Abstract
Over the last few years, microRNAs (miRNA)-controlled cancer stem cells have drawn enormous attention. Cancer stem cells are a small population of tumor cells that possess the stem cell property of self-renewal. Recent data shows that miRNA regulates this small population of stem cells. In the present review, we explained different characteristics of cancer stem cells as well as miRNA regulation of self-renewal and differentiation in cancer stem cells. We also described the migration and tumor formation. Finally, we described the different miRNAs that regulate various types of cancer stem cells, such as prostate cancer stem cells, head and neck cancer stem cells, breast cancer stem cells, colorectal cancer stem cells, lung cancer stem cells, gastric cancer stem cells, pancreatic cancer stem cells, etc. Extensive research is needed in order to employ miRNA-based therapeutics to control cancer stem cell population in various cancers in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extensive research conducted over a decade on the presence of cancer stem cell (CSC) has drawn considerable attention due to its clinical importance. The CSC is defined as a cell inside a tumor which has the ability to self-renew and to cause the mixed lineages of cancer cells comprising the tumor. These cells can be identified experimentally by their capability to produce a tumor. Few other terms such as “tumor-initiating cell” and “tumorigenic cell” are also used to describe CSC [1, 2].
Studies on stem cells and cancer cells have derived many important findings. Both cancer cells and somatic stem have the capacity to self-renew. Self-renewal of somatic stem cells is tightly regulated, but it is not for cancer cells (Fig. 1). General stem cells produce normal mature cells, but cancer cells differentiate abnormally [3, 4]. Organogenic capacity is found in both normal stem cells and cancer cells, but there is a difference. Normal stem cells produce normal tissues, but the cancer cells produce abnormal tissues. Therefore, heterogeneity and plasticity are the two main properties of the CSCs [5]. Research data shows that CSCs show distinct characteristics compared to normal cancer cells such as tumor maintenance, progression, and chemoresistance [6]. Therefore, CSCs are clinically important as they are critical in invasiveness and recurrence of cancer. It is necessary to understand how CSC is controlled at the molecular level.
Diagram showing the comparison between stem cell and cancer stem cell formations. Both stem cell and cancer stem cell have self-renewal capacity, with the former in highly regulated manner while the latter is poorly regulated. a Stem cell originates from embryonic precursors and form mature cells. b Cancer stem cell forms from normal progenitor cell and stem cell
The current discovery of microRNAs (miRNAs) may provide a new explanation on the molecular regulatory mechanisms of CSCs. miRNAs are endogenous, non-coding, small RNAs (approximately 21–25 nucleotides) with gene regulatory activity [7, 8]. miRNAs are generated from primary miRNA transcripts, which are called pri-miRNAs by sequential cleavages with the enzyme like RNase III enzyme Dicer. The pri-miRNAs are often more than a few thousand nucleotides long [7, 8]. miRNA is attached to the RNA-induced silencing complex (RISC), which also contains the GW182 and Argonaute proteins. The miRNA controls gene expression by binding to the sequences in the 3′UTRs of the target region messenger RNAs (mRNAs), which causes either mRNA degradation or translation inhibition [9].
To date, more than 1100 miRNAs have been discovered in humans. The miRNAs regulate various cellular and physiological processes. There is a link between miRNAs and various diseases such as cancer, hypertension, diabetes, and acquired immune deficiency syndrome (AIDS) [10–17]. The expression of miRNA was found to be upregulated or downregulated in different diseases. Hence, miRNA class is a significant class of post-transcriptional gene expression regulator.
In the present review, we described few important characteristics of CSCs. We also illustrated miRNA regulation self-renewal and differentiation properties of CSC.
Important characteristics of CSC
CSCs are a term for cancer cells that have the ability to self-renew. These cells give rise to other malignant stem cells. These cells undergo differentiation process, resulting in non-tumorigenic cancer cells, which are phenotypically different. To date, the mother cell of origin for CSCs remains unclear and it has not been definitively determined yet. These CSCs may or may not be derived from their normal stem cell.
