Abstract
Dishevelled-Axin domain containing 1 (DIXDC1) is a DIX (Dishevelled-Axin) domain possessing protein that acts as a positive regulator of the Wnt pathway. Although DIXDC1 has been investigated in several cancers, it has not yet been studied in human hepatocellular carcinoma (HCC). The purpose of the current study was to investigate the expression pattern of DIXDC1 and assess the clinical significance of DIXDC1 expression in HCC patients. Data containing three independent investigations from Oncomine database demonstrated that DIXDC1 mRNA was downregulated in HCC compared with matched non-cancerous tissues. Similar results were also obtained in 25 paired HCC tissues and corresponding non-cancerous tissues by qPCR and Western blot analysis. Additionally, another independent set of 140 pairs of HCC specimens was evaluated for DIXDC1 expression by IHC and demonstrated that reduced expression of DIXDC1 in 50.7 % (71/140) of HCC tissues was significantly correlated with tumor size (p = 0.024), tumor differentiation (p < 0.001), tumor thrombi (p = 0.019), TNM stage (p = 0.019), and BCLC stage (p = 0.008). Importantly, Kaplan–Meier survival and Cox regression analyses were executed to evaluate the prognosis of HCC patients and found that DIXDC1 protein expression was one of the independent prognostic factors for overall survival of HCC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Hepatocellular carcinoma (HCC) is the fifth most commonly diagnosed cancer and is the third leading cause of cancer death worldwide [1]. Surgical resection and various tumor ablation procedures have provided an opportunity for a cure or prolonged life span [2]. However, the outcome for patients with HCC remains typically poor, primarily because the advanced tumor stage renders it unsuitable for surgical resection and because of intrahepatic metastasis with frequent tumor recurrence after hepatectomy [3]. Therefore, it is imperative to identify high-risk factors and new biomarkers for prognostic prediction in patients with HCC.
Dishevelled-Axin domain containing 1 (DIXDC1), a human homolog of Ccd1, is a scaffold protein containing a Dishevelled-Axin (DIX) domain, a coiled-coil (CC) domain, and an actin-binding calponin homology (CH) domain [4]. It was initially reported to be associated with the nervous systems and neurobiological aspects, such as neural patterning [5], embryonic cortical development [6], and psychiatric disorders [7]. Investigations almost entirely focused on its positively regulatory role in the Wnt signaling pathway through its DIX domain [5, 8]. In addition, DIXDC1 could directly bind to filamentous actin in normal cell via its actin-binding domain, thereby functioning as a cytoskeletal-associated protein [9]. Recently, overexpression of DIXDC1 had been reported in several human malignancies, including gastric cancer [10, 11], lung cancer [12], and colon cancer [13, 14], and it had been shown that DIXDC1 was responsible for cell proliferation, invasion and metastasis, as well as prognosis in gastric cancer and lung cancer [10, 12]. However, a study conducted by Goodwin et al. demonstrated that DIXDC1 depletion led to increased cell invasion in vitro and promotion of metastasis in mice [4]. Moreover, by query of the TCGA TumorScape database, they revealed that DIXDC1 was frequently downregulated in human cancers, which was correlated with poor survival [4]. These discrepant results indicated that the potential significance of DIXDC1 in cancer pathobiology was tissue dependent and varied with the type of malignancy.
Although DIXDC1 had been investigated in several cancers, to the best of our knowledge, it has not yet been studied in human HCC. The expression and clinical significance of DIXDC1 in HCC are completely lacking. Therefore, in this retrospective study, we examined both the messenger RNA (mRNA) and protein expression level of DIXDC1 in HCC clinical samples and further analyzed the relationship of DIXDC1 expression with clinicopathologic parameters, including clinical outcome. We found that DIXDC1 expression was frequently downregulated in HCC tissues. The reduced expression of DIXDC1 in HCC was correlated with tumor progression and might serve as an independent unfavorable prognostic indicator for patients with HCC.
