Abstract
The high resistant rate of Adriamycin (Adr) is associated with a poor prognosis of breast cancer in women worldwide. Since miR-222 might contribute to chemoresistance in many cancer types, in this study, we aimed to investigate its efficacy in breast cancer through PTEN/Akt/p27 kip1 pathway. Firstly, in vivo, we verified that miR-222 was upregulated in chemoresistant tissues after surgery compared with the paired preneoadjuvant samples of 21 breast cancer patients. Then, human breast cancer Adr-resistant cell line (MCF-7/Adr) was constructed to validate the pathway from the parental sensitive cell line (MCF-7/S). MCF-7/Adr and MCF-7/S were transfected with miR-222 mimics, miR-222 inhibitors, or their negative controls, respectively. The results showed that inhibition of miR-222 in MCF-7/Adr significantly increased the expressions of PTEN and p27 kip1 and decreased phospho-Akt (p-Akt) both in mRNA and protein levels (p < 0.05) by using quantitative real-time PCR (qRT-PCR) and western blot. MTT and flow cytometry suggested that lower expressed miR-222 enhanced apoptosis and decreased the IC50 of MCF-7/Adr cells. Additionally, immunofluorescence demonstrated that the subcellular location of p27 kip1 was dislocated resulting from the alteration of miR-222. Conversely, in MCF-7/S transfected with miR-222 mimics, upregulation of miR-222 is associated with decreasing PTEN and p27 kip1 and increasing Akt accompanied by less apoptosis and higher IC50. Importantly, Adr resistance induced by miR-222 overexpression through PTEN/Akt/p27 was completely blocked by LY294002, an Akt inhibitor. Taken together, these data firstly elucidated that miR-222 could reduce the sensitivity of breast cancer cells to Adr through PTEN/Akt/p27 kip1 signaling pathway, which provided a potential target to increase the sensitivity to Adr in breast cancer treatment and further improved the prognosis of breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advances in early diagnosis and therapy, breast cancer is still the most general cancer and the leading cause of cancer-related death in women worldwide [1]. Chemotherapy is the major management of breast cancer, while drug resistance minimizes its effectiveness and is often associated with poor clinical characteristics such as higher migration or invasion [2, 3]. Adriamycin (Adr) is a frequent standard chemotherapeutic agent used in combination with other drugs to treat breast cancer, which has been discovered to improve overall survival and quality of life for patients with breast cancer. However, important clinical problems of Adr resistance had been emphasized. The underlying mechanisms of the resistant-development in breast cancer remain poorly understood; therefore, studies on mechanisms of Adr resistance and how to rescue this resistance to improve cancer chemotherapy are increasingly essential. Luckily, several studies had demonstrated that microRNAs were involved in various biological processes including chemoresistance [4].
microRNAs (miRNAs), whose aberrance had been definitely found to be involved in numerous pathogenesis, such as cell proliferation, invasion, metastasis, apoptosis, and drug resistance among various types of cancers including breast cancer [5–7], could downregulate messenger RNA expression by repressing translation or by cleaving the targeted mRNA directly through targeting its 3′ untranslated region (3'UTR) [8]. There were many researches having reported that miR-222 played a pivotal role in tumorigenesis in diverse cancers, like pancreatic cancer, hepatocellular carcinoma, gastric cancer, and breast cancer [9–12]. In detail, miR-222 directly targeted mRNAs, like phosphatase and tensin homolog (PTEN), BCL-2, Bax, BIM, p27, and p53, through combining with their 3'UTRs [10, 13, 14]. However, to date, little is known about the role of miR-222 in the fundaments of Adr resistance in breast cancer. In our previous study, we verified, in vitro, that miR-222 enhanced the Adr resistance through inhibiting PTEN in Adr-resistant MCF-7 cells (MCF-7/Adr) compared with sensitive cells (MCF-7/S), while further molecular mechanisms remain obscure [15].