Several mutations are necessary for a cell to become cancerous
Multiple mutations are required for a cell to become cancerous, and this becomes necessary for the cellular origin of cancer cells [18, 19] (Fig. 2). Mutations may occur during the short life span of these cells. To maintain the disease line, cancer cells must overcome the genetic constraints on both self-renewal as well as propagation [20]. CSCs possess the capability to self-renew. These cells are consequent either from self-renewing like normal stem cells (which may be produced by altering proliferative pathways) or from progenitor cells. These stem cells have obtained self-renewal capacity as a result of oncogenic mutations. The traditional outlook of cancer is a clonal genetic model. According to this model, cancer cell arises through a series of mutations. The mutation also occurs in oncogenes and tumor suppressor genes, which cause the selective overgrowth of a population of tumor cells. This mutation helps to develop the different properties like invasiveness, metastasis, and drug resistance. A model described that a series of epigenetic changes are necessary for mutations, which help to give rise to tumor proliferation, invasion, metastasis, and drug resistance [21, 22]. However, the mutations must accumulate in stem cells in the tissue where cancers commonly occur and it was noted that cancers commonly occur in the blood, gut epithelium, and skin. Due to the accumulation effect of the mutations, progenitors and differentiated cells tend to have a short life span, and on the other hand, the stem cells persist throughout life in these tissues.
Small population of cell within the cancer cell population which proliferates extensively
Scientists have applied the theory of stem cell biology for tumorigenesis. They describe cancer as a clonally derived population of cells which has the propagative capacity, and it is just like cells within normal tissues. Previously, it was established that when different types of cancer cells were assayed for their proliferative potential, only a small minority of cells were able to proliferate extensively [23, 24]. Therefore, the idea that malignant tumors include both CSC with huge proliferative potential and more differentiated cancer cells with restricted proliferative potential was formed [23, 24]. Anticancer agents may cause apoptosis of cancer cells, but CSCs might survive and regenerate the cancer again (Fig. 3). This is a famous model reproducible in teratocarcinoma, which showcases the totipotency of CSC. On the other hand, it was not clear that whether this model could be applied to describe more common cancers. In short, there is a coexistence of the undifferentiated and differentiated cells in tumor.
CSCs and self-renewal activity
Some postulate that a tumor originates from CSCs derived either from transformed tissue stem cells or transformed progenitor cells that have regained self-renewal capability. As CSCs could self-renew, they should be derived from self-renewing normal stem cells. For instance, leukemic stem cells have a surface marker phenotype similar to normal hematopoietic stem cells, which confirms that they arise from hematopoietic stem cells [25]. Several studies have demonstrated that leukemogenic mutation increases the proliferation of hematopoietic stem, and it blocks the differentiation of normal hematopoietic stem/progenitor cells [26, 27]. Phenotypic differences between leukemia stem cells and hematopoietic stem cells have been recorded [28, 29]. This finding supports the view that the initial mutations occur in hematopoietic stem cells, which change their phenotypes. Many researchers depend exclusively on cell lines for oncogene expression. However, efforts should be invested to determine the effects of oncogene expression on the phenotype and the function of normal stem/progenitor cells, instead of depending solely on cell lines.
CSCs—made by cell fusion
CSCs possess several characteristics of normal stem cells. These features are self-renewal capacity, cell cycle (slow), cell differentiation capacity, enhanced resistance development for different cytotoxic mediators, and radiation. Recently, Dittmar et al. put forward the hypothesis of “oncogenic resistance” [30]. A new type of tumor-initiating cells, the so-called recurrence CSCs, is proposed. They are linked to increased drug resistance, apoptosis, and enhanced malignancy with cell fusion. They concluded that recurrence CSCs originate from the cellular event. However, tumor tissues consist of a variety of cells including tumor cells, CSCs, monocytes, macrophages, and bone marrow-derived stem cells. The characteristic of the tumor is linked to these cells, which is related to the property of cell fusion. This property is called “fusogenic characters.” These characters will then lead to the origin of repetition CSCs. These cells even survive after the first-line therapy. This survival may occur due to the natural selection, and it may follow the Darwinian evolution [31].