Materials and methods
Patients and specimens
Archived formalin-fixed paraffin-embedded HCC samples with corresponding adjacent non-tumor tissue (ANT) were obtained from 140 patients undergoing initial hepatectomy from January 2006 to December 2010 in Sir Run Run Shaw Hospital (SRRSH), School of Medicine, Zhejiang University, China. Clinical and pathological characteristics of the cohort are summarized in Table 1. Tumor differentiation was graded by the Edmondson–Steiner grading system. Tumor stages were classified according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system [15]. HCC clinical stages were defined based on the Barcelona Clinic Liver Cancer (BCLC) staging system [16].
Among the 140 patients with HCC, follow-up information was available for 108 patients (Table S1). All patients were taken on 6-year follow-up. The follow-up period was defined as the interval from the date of surgery to the date of recurrence or death. The last follow-up was updated on December 20, 2015. Patients alive at the end of follow-up were censored. Disease-free survival (DFS) was defined as the interval from the date of surgery until the detection of tumor recurrence. Patients without signs of recurrence at the end of follow-up were censored. Patients who died from diseases other than HCC or unexpected events were excluded from the study cohort.
For quantitative polymerase chain reaction (qPCR) and Western blot analysis, an additional 25 paired fresh-frozen HCC tissues and corresponding non-cancerous tissues were obtained from HCC patients undergoing initial hepatectomy from August 2014 to December 2014 in SRRSH.
Real-time qPCR analysis
Total RNAs from primary tumor and adjacent non-tumor tissue samples were extracted using TRIzol reagent (Ambion, USA) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 2 μg of RNA from each sample using iScript™ cDNA Synthesis Kit (BIO-RAD, USA). Quantitative real-time PCR was performed using a 7500 Real-time PCR system (Applied Biosystems, Inc., USA). Primer sequences sed for DIXDC1 detection were as follows: forward 5′-TGCATGTTATGGAGACGCAGAAG-3′ and reverse 5′-AGGTGCTGCTGACAGTTGGAGA-3′. The relative expression of DIXDC1 was normalized to β-actin (forward 5′-GCCAACACAGTGCTGTCTGG-3′, reverse 5′-GCTCAGGAGGAGCAATGATCTTG-3′). The 2−ΔCt method was used to quantify the relative DIXDC1 expression levels and normalized using the β-actin expression. Each sample was tested in triplicate.
Western blot analysis
Total proteins from frozen tissues were extracted with RIPA lysis buffer containing protease inhibitors (Beyotime, China) and quantified using the Piece BCA Protein Assay Kit (Thermo Scientific, USA). Fourty-microgram protein was separated by 8 % SDS-PAGE. Samples were transferred to PVDF membranes (Millipore, USA) and incubated overnight at 4 °C with antibody against DIXDC1 (1:1000, OriGene, USA) and β-actin (1:1000, Sigma, USA). After incubation with HRP-coupled anti-mouse IgG antibody (1:2000, Beyotime, China) at 37 °C for 2 h, target proteins on PVDF membrane were visualized using Clarity™ Western ECL Substrate (BIO-RAD, USA) and captured using a Luminescent Image Analyzer (FUJIFILM, Japan).
Immunohistochemical analysis
Paraffin-embedded specimens were cut to 4 μm, deparaffinized with xylene, and rehydrated in a graded series of alcohols. Antigen retrieval was performed using 0.01-M citrate buffer for a 3-min boil. Hydrogen peroxide was applied to block peroxidase, and then the slides were incubated with normal goat serum. The primary antibody for DIXDC1 (1:600, OriGene, USA) was incubated overnight at 4 °C. Normal goat serum was used as a negative control. The expression of DIXDC1 was then detected by the Mo&Rb GTVision III Detection System (Gene Tech, China). DAB visualization was then performed, and the slides were counterstained with hematoxylin.