PTEN, a multifunctional tumor suppressor, was a common target of miR-222, which had been proved in various cancers [13, 16, 17]. What’s more, PTEN dephosphorylated phosphatidylinositol-3,4,5-triphosphate (PIP3), the second messenger produced by phosphoinositide 3-kinase (PI3K), to negatively regulate the activity of the serine/threonine protein kinase, Akt, eventually taking part in regulating progression of cancers [18]. Cyclin-dependent kinase inhibitor 1B, also named p27 kip1 (hereafter referred to as p27), inhibited cell cycle progression G1-S transitions [19], and phosphorylation of p27 at different sites altered its distributions in nuclear and cytoplasmic involving in different cancers [20]. Viglietto G et al. described that phospho-Akt (p-Akt) phosphorylated p27 resulting in the change of its location, which influenced apoptosis rate [21]. Especially, PTEN and p27 were two putative tumor suppressors for cancers and had negative correlations with the depth of stromal invasion, tumor size, and clinical stage [22]. However, to date, the combinations among miR-222, PTEN, Akt, and p27 have not been evaluated in breast cancer with Adr resistance. Moreover, according to Sun et.al [23] and Wang et.al [24], p27 kip1 was also a direct target gene of miR-222.
Additionally, in pathway enrichment analysis, we discovered that the miR-222 enriched pathways in cancer pathway, whose subpath PTEN/Akt/p27 had been little studied. Therefore, we aimed at the investigation that whether miR-222 inhibited the pathway leading to the Adr resistance of breast carcinoma cells.
Materials and methods
Tissue collection
All chemoresistant tissues after surgery and paired tissues for preneoadjuvant chemotherapy were obtained from 21 patients at Nanjing Drum Tower Hospital, from 2010 to 2015. The specimens were routinely fixed in 10 % formalin and embedded in paraffin, obtained from Department of Pathology. Before the start of the study, it was approved by the ethics committee of the hospital, with fully informed, written consent from the patients, which were conducted in accordance with International Guidelines for Use of Human Tissues. Tumor response to neoadjuvant chemotherapy was evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines [25].
Cell lines
Human breast cancer cell line MCF-7, used in this study, was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The Adr-resistant subline selected at 500 nM adriamycin (MCF-7/Adr) was successfully established from MCF-7, by exposing MCF-7 to gradually increasing concentrations of Adr, in vitro, in our laboratory. Parental MCF-7 cultured synchronously in the absence of drug was used as a control (called MCF-7/S). The IC50 (inhibitory concentration to produce 50 % cell death) values of Adr were 403.56 and 0.66 μM for MCF-7/Adr and MCF-7/S cells, respectively [15]. The drug-resistant derivative cell line was cultured in drug-free medium for 2 weeks before subsequent experiments to avoid the influence of drug.
MCF-7/S and MCF-7/Adr were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (KeyGEN BioTECH, Jiangsu, China), supplemented with 10 % fetal bovine serum (FBS; Zhejiang, China), 80 U/ml penicillin, and 0.08 mg/ml streptomycin and incubated at 37 °C and 5 % CO2 in a humidified chamber atmosphere.
miRNA mimics and inhibitors
The hsa-miR-222 mimics, hsa-miR-222 inhibitors, negative control miRNA mimics (mimics-NC), and negative control miRNA inhibitors (inhibitors-NC) were chemically synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Transfection experiment
Prior to transfection, cells in logarithmic phase were collected when a confluence of 80–90 % was reached. Then, transfection was performed using a Nepa21 pulse generator (Nepa Gene, Chiba, Japan). Briefly, 1 × 106 cells in 100 ul antibiotic-free DMEM mixed with miR-222 inhibitors or miR-222 mimics at a concentration of 30 nM were added into an electrode champer. The parameters of NEPA 21 electroporator were: poring pulse: voltage, 125 V; pulse length, 5 ms; pulse interval, 5 ms; number of pulses, 2; decay rate, 10 %; polarity +; transfer pulse: voltage, 20 V ; pulse length, 50 ms; pulse interval, 50 ms; number of pulses, 5; decay rate 40 %; polarity +/−. Cells transfected with mimics-NC or inhibitors-NC were used as negative control, and cells transfected without external genes were used as blank control. After transfection, the cells of each electrode chamber were plated into two wells of six-well plate with non-antibiotic-culture medium with FBS for 1 day.
Total RNA extraction, cDNA synthesis, and quantitative real-time PCR (qRT-PCR)
Total RNA, including miRNAs, was extracted using RNAsimple Total RNA kit (TIANGEN BIOTECH, Beijing, China) according to the manufacturer’s instructions. The concentration and quality of the RNA were measured by the UV absorbance at 260 and 280 nm (260/280 nm) on Nanodrop 2000 spectrophotometry (Thermo Scientific, USA). The first strand of complementary DNA was synthesized using the Bu-SuperScript RT Kit (Biouniquer Technology, Nanjing, China) following the manufacturer’s instruction. Then, qRT-PCR was performed using the SYBR Premix Ex Taq system (Roche, Australia).