miRNAs regulate self-renewal and differentiation in CSCs
miRNAs regulate the maintenance of the stem cell line. Recent experiments showed that the knockout of DGCR8 and Dicer1 led to a defect in stem cell differentiation in murine embryonic stem (ES) cells. This shows that miRNAs play important roles in stem cells [32, 33]. Six major factors are required for the effect of pluripotency maintenance. They are such as Nanog, Sox2, Oct4, KLF4 Lin28, and c-Myc [34–37]. It has been observed that several miRNAs such as miR-470, miR-296, and miR-134 may inhibit the self-renewing factors, such as Oct4, Sox2, and Nanog [38]. Conversely, miR-145 helps in cell differentiation through mRNA targeting of KLF4, Sox2, and Oct4 [39]. On the other hand, it has been noted that let-7 suppresses the expression of Lin28 [40]. Inhibition of differentiation in ES by c-Myc is achieved via the miR-200 family [41].
A study by Yu et al. showed that the expression of miRNA let-7 was reduced in breast cancer CSCs [42]. Inducing the expression of this miRNA suppressed the proliferation of cancer cells and tumor formation in non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mice. In vitro self-renewal of non-CSCs was enhanced when let-7 was antagonized. The tumor-suppressing effects of let-7 were achieved via downregulation of H-RAS and HMGA2 [42].
There is a decrease in the stem cell (CD133+) population compared to the non-stem (CD133) cell population in glioblastoma cells. The researchers showed that the upregulation of miR-451 inhibited neurosphere formation and cell growth and finally reduced the tumor growth. The action of miR-451 might be exerted via SMAD signaling [43]. Therefore, it can be concluded that microRNAs regulate the self-renewal and differentiation of CSC [44].
Migration of CSC and tumor formation
Migration of cell is one of the important processes during the development of multicellular organisms [31]. However, data on CSC migration is very limited. Brabletz et al. developed a “migrating CSC” model, which described cancer cell migration [45]. The concept of this model is based on the existence of two forms of CSCs, which helps in tumor progression. These two forms of cancer cells are “stationary CSCs” and “mobile CSCs.” Stationary CSCs are motionless and always surrounded by the epithelial tissues. These cells are active in initiating the precursor lesions.
Adenoma is an example, in which its development covers all the steps of tumor development, but the CSCs do not spread during that time. According to Brabletz et al., the mobile CSCs are located at the tumor–host interface [45]. These cells begin to form CSCs by getting hold of transient epithelial-to-mesenchymal transition (EMT), which is responsible for the property of stemness. A tumor cell has two most important characteristics, which are stemness and mobility, and these two traits hold significant hints for the further perceptive of malignant progression. These cells are migrating CSCs [45]. The collapse of epithelial cell homeostasis leads to migration of CSCs causing destructive cancer progression. Lastly, these cells have lost its epithelial characteristics and acquired migratory phenotype. This phenomenon is an important phenomenon for CSC, known as the EMT [46]. This phenomenon can help to transmit and to spread the cancer cells and also keep on hold the task of stem cell. It can also form metastatic colonies. In colorectal cancer, Brabletz et al. characterized mobile CSC population, whereby they observed that the cell population can overexpress nuclear β-catenin [45]. The isolated tumor cells also overexpress β-catenin at the tumor–host interface. These cell populations were considered as migrating CSCs. These populations were associated with metastasis. They have very low survival time. Its phenomenon has been noted by several authors [45, 47, 48]. This migrating CSC concept provides the explanation for malignant tumor growth and metastasis occurrence. It also assimilated the current tumor initiation and progression concept. However, all these concepts were related to accumulations of genetic alterations in a tumor atmosphere, which were the responsible driving forces for CSC.