Immunostaining was evaluated and scored by two pathologists without prior knowledge of the clinicopatholgical data using the German immunoreactive score (IRS) [17]. Staining intensity was graded as “0” (negative), “1” (weak), “2” (moderate), and “3” (strong). Staining extent was graded as “0” (< 5 %), “1” (5–25 %), “2” (25–50 %), “3” (50–75 %), or “4” (> 75 %). Values of the staining intensity and the staining extent were multiplied as a final IRS of DIXDC1 expression. Using this method of assessment, we evaluated DIXDC1 expression in HCC tissues by the IRS of 0, 1, 2, 3, 4, 6, 8, 9, or 12. The median of all scores was used as cutoff values for DIXDC1. An optimal cutoff value was used as follows: a score of ≥6 was used to define tumors with high DIXDC1 expression and a score of ≤4 indicated low DIXDC1 expression.
Statistical analysis
Statistical analyses and graphical representations were performed using SPSS PASW Statistics 18.0 software (SPSS, Inc., Chicago, IL) and GraphPad Prism 5 (San Diego, CA) software. Pearson chi-square test or Fisher exact test was used to analyze the relationship between DIXDC1 expression and clinical features. Kaplan–Meier method with log-rank test was used to compare patients’ survival between subgroups. The influence of variables on survival was evaluated by univariate and multivariate Cox proportional hazard model. Each variable with statistical significance on univariate analysis was entered into multivariate models for estimating their independent prognostic value on cancer. The analysis of qPCR was performed by paired Student’s t test. A two-sided p value <0.05 was considered statistically significant.
Result
DIXDC1 mRNA expression in HCC
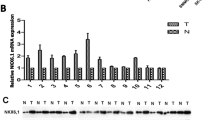
To determine the expression of DIXDC1 in HCC, we first performed data mining and analyzed DIXDC1 mRNA expression level from the publicly available Oncomine database (www.oncomine.org). We found that the level of DIXDC1 mRNA was significantly downregulated in HCC tissues compared with normal liver using Roessler liver dataset (Fig. 1a) and Wurmbach liver dataset (Fig. 1b). Similar result was also observed when compared with liver cirrhosis using Mas liver dataset (Fig. 1c). Furthermore, the expression of DIXDC1 mRNA was analyzed in 25 paired fresh-frozen HCC tissues and corresponding non-cancerous tissues. Downregulation of the DIXDC1 mRNA was observed in about 76 % (19/25) of the HCC samples (Fig. 1d). Statistical analysis revealed the significant difference in the mRNA level of DIXDC1 between HCC tissues and adjacent non-cancerous tissues (p = 0.032, Fig. 1e).
DIXDC1 expression in HCC at mRNA level. a Downregulated of DIXDC1 expression in HCC tissues comparing with normal liver tissues revealed using the Roessler liver dataset from Oncomine dataset. b Reduced DIXDC1 expression in HCC tissues revealed by Wurmbach liver dataset. c Decreased DIXDC1 expression in HCC tissues comparing with liver cirrhosis revealed using the Mas liver. d DIXDC1 mRNA level in 25 HCC samples and paired adjacent non-cancerous tissues. The expression of DIXDC1 mRNA level was expressed as Log2([T]/[N]). T tumor tissues, N non-cancerous tissues. Log2([T]/[N]) < 0 represented the downregulation of DIXDC1 in tumor tissues. Log2([T]/[N]) > 0 represented the upregulation of DIXDC1 in tumor tissues. e DIXDC1 mRNA levels in 25 HCC samples (T) and 25 paired adjacent non-cancerous tissues (ANT) were examined using real-time qPCR. The DIXDC1 expression was normalized to that of β-actin
DIXDC1 protein expression in HCC
DIXDC1 protein levels were evaluated using Western blot or immunohistochemical staining (IHC). To confirm the qPCR result, we performed Western blot of DIXDC1 on protein level in 25 pairs of HCC specimens and found that DIXDC1 expression was downregulated in 80 % (19/25) of the HCC patients (Fig. 2a).