The expression of mature miR-222 was determined by qRT-PCR and U6 served as a housekeeping gene to normalize miRNA expression. Amplification was done on a Light Cycler 480 (Roche, Australia) as follows: 95 °C for 10 min followed by 40 cycles at 95 °C for 5 s and 60 °C for 20 s, followed by melting curve detection. PTEN, Akt, and p27 mRNAs expressions were analyzed using qRT-PCR as follows: 95 °C for 10 min followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s, followed by melting curve detection. β-actin was used as an endogenous control. All qRT-PCR reactions were conducted at least in triplicate, and the 2-ΔΔCt method was used to analyze PCR data. Primer sequences are presented in Table 1.
MTT assay
After transfection, the 100 μL cells were seeded in 96-well plates at a density of 8 × 104/ml and allowed to attach and grow for 24 h. Various concentrations of Adr were added to the cells (quadruplicate wells per condition). Two days later, 20 μl of 5 mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, Germany) was added to each well, and the plates were incubated for another 4 h. After removal of the culture medium, the cells in each well were mixed with 150 μl of dimethyl sulfoxide (DMSO, AMRESCO, America) and the absorbance at 490 nm was measured with CliniBio 128 (ASYS-Hitech, Austria). The half maximal inhibitory concentration (IC50) was calculated using probit analysis with SPSS 22.0 (SPSS Inc., Chicago, USA).
Flow cytometry
Transfected MCF-7/S or MCF-7/Adr cells (5 × 105) were seeded in each well of six-well plates and cultured for 24 h, then added 100 nM Adr to each plate for 24 h. Finally, they stained with 5 μl of APC Annexin V and 5 ul of 7-AAD (BD Pharmingen, America) in the dark for 15 min. Then, each tube was added 400 ul of 1X binding buffer and analyzed with the flow cytometer (FACSVerse/Calibur/AriaII-SORP, BD, America).
Western blot
Total proteins from cells, collected at 24 h after transfection, were harvested using a PIPA lysis buffer (Biouniquer Technology, Nanjing, China) referring to the manufacturer’s instruction. Proteins were measured with Nanodrop 2000 spectrophotometry (Thermo Scientific, USA). Equal amounts of proteins were separated by electrophoresis using 8 % sodium dodecyl sulfate (SDS) polyacrylamide gels before transferring to polyvinylidene difluoride membranes (Sigma, Germany). The membranes were blocked in 5 % skim milk for 2 h and then probed with primary antibodies against PTEN (1:600; Cell Signaling Technology, America), p27 (1:500; Abcam, America), phospho-Akt (p-Akt, 1:800, Cell Signaling Technology, America), total-Akt (t-Akt, 1:800, Cell Signaling Technology, America), or β-actin (1:7000, Arigo, China) at 4 °C overnight, followed by the secondary antibody (1:6000; Kangwei Ltd., China) for 1 h. Finally, after washing, enhanced chemiluminescence (ECL) plus kit (Millipore, America) was applied for visualization and β-actin was utilized as an internal control.
Immunofluorescence microscope
Prepared cells were grown on coverslips for 24 h and fixed in 4 % paraformaldehyde for 30 min, permeabilized in 0.5 % Triton X-100 for 20 min, and blocked in 5 % BSA for 1 h. Primary p27 antibody was used at 1:200 in blocking buffer at 4 °C overnight. Secondly, cells were washed and then incubated with secondary fluorescence antibody at 1:250 in 1 % BSA for 1 h. Nuclei were stained with Hoechst 33258 for 5 min. At last, coverslips were washed and then mounted on slide with antifade mounting medium. Slides were imaged using the confocal microscope and LCS software (Zeiss, Germany).
Statistical analysis
Statistical analysis was performed with SPSS 22.0. All experiments, at least, were carried out in triplicate independently, and the data were presented as mean ± standard error. The representative images were presented. One-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls post hoc test was used to assess the statistical significance of difference between cell groups. The comparison of miRNA expression level between chemoresistance specimens and preneoadjuvant chemotherapy biopsies in individual patients was performed using Wilcoxon matched pairs signed rank test. P value < 0.05 was considered statistically significant.