It is difficult to obtain the confirmation of the reality of CSC in solid tumors for three major reasons, which are the following: (i) solid tumors are not easy to access, (ii) practical assays suitable for identification and quantification of normal stem cells from many organs have not yet been developed, and (iii) specific cell surface markers necessary to isolate such cells have not been identified [1]. Recently, few remarkable reports were published in this area which included the complete regeneration of a complete mammary gland from a single mouse mammary cell. Cells identified from human breast tumors can cause breast cancer in NOD/SCID mice, which suggest the ability for self-renewal. These cells with CD44+ or CD24− were recorded low in eight of the nine patients, and the tumors were recognized in recipient animals. In that experiment, 100 cells were transplanted. On the other hand, tens of thousands of breast cancer cells with a different marker set failed to induce tumors [49–52]. In another experiment, brain tumor stem cells were identified and they caused brain tumor in NOD/SCID mice. These tumor cell populations had CD133+ marker, a marker found on normal neural stem cells. In this experiment, it was found that the transplantation of 100 CD133+ tumor cells was sufficient to begin the development of a tumor in recipient animals [53, 54]. In recent times, from human prostate cancer, cells that may cause prostate tumors in NOD/SCID mice have been identified. Cells expressing CD44 were identified from prostate tumor cells [1, 56]. Using flow cytometry, Hurt et al. identified a population of prostate cells with the characteristic CD44+/CD24− [55]. These cells showed stem cell characteristics. It forecasted survival in prostate cancer patients. CD44+/CD24− prostate cells are stem-like cells accountable for tumor introduction. Hurt et al. provided a genomic definition of these cells and the distinguished cells that they gave rise to [56]. These data were consistent with the CSC hypothesis.
A report was published by Zaidi et al. regarding the origin of brain tumor stem cell (BTSC) hypotheses [57]. In the hypothesis, the researchers stated about the clinical implication of BTSC and its origin. Furthermore, the hypothesis described that the fatal characteristics of human brain tumors can be initiated by BTSC, which were resistant to chemotherapy and radiotherapy treatment [58]. These BTS cells possess the ability to invade the local brain parenchyma. However, the origin of these BTS cells is still controversial. The emergence of these tumor-initiating cells could be described by three steps. First, adult glial cell (in BTSC) obtains mutations, thus possessing unregulated “stem cell”-like characteristics. Second, there are some restricted neural progenitor cells. These cells acquired mutations after successive divisions and give them unregulated stem cell-like characteristics. Third, the mature cells are regulated by intrinsic regulations for their division and proliferation. These cells provide them a tumorigenic property after obtaining mutations [59, 60].
Glial cells act as the precursors of tumor-initiating BTSCs. Recently, few studies were performed to reprogram adult skin cells to induce pluripotent stem (ips) cells [61–64]. These studies used some nuclear reprogramming factors. These studies suggested that cells had stem cell-like properties, which were dormant and could be experimentally endowed. The question arises whether a similar process occurs during brain tumor pathogenesis or not. Various experiments used animal models for tumor generation. These study models established that cortical astrocytes could be targeted with oncogenes or signaling proteins. In these studies, the glioma-like histological structure was found [65–72]. Bachoo et al. performed an experiment where researchers used rodents with Ink-4a-Arf knockout mature glial cells. Ink-4a-Arf is cell cycle regulator gene. Gliomas were developed as a result of the study [65]. Furthermore, the overexpression of a c-Myc gene in astrocytes results in the downregulation of astrocytic cell type marker glial fibrillary acidic protein and the upregulation of BTSC marker nestin [73].
