates in naturally acidic streams increased in response to enrichment despite decreased fungal OTU richness. It is thus possible that the lost OTUs were less efficient decomposers than those that remained in the enriched sections. In our previous study (Tolkkinen et al. 2015), decomposition rate was lower in naturally acidic than in circumneutral streams but only if they were also affected by high metal concentrations from catchment land use. Fungal richness was not reduced in that study, but communities were strongly homogenized, suggesting that functionally important fungi were replaced by tolerant but less efficient taxa (Tolkkinen et al. 2015). Together these two studies show that different stressors (increased metals vs. nutrient enrichment) may have strongly divergent impacts on stream biodiversity and ecosystem processes. At a very high level of enrichment, leaf decomposition rate may be rapidly reduced: for example, Woodward et al. (2012) showed that decomposition rate increased until nutrient concentrations were almost 1000-fold higher than in their oligotrophic streams. In our experiment, nutrient concentrations likely increased much less than would have been needed for decomposition rate to have slowed down markedly. The observed increase (14%) in decomposition rate was overall low compared to that typically observed (50%) in experiments measuring the effects of nutrient enrichment on decomposition (Ferreira et al. 2015).
In circumneutral streams, the only significant effect of nutrient enrichment on biological communities was increased richness of diatoms. This is in agreement with the hypothesis that nutrient enrichment should increase richness of primary producers (Hillebrand et al. 2007). However, diatom communities exhibited random variation in both control and enriched sections, suggesting that increased richness in nutrient-enriched sections resulted from stochastic processes (Chase 2010). By contrast, fungal communities in circumneutral streams were less dissimilar than expected by chance, implying that these streams were similar in some unmeasured environmental dimension of importance for fungi and that nutrient enrichment was not large enough to affect fungal community assembly. Chlorophyll a concentrations increased in response to nutrients in both stream types, but algal accrual rate was overall very low, only 12–25% of the values reported for control treatments in N- and P-limitation experiments (Francoeur 2001). Such a low algal productivity reflects the characteristics of these highly oligotrophic, shaded forest streams but also environmental conditions, particularly cold water, during our early-autumn experiment, as well as the relatively short duration of the experiment.
The effect of nutrient enrichment on water chemistry was less pronounced in circumneutral than in naturally acidic streams. The lower water temperature of circumneutral streams may have influenced nutrient release from the pellets, causing concentrations of nutrients, particularly of nitrate, to be highly variable. Clearly, flow-proportional nutrient dipping would have yielded more constant nutrient release rate but as pellets were replaced regularly throughout the experiment, we do not think our method of adding nutrients could have masked treatment effects. Also, roughly half of the phosphate was added in a clay matrix which likely allowed a more constant release in both stream types. Decomposition (Ferreira et al. 2015) and primary production (Francoeur 2001) are typically influenced more by enrichment of both N and P than by a single nutrient. Respiration of stream biofilms is, however, primarily N-limited in Fennoscandian streams (Burrows et al. 2015), suggesting that even small increases of N should influence microbial metabolism. It is possible that a more marked increase of nitrate concentrations might have resulted in a stronger synergistic effect by the two nutrients on leaf decomposition (Stelzner et al. 2003). Also, the experimental duration of 19 days means that our results cannot be extrapolated to situations where nutrient enrichment functions as a press disturbance. Nevertheless, our experiment was planned specifically to mimic a pulse disturbance and our results do show that even a modest nutrient enrichment can alter the rates of decomposition and primary production in boreal streams. From a bioassessment perspective, it is exactly such subtle environmental impacts deriving from pulse-type disturbances that need to be detected for effective proactive management actions.
Conclusions
Aquatic fungi contribute importantly to decomposition and downstream transport of organic matter in stream networks, and they thus play a critical role in facilitating the flow of nutrients and energy through the detrital pathway. Nutrient enrichment may increase the role of microbes in decomposition, with a consequent increase in the production of FPOM by leaf-shredding invertebrates, eventually enhancing the availability of organic matter for other consumers (Tant et al. 2015). Our results suggest that such cascading effects of nutrient enrichment may be even stronger in naturally acidic streams that support unique microbial communities. Increased primary production can further modify these effects, potentially shifting these streams towards autotrophy (Holland et al. 2012). Interactions between green and brown food webs are mediated by higher-lever consumers many of which are dietary generalists that shift opportunistically between resources, particularly in acidic streams (Ledger and Hildrew 2005). Therefore, future studies should incorporate multiple trophic levels in field experiments manipulating nutrient inputs in widely contrasting environmental settings and for longer periods of time.