Results
miR-222 is upregulated in postsurgical tissues
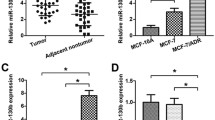
The expression levels of miR-222 were obtained from 21 paired specimens by qRT-PCR. The result revealed that miR-222 expression was upregulated in chemoresistant tissues following surgery, compared with the preneoadjuvant chemotherapeutic tissues (Fig. 1a, *P < 0.05), indicating a potential role for miR-222 in drug-resistant breast cancer. Moreover, the 21 patients received partial response (PR) (n = 13) and stable disease (SD) (n = 4) or progression disease (PD) (n = 4). The expression of miR-222 had no statistical significance between PR and SD + PD both in preneoadjuvant chemotherapeutic tissues (P = 0.1407) and chemoresistant tissues after surgery (P = 0.711).
qRT-PCR analysis of miR-222 expression in tissues and transfected MCF-7 cell lines. a The expressions of miR-222 in chemo-resistant tissues after surgery and paired tissues for preneoadjuvant chemotherapy from 21 patients (*P < 0.05). b Relative expression of miR-222 in MCF-7/Adr cells with miR-222 inhibitors. c Relative expression of miR-222 in MCF-7/S cells with miR-222 mimics. Cells transfected without external genes were used as blank control, which has no statistical significance with negative control. Bars indicate the mean ± standard deviation from at least three independent experiments. *P < 0.05 or **P < 0.01, compared with the NC. ΔCt values for each miRNA studied are shown referenced to the expression of the endogenous control U6
miR-222 expression in transfected MCF/S and MCF-7/Adr cells
Initially, in our laboratory, miRNA assay evaluated that miR-222 was upregulated in MCF-7/Adr compared with MCF-7/S, which was confirmed in vitro [15]. In this study, after transfecting miR-222 inhibitors into MCF-7/Adr and miR-222 mimics into MCF-7/S, the expressions of miR-222 were examined by qRT-PCR. The results factually suggested that miR-222 expression was downregulated in MCF-7/Adr after transfected with miR-222 inhibitors, compared with inhibitors-NC (Fig. 1b, **P < 0.01). Meanwhile, miR-222 was upregulated in MCF-7/S after transfected with miR-222 mimics, compared with mimics-NC (Fig. 1c, *P < 0.01).
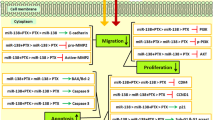
miR-222 negatively correlates with PTEN/Akt/p27 pathway
In order to further investigate the associations between miR-222 and PTEN/Akt/p27 pathway, the mRNA and protein levels of PTEN, total-Akt (t-Akt), phosphor-Akt (p-Akt), and p27 were determined by qRT-PCR and western blot. The results showed that the expressions of PTEN were significantly increased, p-Akt was decreased, and p27 was increased in both mRNA and protein levels of miR-222-inhibitor-transfected MCF-7/Adr, compared with controls (Fig. 2a, c; **P < 0.01). Conversely, transfected miR-222 mimics to MCF-7/S induced the downregulation of PTEN, upregulation of p-Akt, and downregulation of p27 in the mRNA and protein levels, compared with negative controls (Fig. 2b, d; **P < 0.01). Moreover, the expressions of t-Akt in protein level had no changes in all groups (Fig. 2c, d).
RT-qPCR and western blot analysis of PTEN, p-Akt, and p27 expressions in MCF-7/Adr and MCF-7/S cells after transfection for 24 h. a and c Relative expressions of PTEN, p-Akt, t-Akt, and p27 in MCF-7/Adr cells after transfected with miR-222 inhibitors. b Relative expressions of PTEN, p-Akt, t-Akt, and p27 in MCF-7/S cells after transfected with miR-222 mimics. Cells transfected without external genes were used as blank control, which has no statistical significance with negative control. Bars indicate the mean ± standard deviation from at least three independent experiments. *P < 0.05 or **P < 0.01, compared with the NC. ΔCt values for each mRNA studied are shown referenced to the expression of the endogenous control β-actin. Western blot figures show one representative experiment
Involvement of miR-222 in the resistance of MCF-7/S and MCF-7/Adr cells to Adr
To determine the effect of miR-222 expression on chemosensitivity to Adr, miR-222 inhibitors were transfected into MCF-7/Adr. miR-222 inhibitors could enhance their sensitivity to Adr, compared with the controlled cells (IC50: 282.016 vs 372.110; Fig. 3a,**P < 0.01). Similarly, miR-222 mimics were transfected into MCF-7/S cells. The IC50 values from MTT of Adr for transfected MCF-7/S cells were significantly increased, compared with those of the controlled cells (IC50: 3.525 vs 2.732; Fig. 3b, *P < 0.05). These results suggest that miR-222 could modulate Adr resistance in MCF-7/Adr and MCF-7/S cells.