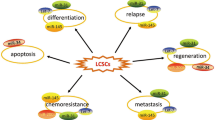
Lastly, the introduction of a gene into astrocytes resulted in the overexpression of platelet-derived growth factor (a molecule that maintains glial precursors in an undifferentiated condition), which helped informed gliomas [74]. All these mentioned findings suggest that differentiated brain cells can obtain stem cell-like properties. On the other hand, the brain cells also have the capability to initiate tumor formation. The regulatory functions of miRNAs on CSCs were summarized (Fig. 4).
miRNA regulates different CSC functions
Recent studies concluded that various CSC-associated cancers were regulated by miRNAs (Table 1). Few examples are stated below:
Prostate CSCs
miRNA controls the prostate CSC functions. Liu et al. illustrated that miR-34a suppressed prostate CSCs via CD44. They found that enforced expression of miR-34a in prostate cancer cells with CD44+ inhibited tumor regeneration and metastasis. The effects of knocking down CD44 were found to be similar to overexpression of miR-34a. Administration of miR-34a increased the survival of prostate cancer-bearing mice and retarded the progression of cancer [75]. The results of this study were reproduced by Li et al., showing that miR-34a reduced the level of CD44 expression in tumors [76]. In another study by Chang et al., it was concluded that miR-7 controlled the stemness of prostate cancer stem-like cells. It suppressed the CSC properties and tumorigenesis through inhibiting the KLF4/PI3K/Akt/p21 pathway [77]. Zoni et al. showed that expression of miR-25 was low in prostate CSCs. Overexpression of miR-25 affected the cytoskeleton of cancer cell responsible for invasion, particularly the expression of αv- and α6-integrin through communication with the 3′-untranslated regions of the genes involved [78].
Head and neck CSCs
miRNA microarray study revealed that the lower expressions of miR-424, let-7a, miR-6836, miR-6873, miR-7152, and miR-147b were overexpressed in CSCs in subtype of head and neck squamous cell carcinomas with high aldehyde dehydrogenase 1 [79]. Sun et al. illustrated that miRNA34a reduced epithelial–mesenchymal transition, aldehyde dehydrogenase activity, invasiveness, and clonogenicity of CSC of head and neck squamous cell carcinoma. This implied that the therapeutic modulation of miR-34a in CSCs in head and neck squamous cell carcinomas might reduce the rate of metastasis [80].
Breast CSCs
miRNA also regulates the breast CSC functions. Shimono et al. indicated that 37 microRNA expressions were altered in human breast tumors containing breast cancer stem cell (BCSC). Among the 37 miRNAs, three clusters were downregulated, such as miR-200c-141, miR-200b-200a-429, and miR-183-96-182. miR-200c targeted BMI1, a regulator of stem cell renewal. They also showed that miR-200c suppressed clonal expansion, growth, and differentiation of CSCs [81]. Feng et al. found that miR-200c suppresses self-renewal of breast and mammary epithelial stem cells. This miRNA also regulated programmed cell death 10 protein responsible for cell death and angiogenesis in both stem cells [82].
Colorectal CSCs
Recent data suggested that colorectal CSCs are also regulated by miRNA. Hwang et al. showed that miRNA-146a regulated the snail-dependent symmetric division of colorectal CSCs. Snail-miR-146a–β-catenin loop was significant in the symmetric division of colorectal CSCs. Its disruption inhibited the MEK or Wnt activity and reduced the symmetrical division of colorectal CSCs [83]. Xu et al. showed that expression of miR-328 was low in side population of colorectal CSCs. Overexpression of miR-328 suppressed properties of side population cells, such as invasiveness and drug resistance. The direct targets of miR-318 were found to be ABCG2 and MMP16. The researchers concluded that miR-328 might be a potential target for effective CRC therapy [84]. Bitarte et al. found that miR-451 suppressed the self-renewal and tumorigenicity of colorectal CSCs. These actions were contributed to its suppression on cyclooxygenase-2 responsible for tumor growth and ATP-binding cassette drug transporter ABCB1 responsible for sensitization to anticancer agent. This study concluded that miR-451 was related to recurrence and drug resistance in colorectal cancer, and it could be used as a marker to forecast response of patients with colon carcinoma toward anticancer agents [85].