DIXDC1 expression in HCC at protein level. a Protein expression levels of DIXDC1 in 25 paired HCC tissues (T) and corresponding adjacent non-cancerous tissues (ANT) were determined by Western blot analysis. β-Actin was used as a loading control. b Representative immunohistochemical images of DIXDC1 protein expression in HCC tissues (T) and adjacent non-cancerous tissues (ANT) (magnification ×100). The region in the black box was further enlarged and displayed to the bottom side (magnification ×400)
To further evaluate the phenotypic expression of DIXDC1 in HCC clinical samples, immunohistochemical analysis was performed in another independent set of 140 pairs of HCC specimens. Each pair consisted of cancerous and adjacent non-cancerous tissues derived from the same patient. The positive signal for DIXDC1 was observed primarily in the cytoplasm. The staining intensities were classified into four levels: level 1 with negative staining, level 2 with weak staining, level 3 with moderate staining, and level 4 with strong staining (Fig. 3a). We found significantly higher rates of “low DIXDC1 expression” in HCC tissues (50.7 %, 71/140) compared with adjacent non-cancerous tissues (15.0 %, 21/140) (Fig. 2b). In detail, 29.3 % (41/140) of the cancerous specimens showed negative staining, 21.4 % (30/140) of the cases showed weak staining, 25.7 % (36/140) of the cases showed moderate staining, and 23.6 % (33/140) of the cases showed strong staining of DIXDC1 protein. In striking contrast, only 0.7 % (1/140) of the non-cancerous tissues showed negative staining, 14.3 % (20/140) of the cases showed weak staining, 38.6 % (54/140) of cases showed moderate staining, and 46.4 % (65/140) of cases showed strong staining of DIXDC1 (p < 0.001, Fig. 3b). Thus, DIXDC1 was frequently downregulated in HCC tissues.
Immunohistochemical staining of DIXDC1 protein in human HCC and adjacent non-cancerous tissues. a Representative immunohistochemical expression patterns of DIXDC1 in cancerous (T) and adjacent non-cancerous (ANT) specimens were shown (magnification: upper panel, ×100; lower panel, ×400). b Percentage of cases with different staining intensity of DIXDC1 in the tumor (T) or adjacent normal tissues (ANT) in the study cohort. (n = 140, p < 0.001)
Correlation of DIXDC1 expression with clinicopathologic features
To evaluate the clinical significance of DIXDC1 expression in HCC, the chi-square test was used to assess the correlations between DIXDC1 protein expression and clinicopathologic parameters. As shown in Table 1, DIXDC1 expression was significantly correlated with tumor size (p = 0.024), tumor differentiation (p < 0.001), tumor thrombi (p = 0.019), TNM stage (p = 0.019), and BCLC stage (p = 0.008), while there were no significant associations between DIXDC1 expression and patient age (p = 0.116), gender (p = 0.560), HBsAg (p = 0.523), γ-GT (p = 0.375), AFP (p = 0.179), liver cirrhosis (p = 0.845), and tumor number (p = 0.074).
Prognostic values of DIXDC1 expression for patients with HCC
To determine the value of DIXDC1 for the prognosis of post-surgical HCC patients, 5-year overall (OS) and disease-free survival (DFS) rates were analyzed by Kaplan–Meier analysis and log-rank test. Survival data were available for 108 patients. The 5-year overall and disease-free survival rates were 47.9 and 35.4 % in low DIXDC1 expression and 78.2 and 50.0 % in high DIXDC1 expression. The results indicated that patients with low DIXDC1 expression had much shorter survival time (p = 0.002, Fig. 4a) and a higher tendency of disease recurrence (p = 0.036, Fig. 4b).