Effect of miR mimics ad miR inhibitors on the sensitivity of MCF-7 cell lines to Adr. a IC50 values of Adr were determined after MCF-7/Adr were transfected with miR-222 inhibitors or negative control for 24 h. b IC50 values of Adr was determined after MCF-7/S were transfected with miR-222 mimics or negative control for 24 h. Cells transfected without external genes were used as blank control, which has no statistical significance with negative control. Bars indicate the mean ± standard deviation from at least three independent experiments. *P < 0.05 or **P < 0.01, compared with the NC
MiR-222 influences the apoptosis rates of MCF-7/S and MCF-7/Adr
Apoptosis assay reveals markedly higher Adr-induced apoptosis cells in miR-222-inhibitor-transfected MCF-7/Adr cells compared to negative controls (Fig. 4a, b; *P < 0.05). Moreover, overexpression of endogenous miR-222 decreases Adr-induced apoptosis in MCF-7/S cells (Fig. 4c, d; **P < 0.01). These results disclosed that miR-222 could confer Adr resistance in MCF-7 cells.
Flow cytometry assessment of apoptotic cells induced by Adr. Twenty-four hours after transfection, cells were treated with Adr for 48 h before flow cytometry analysis. a, b Adr was added into MCF-7/Adr cells with a final concentration of 100 μM transfected with miR-222 inhibitors or negative control. c, d Adr was added into MCF-7/S cells with a final concentration of 0.5 μM with transfected miR-222 mimics or negative control. e, f MCF-7/Adr cells were pretreated with 50 uM LY294002 or DMSO for 1 h prior to cotreatment with Adr. Cells transfected without external genes were used as blank control, which has no statistical significance with negative control. Bars indicate the mean ± standard deviation from at least three independent experiments. *P < 0.05 or **P < 0.01, compared with the NC. Flow cytometry figures show one representative experiment
Akt inhibitor (LY294002) increases the apoptosis caused by PTEN/Akt/p27 pathway
To examine the effect of LY294002 on PTEN/Akt/p27 signaling pathway, MCF-7/Adr cells were pretreated with 50 μM LY294002 for 1 h prior to cotreatment with Adr for 24 following by flow cytometry. The apoptosis rate of MCF-7/Adr was increased, compared with the controlled cells (Fig. 4e, f;*P < 0.05).
p27 location dysregulated by miR-222/PTEN/Akt/p27 pathway
The subcellular locations of p27 were altered by miR-222, which was detected by immunofluorescence. After transfected miR-222 inhibitors into MCF-7/Adr, the expression of p27 in the nucleus is increased compared to the controls (Fig. 5a, b). Furthermore, its expression in the nucleus is decreased after transfected miR-222 mimics in to MCF-7/S compared to the controls (Fig. 5c, d).
After transfection for 24 h, cells were incubated primary antibody overnight and fluorescence-conjugated secondary antibody for 1 h. Immunofluorescence microscope disclosed the subcellular locations of p27. a After transfected with miR-222 inhibitors or negative control, the subcellular location of p27 was detected in MCF-7/Adr cells. b After transfected with miR-222 mimics or negative control, the subcellular location of p27 was detected in MCF-7/S. Cells transfected without external genes were used as blank control, which has no statistical significance with negative control. All these results were confirmed in three independent experiments. Immunofluorescence figures show one representative experiment
Discussion
Obviously, Adr-based chemotherapy remains an effective method for treatments of breast cancer, and Adr inhibits topoisomerase II resulting in DNA double strand and single strand breaking [26]. Nevertheless, Adr resistance has become a predominant chemotherapeutic obstacle in breast cancer. Emerging reports have demonstrated that miRNAs are becoming hotspots involving in the development and progression of cancers via regulating the expressions of multiple target genes [7]. Among them, miR-222 had been well established as a novel class regulator in cell proliferation, apoptosis, invasion, and drug resistance of various cancers [5, 6, 27, 28] through targeting PTEN, Bax, p57, or p27. In our previous study, we found that miR-222 decreased the susceptibility of breast cancer cells to Adr [15]. However, its concrete role in Adr-resistant breast cancer has not been well elucidated.