Lung CSCs
Several miRNAs have been found to regulate lung CSCs. A recent study found that miRNA-200b exerted inhibitory effects on human lung adenocarcinoma cancer stem-like cells by reversing the maintenance and chemoresistance of these cells. It was shown that HDAC1/miR-200b/Suz-12-E-cadherin signaling was accounted for this property [86]. Shi et al. illustrated that miR-34a was a negative regulator of the tumor-regenerating properties of CD44hi stem-like non-small cell lung cancer cells (NSCLC). On the other hand, antagonist of miR-34a promoted growth of tumor in CD44lo cells. This suggested that CD44 was the target of miR-34a, and it served as a novel therapeutic agent against NSCLC [87].
Gastric CSCs
Liu et al. demonstrated the miRNA expression profile of gastric CSCs using miRNA microarray techniques on MKN-45 cancer cell line. In this experiment, an array with 1887 human miRNAs probes was used to understand the expression profiles of the gastric CSCs. They found that nine miRNAs were upregulated and 173 were downregulated, indicating that most of the miRNAs possessed tumor-suppressing activity [88].
Pancreatic CSCs
miRNA controls the function of pancreatic CSCs. Ma et al. observed that overexpression of miR-200c decreased clonogenicity, invasiveness, and chemoresistance of human pancreatic CSCs. These inhibitory activities were attributed to downregulation of zinc-finger E-box binding homeobox 1 (ZEB1) and Vimentin and upregulation of E-cadherin. miR-200c also inhibited epithelial–mesenchymal transition in nude mice. It was a potential therapeutic target for pancreatic CSCs [89, 90].
Leukemia-associated CSCs
There is a profound link between the miRNA and leukemia-associated CSC function. Babashah et al. showed that the relationship between Smos links miRNA-326 and CD34+ CML stem/progenitor cells. Overexpression of miRNA-326 decreased the proliferation and increased the apoptosis of CD34+ cells by downregulating Hh smoothened (Smo) signal transducer. Restoration of Smo level reversed these effects. Therefore, miR-326 is a therapeutic target for leukemia [91].
Liver CSCs
An association has been noted between the miRNA and the function of liver CSCs. Zhang et al. illustrated that miRNA-150 inhibited human CD133+ liver cancer stem by inhibiting the cell growth and formation of tumorsphere and inducing cell cycle arrest and apoptosis. This miRNA worked via interacting with the mRNA of the transcription factor c-Myb [92]. Liu et al. found that miR-155 promoted liver CSC self-renewal property indicated by an increase in the proportion of CD90+ and CD133+ cells. miR-155 regulated liver CSC via TP53INP1 [93].
Ovary CSCs
Several miRNAs control the characteristics and functions of ovary CSCs. miRNA-17 helped CSC development as well as normal ovarian cancer cell development. It was shown that the miRNA-17 suppressed the LKB1-p53-p21/WAF1 pathway [94]. miRNA-98 promoted ovarian cancer stem (OCS) cell proliferation via enhancer of zeste homolog 2 protein in regulating other transcription factors important in cell proliferation. Inhibition of this miRNA caused cell cycle arrest in OCS by upregulation of p21 and downregulation of CDK2/cyclin E and c-Myc. These activities were conveyed through the pRb-E2F pathway [95]. These findings helped the identification of novel therapeutic targets in human ovarian cancer.
Conclusion
miRNA-controlled CSC possesses few properties of CSC. The miRNA-controlled CSC has significant implications for future studies aimed at diagnosis of cancer and identification of individuals at risk. Better understanding of miRNA-controlled CSC may help us to develop better cancer therapy. Further studies on the miRNA-related origin and migration of CSC related to tumorigenic population may shed considerable light on the miRNA-controlled metastasis process. This knowledge may allow for the development of diagnostic kits that enable us to identify patients at risk for metastatic disease. More insights about the miRNA-controlled stem cell may identify the origin, mechanism of self-renewal, and migration of CSC. This knowledge of miRNA-controlled CSCs may significantly alter the landscape of oncology.