The prognostic value of DIXDC1 was further confirmed by stratified survival analysis. Low DIXDC1 expression was found in 41 cases from the 95 (43.2 %) HCC patients with single tumor, and DIXDC1 was a predictor for overall survival (p = 0.001, Fig. 5a) and a tendency towards statistical significance for recurrence-free survival (p = 0.058, Fig. 5b). Similarly, low levels of DIXDC1 also significantly predicted poor overall survival in patients with early stage (stages I–II) tumors (p = 0.004, Fig. 5c) and closely correlated with shorter disease-free survival (p = 0.095, Fig. 5d).
Stratified survival analysis for HCC subgroups. a, b Kaplan–Meier analysis of overall survival and disease-free survival of patients with single tumor according to DIXDC1 expression status (n = 95). c, d Kaplan–Meier analysis of overall survival and disease-free survival of patients with early stage tumors (TNM stages I–II) according to DIXDC1 expression status (n = 98)
To assess whether DIXDC1 expression represented an independent prognostic indicator in HCC, the effect of each variable on survival was determined by the Cox regression analysis. Univariate analysis indicated that DIXDC1, as well as tumor size, tumor thrombi, TNM stage, and BCLC stage were significantly associated with the overall and recurrence-free survival of HCC patients (Table 2). The variables that significantly correlated with survival in the univariate analysis were further assessed by multivariate analysis. Results revealed that DIXDC1 was an independent prognostic marker for overall survival in HCC patients (hazard ratio (HR) = 0.439, p = 0.011, Table 2).
Discussion
Despite improvements in surveillance and treatment strategies, the long-term survival of HCC remains dismal due partly to post-treatment relapse and distant metastasis [3, 18]. The BCLC staging classification and the pathological TNM stage have been established as clinical practice guidelines for HCC [19]. Once a diagnosis has been made, patients’ prognosis will vary according to disease stage and treatment received. However, due to the tumor heterogeneity, patients with the same clinical stage or pathological status still have different clinical outcomes [20]. Hence, it is of particular importance to identify novel molecular markers that can effectively distinguish between HCC patients with favorable or unfavorable prognoses in the same stage or grade. Therefore, in this study, we presented for the first time the downregulated expression of DIXDC1 in HCC and its potential role in predicting the tumor progression outcome and unfavorable prognosis of HCC patients.
DIXDC1 was initially reported to exert its regulatory role in the Wnt signaling during neural development [5, 8]. As Wnt/β-catenin pathway also plays an important role in tumorigenesis [21], subsequent studies revealed that overexpression of DIXDC1 might promote tumor progression and predict poor survival in colon cancer, gastric cancer, and lung cancer [10–14]. However, Goodwin et al. demonstrated that DIXDC1 was downregulated in human cancers and correlated with poor survival [4]. The above contradictory results indicated that the functions of DIXDC1 in cancer genesis were context dependent. What is more, the exact role of DIXDC1 in HCC has not been assessed yet.
In the current study, we firstly collected DIXDC1 information through bioinfomatic analysis from Oncomine database. Three independent datasets showed that DIXDC1 mRNA was reduced in HCC tissues compared with normal or cirrhosis liver tissues. Then, by using qPCR and Western blot analysis, we detected low DIXDC1 expression at both the mRNA and protein levels in 25 paired HCC samples. The preliminary result supported the note that DIXDC1 was downregulated in human cancers [4]. Furthermore, our subsequent immunohistochemical analysis of 140 paired HCC specimens demonstrated that expression of DIXDC1 was significantly downregulated in HCC, in which 50.7 % (71/140) of the cancerous tissues presented low DIXDC1 expression, whereas only 15.0 % (21/140) of the adjacent non-cancerous tissues showed the same expression pattern. Of note, we observed low DIXDC1 expression in 76 % (19/25) of HCC tissues by qPCR and 80 % (19/25) by Western blot analysis, whereas only 50.7 % (71/140) of HCC samples showed decreased expression by IHC analysis. This discrepancy might result from the limited quantity of HCC fresh samples and the relatively low DIXDC1 expression in the high DIXDC1 group. For example, as Fig. 2b showed, some evaluated “high DIXDC1 expression” specimens by IHC analysis showed relatively low DIXDC1 expression in HCC compared with adjacent non-cancerous tissues, of which the differential expression pattern could be decided as “low DIXDC1 expression” by qPCR or Western blot analysis. Taken together, our results unambiguously confirmed the significant downregulation of DIXDC1 expression in HCC.