Chiefly, we showed that miR-222 expression was significantly increased in postchemotherapeutic breast cancer tissues compared with the preadjuvant chemotherapy tissues, which significantly indicated that miR-222 was a significant regulator in drug-resistant breast cancer. However, we found that the expression of miR-222 had no significance in PR group compared with SD + PD groups. We speculated that the reasons for these results were the small sample size, various characters of patients, or the differential expression in vivo and in vitro. In addition, Zeng et.al disclosed that, in bladder cancer, miR-222 enhanced cisplatin resistance through protein phosphatase 2A subunit B (PPP2R2A)/Akt/mTOR axis [28]. Zhao et.al suggested that miR-222-ABCG2 pathway was in the correlation between cisplatin resistance and tongue squamous cell carcinoma [6]. Moreover, miR-222 negatively regulated metalloproteinase-3 (TIMP3)-enhanced tamoxifen resistance to breast cancer [29]. In acquired fulvestrant-resistant breast cancer, Rao et.al found that miR-222 played a crucial role through regulating beta-catenin or TGF-beta [30]. Interestingly, Zhang et.al found that miR-222 regulated treatment sensitivity, cell growth, and invasion via direct modulation of PTEN in gastric cancer cells [16]. All these findings had the same standpoint with us that miR-222 was truly correlated with the chemoresistance, and further investigation of more mechanisms is warranted.
Moreover, in vitro, we further confirmed that overexpression miR-222 enhanced Adr resistance in MCF-7/S and miR-222 inhibitors transfected into MCF-7/Adr decreased the Adr resistance, through altering PTEN/Akt/p27 pathway. Notably, Akt inhibitor (LY294002) increased the apoptosis rate after adding Adr to MCF-7/Adr through blocking the PTEN/Akt/p27 signing pathway. Therefore, our data suggested that miR-222 could reduce the sensitivity of breast cancer cells to Adr through PTEN/Akt/p27 signaling pathway and miR-222 inhibitors might rescue the resistance. Lau MT et.al reported that PTEN, an essential tumor suppressor gene, inhibited phosphoinositide 3-kinase (PI3K)/Akt signaling [31]. p27, as a cyclin-dependent kinase inhibitor, was found to obstruct the cell cycles into S phase and had been related with a tamoxifen-resistance phenotype [32, 33]. The activity of p27 was influenced by two mechanisms. One is the level of transcription and protein stability and another is its subcellular localization [34]. Several studies had proved that p-Akt was associated with the activation of the p27 resulting in the changes of its subcellular location [20]. Here, we found that p-Akt negatively regulated the subcellular localization of p27 in nucleus, which represented more drug resistance. Besides, there were three phosphorylation sites, concluding Ser10, Thr157, and Thr198, which were enrolled in cellular localization [21, 35, 36]. However, the specific molecular mechanisms of the phosphorylation regulation of p27 remain largely elusive, which need a better understanding.
Actually, clinically, many patients suffered resistance from different drugs in breast cancer, such as paclitaxel, cyclophosphamide, epirubicin, tamoxifen, and fulvestrant, and the mechanisms of multidrug-resistance are also illegible. In this study, we exclusively focused on Adr resistance in breast cancer and more researches are demanded in the follow-up period especially on animals.
Although many studies confirmed that miR-222 inhibited the expression of PTEN/Akt, Calderaro et.al found that PTEN and p-Akt expression had no inverse correlation, in vivo, in human bladder urothelial carcinomas [37]. From this research, we suggest that the association between miR-222 and PTEN/Akt/p27 pathway was different from various cancers or had racial differences and might have differences in vivo and in vitro, which urge us to uncover its further potentiality. Mechanisms involved in the regulation of miR-222 and pathways continue to be identified in drug-resistant breast cancer.
Furthermore, in our previous research, the result showed that miR-222 might spread Adr resistance capacity through exosomes, nano-sized vesicles [38], while the extensional mechanisms are still unclear. On the basis of these results, we conjecture that exosomes mediated drug resistance extremely through PTEN/Akt/p27 pathway, which deserves further researches. However, the tumorigenesis of cancers was determined by gene-regulating networks, and miRNAs exclusively take part in partial functions especially of miR-222. For example, Pichiorri et.al found that nucleolin (NCL) regulated the expression of miR-222 inducing breast cancer progression and drug resistance [39]. Stinson et.al identified that miR-222 triggered epithelial-to-mesenchymal transition (EMT) and acted downstream of the oncogenic RAS-RAF-MEK pathway, which all were involved in promotion of clinically aggressive metastatic breast cancer [40]. Indeed, cell proliferation, invasion, metastasis, and angiogenesis are closely related with chemoresistance in cancers. Therefore, PTEN/Akt/p27 partly participated in the progression, and later we could unearth the potential of miR-222 in other novel pathways as mentioned above and additional experiments are necessary including in vivo studies.