References
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells—perspectives on current status and future directions: ACCR workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–44.
Baker M. Cancer stem cells, becoming common. Nat Rep Stem Cells. 2008. doi:10.1038/stemcells.2008.153.
Sell S, Pierce GB. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab Investig. 1994;70(1):6–22.
Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895–902.
Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22(3):457–72.
Han L, Shi S, Gong T, Zhang Z, Sun X. Cancer stem cells: therapeutic implications and perspectives in cancer therapy. Acta Pharm Sin B. 2013;3(2):65–75.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–24.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4(3):143–59.
Chakraborty C, George Priya Doss C, Bandyopadhyay S. miRNAs in insulin resistance and diabetes-associated pancreatic cancer: the ‘minute and miracle’ molecule moving as a monitor in the ‘genomic galaxy’. Curr Drug Targets. 2013;14(10):1110–7.
Carè A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13(5):613–8.
Mishima Y, Stahlhut C, Giraldez AJ. miR-1-2 gets to the heart of the matter. Cell. 2007;129(2):247–9.
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, Zhang Y, Xu C, Bai Y, Wang H. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13(4):486–91.
Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and in mice lacking miRNA-1-2. Cell. 2007;129(2):303–17.
Naraba H, Iwai N. Assessment of the microRNA system in salt sensitive hypertension. Hypertens Res. 2005;28(10):819–26.
Chakraborty C, Doss CG, Bandyopadhyay S, Agoramoorthy G. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip Rev RNA. 2014;5(5):697–712.
Huang J, Wang F, Argyris E, Chen K, Liang Z, Tian H, Huang W, Squires K, Verlinghieri G, Zhang H. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4 T lymphocytes. Nat Med. 2007;13(10):1241–7.
Knudson Jr AG, Strong LC, Anderson DE. Heredity and cancer in man. Prog Med Genet. 1973;9:113–58.
Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5320–5.
Morrison SJ, Qian D, Jerebek L, Thiel BA, Park I-K, Ford PS, Kiel MJ, Schork NJ, Weissman IL, Clarke MF. A genetic determinant that specifically regulates the frequency of hematopoietic stem cells. J Immunol. 2002;168:635–42.
Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7:21–33.
Nowell PC. The clonal nature of neoplasia. Cancer Cells. 1989;1:29–30.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11.
Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–3.
Schmid M, Haaf T, Grunert D. 5-Azacytidineinduced undercondensations in human chromosomes. Hum Genet. 1984;67:257–63.
Pereira DS et al. Retroviral transduction of TLS-ERG initiates a leukemogenic program in normal human hematopoietic cells. Proc Natl Acad Sci U S A. 1998;95:8239–44.
Kelly LM, Gilliland DG. Genetics of myeloid leukemias. Annu Rev Genomics Hum Genet. 2002;3:179–98.
Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997;89:3104–12.
Jordan CT, Upchurch D, Szilvassy SJ, et al. The interleukin-3 receptor α chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–84.
Dittmar T, Nagler C, Schwitalla S, Reith G, Niggemann B, Zänker KS. Recurrence cancer stem cells—made by cell fusion? Med Hypotheses. 2009;73:542–7.
Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69.
Kanellopoulou C, Muljo SA, Kung ALT, et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005;19:489–501.
Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet. 2007;39:380–5.
Chakraborty C, Agoramoorthy G. Stem cells in the light of evolution. Indian J Med Res. 2012;135(6):813–9.
Chakraborty C, Roy SS, Hsu JM, Agoramoorthy G. Network analysis of transcription factors for nuclear reprogramming into induced pluripotent stem cell using bioinformatics. Cell J. 2014;15(4):332–9.