References
Anderson, M.J. 2006. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62: 245–253.
Annala, M.J., H. Mykrä, M. Tolkkinen, T. Kauppila, and T. Muotka. 2014. Are biological communities in naturally unproductive streams resistant to additional anthropogenic stressors? Ecological Applications 24: 1887–1897.
Baldy, V., V. Gobert, F. Guerold, E. Chauvet, D. Lambrigot, and J.-Y. Chargosset. 2007. Leaf litter breakdown budgets in streams of various trophic status: Effects of dissolved nutrients on microorganisms and invertebrates. Freshwater Biology 52: 1322–1335.
Bärlocher, F. 2005. Freshwater fungal communities. In The fungal community: Its organization and role in the ecosystem, ed. J. Deighton, J.F. White, and P. Oudemans, 39–59. Boca Raton, FL: Taylor and Francis Group/CRC Press.
bbe Moldaenke. 2013. http://www.bbe-moldaenke.de/chlorophyll/benthotorch.
Benfield, E.F. 1996. Leaf breakdown in stream ecosystems. In Methods in stream ecology, ed. F.R. Hauer, and G.A. Lamberti, 579–589. San Diego, California: Academic Press.
Bondar-Kunze, E., S. Maier, D. Schönauer, N. Bahl, and T. Hein. 2016. Antagonistic and synergistic effects on a stream periphyton community under the influence of pulsed flow velocity increase and nutrient enrichment. Science of the Total Environment 573: 594–602.
Burkepile, D.E., and M.E. Hay. 2006. Herbivore vs. nutrient control of marine primary producers: Context-dependent effects. Ecology 87: 3128–3139.
Burrows, R.M., E.R. Hotchkiss, M. Jonson, H. Laudon, B.G. Mckie, and R.A. Sponseller. 2015. Nitrogen limitation of heterotrophic biofilms in boreal streams. Freshwater Biology 60: 1237–1251.
Caporaso, J.G., J. Kuczynski, J. Stombaugh, K. Bittinger, F.D. Bushman, E.K. Costello, N. Fierer, A.G. Pena, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7: 335–336.
Chase, J.M. 2010. Stochastic community assembly causes higher biodiversity in more productive environments. Science 328: 388–1391.
Chase, J.M., and J.A. Myers. 2011. Disentangling the importance of ecological niches from stochastic processes across scales. Philosophical Transactions of the Royal Society B. 366: 2351–2363.
Clements, W.H., D. Kashian, P.M. Kiffney, and R.E. Zuellig. 2016. Perspectives on the context-dependency of stream community responses to contaminants. Freshwater Biology 61: 2162–2170.
De Cáceres, M., and F. Jansen. 2015. indiscspecies: Relationship between species and groups of sites. R package version 1.7.6. https://cran.r-project.org/web/packages/indicspecies.
Dufrêne, M., and P. Legendre. 1997. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366.
Dunck, B., E. Lima-Fernandes, F. Cássio, A. Cunha, L. Rodrigues, and C. Pascoal. 2015. Responses of primary production, leaf litter decomposition and associated communities to stream eutrophication. Environmental Pollution 202: 32–40.
Edgar, R.C. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461.
Ferreira, V., B. Castagneyrol, J. Koricheva, V. Gulis, E. Chauvet, and M.A.S. Craça. 2015. A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams. Biological Reviews 90: 669–688.
Francoeur, S.N. 2001. Meta-analysis of lotic nutrient amendment experiments: Detecting and quantifying subtle responses. Journal of the North American Benthological Society 20: 358–368.
Garcia, L., I. Pardo, W.F. Cross, and J.S. Richardson. 2017. Moderate nutrient enrichment affects algal and detritus pathways differently in a temperate rainforest stream. Aquatic Sciences 79: 941–952.