In summary, our study underscored that miR-222 decreased Adr sensitivity at least partly through PTEN/Akt/p27 pathway in human breast cancer cells. We also provided direct evidences that miR-222 inhibitors might be a novel therapeutic approach to improve Adr sensitivity in breast cancer. We believed that this novel pathway should be prioritized for utilization in the management of chemoresistance in breast cancer in the future.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262.
Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. doi:10.1146/annurev.med.53.082901.103929.
Wind NS, Holen I. Multidrug resistance in breast cancer: from in vitro models to clinical studies. Int J Breast Cancer. 2011;2011:967419. doi:10.4061/2011/967419.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Brognara E, Fabbri E, Montagner G, Gasparello J, Manicardi A, Corradini R, et al. High levels of apoptosis are induced in human glioma cell lines by co-administration of peptide nucleic acids targeting miR-221 and miR-222. Int J Oncol. 2015. doi:10.3892/ijo.2015.3308.
Zhao L, Ren Y, Tang H, Wang W, He Q, Sun J, et al. Deregulation of the miR-222-ABCG2 regulatory module in tongue squamous cell carcinoma contributes to chemoresistance and enhanced migratory/invasive potential. Oncotarget. 2015. doi:10.18632/oncotarget.6253.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi:10.1016/j.cell.2009.01.002.
Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11(4):252–63. doi:10.1038/nrm2868.
Liu W, Song N, Yao H, Zhao L, Liu H, Li G. miR-221 and miR-222 simultaneously target RECK and regulate growth and invasion of gastric cancer cells. Med Sci Monit. 2015;21:2718–25. doi:10.12659/msm.894324.
Zhao Y, Wang Y, Yang Y, Liu J, Song Y, Cao Y, et al. MicroRNA-222 controls human pancreatic cancer cell line Capan-2 proliferation by P57 targeting. Journal of Cancer. 2015;6(12):1230–5. doi:10.7150/jca.12546.
Sohn W, Kim J, Kang SH, Yang SR, Cho JY, Cho HC, et al. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;47:e184. doi:10.1038/emm.2015.68.
Chen WX, Hu Q, Qiu MT, Zhong SL, JJ X, Tang JH, et al. miR-221/222: promising biomarkers for breast cancer. Tumour Biol. 2013;34(3):1361–70. doi:10.1007/s13277-013-0750-y.
Zhou S, Shen D, Wang Y, Gong L, Tang X, Yu B, et al. microRNA-222 targeting PTEN promotes neurite outgrowth from adult dorsal root ganglion neurons following sciatic nerve transection. PLoS One. 2012;7(9):e44768. doi:10.1371/journal.pone.0044768.
Zhang C, Zhang J, Zhang A, Wang Y, Han L, You Y, et al. PUMA is a novel target of miR-221/222 in human epithelial cancers. Int J Oncol. 2010;37(6):1621–6.
Zhong S, Li W, Chen Z, Xu J, Zhao J. MiR-222 and miR-29a contribute to the drug-resistance of breast cancer cells. Gene. 2013;531(1):8–14. doi:10.1016/j.gene.2013.08.062.
Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F, Xiao Y, Guang-Xiu W, et al. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer. 2010;10:367. doi:10.1186/1471-2407-10-367.
Wang Y, Dai W, Chu X, Yang B, Zhao M, Sun Y. Metformin inhibits lung cancer cells proliferation through repressing microRNA-222. Biotechnol Lett. 2013;35(12):2013–9. doi:10.1007/s10529-013-1309-0.
de Araujo WM, Robbs BK, Bastos LG, de Souza WF, Vidal FC, Viola JP, et al. PTEN overexpression cooperates with lithium to reduce the malignancy and to increase cell death by apoptosis via PI3K/Akt suppression in colorectal cancer cells. J Cell Biochem. 2016;117(2):458–69. doi:10.1002/jcb.25294.
Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78(1):67–74.
Hnit SS, Xie C, Yao M, Holst J, Bensoussan A, De Souza P, et al. p27(Kip1) signaling: transcriptional and post-translational regulation. Int J Biochem Cell Biol. 2015;68:9–14. doi:10.1016/j.biocel.2015.08.005.
He W, Wang X, Chen L, Guan XA. Crosstalk imbalance between p27(Kip1) and its interacting molecules enhances breast carcinogenesis. Cancer Biother Radiopharm. 2012;27(7):399–402. doi:10.1089/cbr.2010.0802.