Interestingly, according to our results, low DIXDC1 expression in HCC was significantly correlated with tumor size, tumor differentiation, tumor thrombi, TNM stage, and BCLC stage, as tumor size, vascular invasion, and tumor thrombi are critical tumor status-related variables in the TNM stage and BCLC stage system. Tumor differentiation is a crucial indicator of the degree of tumor cell malignancy. The results strongly supported that DIXDC1 could be used as a biomarker to evaluate the progression of HCC and then to distinguish disease aggressiveness between HCC patients. Loss of DIXDC1 might facilitate proliferation, differentiation, and motility of the primary tumor cells. Although previous studies found that overexpression of DIXDC1 promote cell proliferation and invasion in some cancer types [10, 12, 13], Goodwin et al. showed that knockdown of DIXDC1 led to increased cell invasion in vitro and in vivo [4]. Thus, these characters of DIXDC1 were further needed to confirm in HCC cells for detailed phenotypes.
The most important finding of the present study was the prognostic value of DIXDC1 in HCC patients. Patients with a low level of DIXDC1 expression had significantly shorter overall and disease-free survival time compared to those with a high level of DIXDC1 expression. Univariate analysis showed that reduced DIXDC1 expression was significantly associated with the overall and disease-free survival rate in HCC patients. Multivariate analysis indicated that DIXDC1 expression was an independent risk factor for poor overall survival of HCC patients. Moreover, a stratified survival analysis revealed that the decreased expression of DIXDC1 protein in tumors significantly predicted poor overall survival for patients with single tumor as well as for those with early stage tumors. Tumor characteristics such as tumor number and tumor stage are important determinants of prognosis in HCC patients [25, 26]. Although patients with single tumor or early clinical stage have relatively better prognosis, individuals still have a highly variable clinical outcomes which probably reflects a molecular heterogeneity [20]. Thus, molecular biomarkers extend its prognostic usefulness to identify high-risk subgroup of patients with HCC. Results from our study suggested that DIXDC1 expression patterns could serve as a promising biomarker not only to predict prognosis in HCC patients but also to classify patients with some early clinical status into distinct risk subgroups and guide individualized therapeutic strategy.
Notably, the immunohistochemistry results showed that DIXDC1 protein was mainly localized in the cytoplasm. As a positive regulator in the Wnt/β-catenin pathway, DIXDC1 could interact with axin and dishevelled [22, 23]. As a scaffolding protein, DIXDC1 co-localized with actin and γ-tubulin [24], involving in the PI3K-AKT pathway [12, 13]. The abovementioned interaction or signal transduction mainly occurred in the cytoplasm which is reasonable for DIXDC1 acting as an oncogene in an overexpression fashion. Despite the Wnt signaling frequently activated in hepatocarcinogenesis [27], in the context of low DIXDC1 expression in HCC tissues, we speculated that other mechanisms independent of Wnt signaling may be involved in the HCC progression. Clinicopathologic relevance analysis revealed that low DIXDC1 was associated with tumor size, tumor differentiation, and tumor thrombi, all of which reflected the tumor potential of proliferation and metastasis. Goodwin et al. recently found that depletion of DIXDC1 could trigger an upregulation of Snail levels and affect focal adhesion dynamics [4]. Snail is a critical transcription factor in the process of epithelial–mesenchymal transition (EMT) during which epithelial cell layers lose polarity and cell–cell contact, undergo dramatic cytoskeletal remodeling, and ultimately convert into a more aggressive mesenchymal-like state [28]. Moreover, loss of DIXDC1 results in an accumulation of nascent focal adhesions which display increased rates of cell motility as well as hyperactivation of the FAK [4]. As a scaffolding protein, FAK orchestrates a diverse range of cellular processes, such as cell survival, proliferation, migration, invasion, epithelial–mesenchymal transition, and angiogenesis [29]. Interesting, DIXDC1 is also an scaffolding protein related to signaling connection and cytoskeleton. Thus, we postulated that decreased DIXDC1 might be related to EMT, activation FAK/Src signaling module, and cytoskeleton remodeling. High potential of tumor dissemination or de novo tumor formation is often the main biological basis for postoperative recurrence and poor prognosis of HCC [30, 31]. Low level of DIXDC1 may promote metastasis and proliferation in HCC progression. However, the underlying mechanism by which DIXDC1 affects HCC progression remains elusive and will require future investigation.