Gardes, M., and T.D. Bruns. 1993. ITS primers with enhanced specificity for Basidiomycetes—application to the identification of Mycorrhizae and rusts. Molecular Ecology 2: 113–118.
Gevrey, M., F. Rimet, Y. Seuk, J.-L. Giraudel, L. Ector, and S. Lek. 2004. Water quality assessment using diatom assemblages and advanced modeling techniques. Freshwater Biology 49: 208–220.
Gulis, V., and K. Suberkropp. 2003. Interactions between stream fungi and bacteria associated with decomposing leaf litter at different levels of nutrient availability. Aquatic Microbial Ecology 30: 149–157.
Heino, J., M. Tolkkinen, A.M. Pirttilä, H. Aisala, and H. Mykrä. 2014. Microbial diversity and community–environment relationships in boreal streams. Journal of Biogeography 41: 2234–2244.
Hillebrand, H., D.S. Gruner, E.T. Borer, M.E.S. Bracken, E.E. Cleland, J.J. Elser, W.S. Harpole, J.T. Ngai, et al. 2007. Consumer versus resource control of producer diversity depends on ecosystem type and producer community structure. Proceedings of the National Academy of Sciences of United States of America 26: 10904–10909.
Holland, A., L.J. Duivenvoorden, and S.H. Kinnear. 2012. Naturally acidic waterways: Conceptual food webs for better management and understanding of ecological functioning. Aquatic Conservation 22: 836–847.
Jansson, M., A.-K. Bergström, S. Drakare, and P. Blomqvist. 2001. Nutrient limitation of bacterioplankton and phytoplankton in humic lakes in northern Sweden. Freshwater Biology 46: 653–666.
Kahlert, M., and B.G. McKie. 2014. Comparing new and conventional methods to estimate benthic algal biomass and composition in freshwaters. Environtal Science: Processes & Impacts 16: 2627–2634.
Kominoski, J.S., A.D. Rosemond, I. Jonathan, P. Benstead, V. Gulis, J.C. Maerz, and D.W.P. Manning. 2015. Low-to-moderate nitrogen and phosphorus concentrations accelerate microbially driven litter breakdown rates. Ecological Applications 25: 856–865.
Langenheder, S., E.S. Lindström, and L.J. Tranvik. 2005. Weak coupling between community composition and functioning of aquatic bacteria. Limnology and Oceanography 50: 957–967.
Langenheder, S., E.S. Lindström, and J.L. Tranvik. 2006. Structure and function of bacterial communities emerging from different sources under identical conditions. Applied Environmental Microbiology 72: 212–220.
Ledger, M.E., and A.G. Hildrew. 2005. The ecology of acidification and recovery: Changes in herbivore–algal food web linkages across a stream gradient. Environmental Pollution 137: 103–118.
Loukola-Ruskeeniemi, K., A. Uutela, M. Tenhola, and T. Paukola. 1998. Environmental impact of metalliferous black shales at Talvivaara in Finland, with indication of lake acidification 9000 years ago. Journal of Geochemical Exploration 64: 395–407.
Miklós, I., and J. Podani. 2004. Randomization of presence-absence matrices: Comments and new algorithms. Ecology 85: 86–92.
Mustonen, K., H. Mykrä, P. Louhi, A. Markkola, A. Huusko, N. Alioravainen, S. Lehtinen, and T. Muotka. 2016. Sediments and flow have mainly independent effects on multitrophic stream communities and ecosystem functions. Ecological Applications 26: 2116–2129.
National Board of Waters. 1981. The Analytical Methods Used by National Board of Waters. Report 213. National Board of Waters, Helsinki, Finland.
Nylund, J.E., and H. Wallander. 1992. Ergosterol analysis as a means of quantifying mycorrhizal biomass. Methods in Microbiology 24: 77–88.
Oksanen, J., F.G. Blanchet, R. Kindt, P. Legendre, P.R. Minchin, R.B. O’Hara, G.L. Simpson, P. Solymos, et al. 2015. vegan: Community Ecology Package. R package version 2.4-1.