Li LQ, Li XL, Wang L, WJ D, Guo R, Liang HH, et al. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell Physiol Biochem. 2012;30(3):631–41. doi:10.1159/000341444.
Sun C, Li N, Zhou B, Yang Z, Ding D, Weng D, et al. miR-222 is upregulated in epithelial ovarian cancer and promotes cell proliferation by downregulating P27. Oncol Lett. 2013;6(2):507–12. doi:10.3892/ol.2013.1393.
Wang X, Xu Y, Zhu H, Ma C, Dai X, Qin C. Downregulated microRNA-222 is correlated with increased p27Kip1 expression in a double transgenic mouse model of Alzheimer’s disease. Mol Med Rep. 2015;12(5):7687–92. doi:10.3892/mmr.2015.4339.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16.
Lage H, Aki-Sener E, Yalcin I. High antineoplastic activity of new heterocyclic compounds in cancer cells with resistance against classical DNA topoisomerase II-targeting drugs. Int J Cancer. 2006;119(1):213–20. doi:10.1002/ijc.21792.
Li Y, Zhao L, Shi B, Ma S, Xu Z, Ge Y, et al. Functions of miR-146a and miR-222 in tumor-associated macrophages in breast cancer. Scientific reports. 2015;5:18648. doi:10.1038/srep18648.
Zeng LP, Hu ZM, Li K, Xia K. miR-222 attenuates cisplatin-induced cell death by targeting the PPP2R2A/Akt/mTOR Axis in bladder cancer cells. J Cell Mol Med. 2016. doi:10.1111/jcmm.12760.
Gan R, Yang Y, Yang X, Zhao L, Lu J, Meng QH. Downregulation of miR-221/222 enhances sensitivity of breast cancer cells to tamoxifen through upregulation of TIMP3. Cancer Gene Ther. 2014;21(7):290–6. doi:10.1038/cgt.2014.29.
Rao X, Di Leva G, Li M, Fang F, Devlin C, Hartman-Frey C, et al. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene. 2011;30(9):1082–97. doi:10.1038/onc.2010.487.
Lau MT, Klausen C, Leung PCE. Cadherin inhibits tumor cell growth by suppressing PI3K/Akt signaling via beta-catenin-Egr1-mediated PTEN expression. Oncogene. 2011;30(24):2753–66. doi:10.1038/onc.2011.6.
Chu I, Sun J, Arnaout A, Kahn H, Hanna W, Narod S, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128(2):281–94. doi:10.1016/j.cell.2006.11.049.
Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78(1):59–66.
le Sage C, Nagel R, Agami R. Diverse ways to control p27Kip1 function: miRNAs come into play. Cell Cycle. 2014;6(22):2742–9. doi:10.4161/cc.6.22.4900.
Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8(10):1145–52. doi:10.1038/nm759.
Fujita N, Sato S, Tsuruo T. Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2003;278(49):49254–60. doi:10.1074/jbc.M306614200.
Calderaro J, Rebouissou S, de Koning L, Masmoudi A, Herault A, Dubois T, et al. PI3K/AKT pathway activation in bladder carcinogenesis. International Journal of Cancer Journal International du Cancer. 2014;134(8):1776–84. doi:10.1002/ijc.28518.
Chen WX, Liu XM, Lv MM, Chen L, Zhao JH, Zhong SL, et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One. 2014;9(4):e95240. doi:10.1371/journal.pone.0095240.
Pichiorri F, Palmieri D, De Luca L, Consiglio J, You J, Rocci A, et al. In vivo NCL targeting affects breast cancer aggressiveness through miRNA regulation. J Exp Med. 2013;210(5):951–68. doi:10.1084/jem.20120950.
Stinson S, Lackner MR, Adai AT, Yu N, Kim HJ, O’Brien C, et al. TRPS1 targeting by miR-221/222 promotes the epithelial-to-mesenchymal transition in breast cancer. Sci Signal. 2011;4(177):ra41. doi:10.1126/scisignal.2001538.
Acknowledgments
We thank Shan-liang Zhong and Wei-xian Chen for useful discussions and help in revision of the present paper. This study was funded by the National Natural Science Foundation of China (grant number 81272470).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Wang, Dd., Yang, Sj., Chen, X. et al. miR-222 induces Adriamycin resistance in breast cancer through PTEN/Akt/p27kip1 pathway. Tumor Biol. 37, 15315–15324 (2016). https://doi.org/10.1007/s13277-016-5341-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5341-2