There are some limitations in this study. First, the major limitation of this study is the relatively small cohort size with follow-up information. Of the 32 patients excluded from prognosis analysis, 23 patients demonstrated low DIXDC1 expression, which might have some impacts on the prognosis analysis. Second, our study included HCC patients from a single center, in which liver transplantation was not an initial treatment modality. Thus, this data might not be relevant to medical centers that performed many liver transplants. Finally, the potential role of DIXDC1 in the development of HCC had not been elucidated. Further studies are necessary to confirm our findings and clarify the function and mechanism of DIXDC1 in the development of HCC.
In conclusion, our study for the first time revealed that DIXDC1 expressionis was frequently downregulated in HCC tissues. Low expression of DIXDC1 was significantly associated with disease progression and poor postoperative outcome of HCC patients. Our results suggested that DIXDC1 might serve as a novel prognostic molecular marker for patients with HCC and encourage further investigation of its potential role in HCC pathobiology.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics. CA Cancer J Clin. 2012;2015(65):87–108.
Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
Pan HW, YH O, Peng SY, Liu SH, Lai PL, Lee PH, Sheu JC, Chen CL, Hsu HC. Overexpression of osteopontin is associated with intrahepatic metastasis, early recurrence, and poorer prognosis of surgically resected hepatocellular carcinoma. Cancer. 2003;98:119–27.
Goodwin JM, Svensson RU, Lou HJ, Winslow MM, Turk BE, Shaw RJ. An AMPK-independent signaling pathway downstream of the LKB1 tumor suppressor controls Snail1 and metastatic potential. Mol Cell. 2014;55:436–50.
Shiomi K, Uchida H, Keino-Masu K, Masu M. Ccd1, a novel protein with a DIX domain, is a positive regulator in the Wnt signaling during zebrafish neural patterning. Curr Biol. 2003;13:73–7.
Singh KK, Ge X, Mao Y, Drane L, Meletis K, Samuels BA, Tsai LH. Dixdc1 is a critical regulator of DISC1 and embryonic cortical development. Neuron. 2010;67:33–48.
Kivimäe S, Martin PM, Kapfhamer D, Ruan Y, Heberlein U, Rubenstein JL, Cheyette BN. Abnormal behavior in mice mutant for the Disc1 binding partner, Dixdc1. Transl Psychiatry. 2011;1:e43.
Shiomi K, Kanemoto M, Keino-Masu K, Yoshida S, Soma K, Masu M. Identification and differential expression of multiple isoforms of mouse Coiled-coil-DIX1 (Ccd1), a positive regulatorof Wnt signaling. Mol Brain Res. 2005;135:169–80.
Wang X, Zheng L, Zeng Z, Zhou G, Chien J, Qian C, Vasmatzis G, Shridhar V, Chen L, Liu W. DIXDC1 isoform, l-DIXDC1, is a novel filamentous actin-binding protein. Biochem Biophys Res Commun. 2006;347:22–30.