Pascoal, C., and F. Cassio. 2004. Contribution of fungi and bacteria to leaf litter decomposition in a polluted river. Applied Environmental Microbiology 70: 5266–5273.
Pinheiro, J., D. Bates, S. DebRoy, D. Sarkar, and the R Development Core Team. 2014. nlme: Linear and nonlinear mixed effects models. R package version 3.1-118.
Reisinger, A.J., J.L. Tank, and M.M. Dee. 2016. Regional and seasonal variation in nutrient limitation of river biofilms. Freshwater Science 35: 474–489.
Rosemond, A.D., J.P. Benstead, P.M. Bumpers, V. Gulis, J.S. Kominoski, D.W. Manning, K. Suberkropp, and J.B. Wallace. 2015. Experimental nutrient additions accelerate terrestrial carbon loss from stream ecosystems. Science 347: 1142–1145.
Sabater, S., J. Artigas, A. Gaudes, I. Muñoz, G. Urrea, and A.M. Romani. 2011. Long-term moderate nutrient inputs enhance autotrophy in a forested Mediterranean stream. Freshwater Biology 56: 1266–1280.
Schneider, S.C., M. Kahlert, and M.G. Kelly. 2013. Interactions between pH and nutrients on benthic algae in streams and consequences for ecological status assessment and species richness patterns. Science of the Total Environment 444: 73–84.
Slavik, K., B.J. Peterson, L.A. Deegan, W.B. Bowden, A.E. Hershey, and J.E. Hobbie. 2004. Long-term responses of the Kuparuk River ecosystem to phosphorus fertilization. Ecology 85: 939–954.
Stelzner, R.S., J. Heffernan, and G.E. Likens. 2003. The influence of dissolved nutrients and particulate organic matter quality on microbial respiration and biomass in a forest stream. Freshwater Biology 48: 1925–1937.
Tant, C.J., A.D. Rosemond, A.S. Mehring, K.A. Kuehn, and J.M. Davis. 2015. The role of aquatic fungi in transfromations of organic matter mediated by nutrients. Freshwater Biology 60: 1354–1363.
Tolkkinen, M., H. Mykrä, M. Annala, A. Markkola, K.-M. Vuori, and T. Muotka. 2015. Multi-stressor impacts on fungal diversity and ecosystem functions in streams: Natural vs. anthropogenic stress. Ecology 96: 672–683.
Vörösmarty, C.J., P.B. McIntyre, M.O. Gessner, D. Dudgeon, A. Prusevich, P. Green, S. Glidden, S.E. Bunn, et al. 2010. Global threats to human water security and river biodiversity. Nature 467: 555–561.
Warren, D.R., S.M. Collins, E.M. Purvis, M.J. Kaylor, and H.A. Bechtold. 2017. Spatial variability in light yields colimitation of primary production by both light and nutrients in a forested stream ecosystem. Ecosystems 20: 198–210.
Woodward, G., M.O. Gessner, P.S. Giller, V. Gulis, S. Hlady, A. Lecerf, B. Malmqvist, B.G. McKie, et al. 2012. Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336: 1438–1440.
Worm, B., T.B. Reusch, and H.K. Lotze. 2000. In situ nutrient enrichment: Methods for marine benthic ecology. International Review of Hydrobiology 85: 359–375.
Zou, K., E. Thébault, G. Lacroix, and S. Barot. 2016. Interactions between the green and brown food web determine ecosystem functioning. Functional Ecology 30: 1454–1465.
Acknowledgements
We thank Tarja Törmänen for laboratory assistance and Riccardo Fornaroli for assistance in field work. Our research was funded by University of Oulu (Thule Institute), Academy of Finland and MARS project (Managing Aquatic ecosystems and water Resources under multiple Stress) funded under the 7th EU Framework Programme, Theme 6 (Environment including Climate Change), Contract No.: 603378 (http://www.mars-project.eu).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mykrä, H., Sarremejane, R., Laamanen, T. et al. Local geology determines responses of stream producers and fungal decomposers to nutrient enrichment: A field experiment. Ambio 48, 100–110 (2019). https://doi.org/10.1007/s13280-018-1057-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13280-018-1057-4