Tan C, Qiao F, Wei P, Chi Y, Wang W, Ni S, Wang Q, Chen T, Sheng W, Du X, Wang L. DIXDC1 activates the Wnt signaling pathway and promotes gastric cancer cell invasion and metastasis. Mol Carcinog. 2016;55:397–408.
Wang L, Tan C, Qiao F, Wang W, Jiang X, Lian P, Chang B, Sheng W. Upregulated expression of DIXDC1 in intestinal-type gastric carcinoma co-localization with β-catenin and correlation with poor prognosis. Cancer Cell Int. 2015;15:120.
Xu Z, Liu D, Fan C, Luan L, Zhang X, Wang E. DIXDC1 increases the invasion and migration ability of non-small-cell lung cancer cells via the PI3K-AKT-AP-1 pathway. Mol Carcinog. 2014;53:917–25.
Wang L, Cao XX, Chen Q, Zhu TF, Zhu HG, Zheng L. DIXDC1 targets p21 and cyclin D1 via PI3K pathway activation to promote colon cancer cell proliferation. Cancer Sci. 2009;100:1801–8.
Wang L, Li H, Chen Q, Zhu T, Zhu H, Zheng L. Wnt signaling stabilizes the DIXDC1 protein through decreased ubiquitin-dependent degradation. Cancer Sci. 2010;101:700–6.
Edge SB, Byrd DR, Compton CC et al (eds) (2009) AJCC cancer staging manual, 7th edn. Springer, New York
Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711.
Tang L, Tan YX, Jiang BG, Pan YF, Li SX, Yang GZ, et al. The prognostic significance and therapeutic potential of hedgehog signaling in intrahepatic cholangiocellular carcinoma. Clin Cancer Res. 2013;19:2014–24.
Luo RZ, Cai PQ, Li M, Fu J, Zhang ZY, Chen JW, Cao Y, Yun JP, Xie D, Cai MY. Decreased expression of PTPN12 correlates with tumor recurrence and poor survival of patients with hepatocellular carcinoma. PLoS One. 2014;9(1):e85592.
Llovet JM1, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX,et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008; 100: 698–711.
Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–6.
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–205.
Liu YT, Dan QJ, Wang J, Feng Y, Chen L, Liang J, Li Q, Lin SC, Wang ZX, Wu JW. Molecular basis of Wnt activation via the DIX domain protein Ccd1. J Biol Chem 2011; 286: 8597–8608.
Wong CK, Luo W, Deng Y, Zou H, Ye Z, Lin SC. The DIX domain protein coiled-coil-DIX1 inhibits c-Jun N-terminal kinase activation by axin and dishevelled through distinct mechanisms. J Biol Chem. 2004;279:39366–73.
Wu Y, Jing X, Ma X, Wu Y, Ding X, Fan W, Fan M. DIXDC1 co-localizes and interacts with gamma-tubulin in HEK293 cells. Cell Biol Int. 2009;33:697–701.
Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–24.
Ajay D. Staging of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S74–9.
Aravalli RN, Cressman EN, Steer CJ. Cellular and molecular mechanisms of hepatocellular carcinoma: an update. Arch Toxicol. 2013;87:227–47.
Funasaka T, Hogan V, Raz A. Phosphoglucose isomerase/autocrine motility factor mediates epithelial and mesenchymal phenotype conversions in breast cancer. Cancer Res. 2009;69:5349–56.
Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharmacol Ther. 2015;146:132–49.
Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–9.
A Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. EASL panel of experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30.
Author information
Authors and Affiliations
Corresponding author
Additional information
Senjun Zhou, Jiliang Shen, and Shuang Lin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, S., Shen, J., Lin, S. et al. Downregulated expression of DIXDC1 in hepatocellular carcinoma and its correlation with prognosis. Tumor Biol. 37, 13607–13616 (2016). https://doi.org/10.1007/s13277-016-5213-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5213-9