Roy SS, Hsu CH, Wen ZH, Lin CS, Chakraborty C. A hypothetical relationship between the nuclear reprogramming factors for induced pluripotent stem (iPS) cells generation—bioinformatic and algorithmic approach. Med Hypotheses. 2011;76(4):507–11.
Chakraborty C, Shah KD, Cao WG, Hsu CH, Wen ZH, Lin CS. Potentialities of induced pluripotent stem (iPS) cells for treatment of diseases. Curr Mol Med. 2010;10(8):756–62.
Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–8.
Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–58.
Peter ME. Let-7 and miR-200 microRNAs: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–52.
Lin CH, Jackson AL, Guo J, Linsley PS, Eisenman RN. Myc-regulated microRNAs attenuate embryonic stem cell differentiation. EMBO J. 2009;28:3157–70.
Yu F, Yao H, Zhu P, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109–23.
Gal H, Pandi G, Kanner AA, Ram Z, Lithwick-Yanai G, Amariglio N, Rechavi G, Givol D. MIR-451 and Imatinib mesylate inhibit tumor growth of glioblastoma stem cells. Biochem Biophys Res Commun. 2008;376(1):86–90. doi:10.1016/j.bbrc.2008.08.107.
Wang ZM, Du WJ, Piazza GA, Xi Y. MicroRNAs are involved in the self-renewal and differentiation of cancer stem cells. Acta Pharmacol Sin. 2013;34(11):1374–80. doi:10.1038/aps.2013.134.
Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–9.
Thiery JP. Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–6.
Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. 2002;40:127–32.
Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC. A new prognostic staging system for rectal cancer. Ann Surg. 2004;240:832–9.
Dick JE. Breast cancer stem cells revealed. Proc Natl Acad Sci U S A. 2003;100:3547–9.
Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005;7:86–95.
Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–8.
Farnie G, Clarke RB. Breast stem cells and cancer. In: Wiestler OD, Haendler B, Mumberg D, editors. Cancer stem cells: novel concepts and prospects for tumor therapy. Berlin Heidelberg: Springer; 2007. p. 141–54.
Ward RJ, Dirks PB. Cancer stem cells: at the headwaters of tumor development. Annu Rev Pathol. 2007;2:175–89.
Chong YK, Toh TB, Zaiden N. Cryopreservation of neurospheres derived from human glioblastoma multiforme. Stem Cells. 2009;27:29–39.
Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98:756–65.
Donnenberg VS, Luketich JD, Landreneau RJ, et al. Tumorigenic epithelial stem cells and their normal counterparts. In: Wiestler OD, Haendler B, Mumberg D, editors. Cancer stem cells: novel concepts and prospects for tumor therapy. Berlin Heidelberg: Springer; 2007. p. 245–63.
Zaidi HA, Kosztowski T, DiMeco F, Quiñones-Hinojosa A. Origins and clinical implications of the brain tumor stem cell hypothesis. J Neuro-Oncol. 2009;93:49–60.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60.
Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206.
Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A. Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neuro-Oncol. 2008;89:219–24.
Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6.
Okita K, Ichisaka T, Yamanaka S. Generation of germ-line-competent induced pluripotent stem cells. Nature. 2007;448:313–7.
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–24.
Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–77.
Ding H, Roncari L, Shannon P, Wu X, Lau N, Karaskova J, Gutmann DH, Squire JA, Nagy A, Guha A. Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas. Cancer Res. 2001;61:3826–36.
Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–85.
Rich JN, Guo C, McLendon RE, Bigner DD, Wang XF, Counter CM. A genetically tractable model of human glioma formation. Cancer Res. 2001;61:3556–60.
Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61:6674–8.
Uhrbom L, Kastemar M, Johansson FK, Westermark B, Holland EC. Cell type-specific tumor suppression by Ink4a and Arf in Kras-induced mouse gliomagenesis. Cancer Res. 2005;65:2065–9.
Weiss WA, Burns MJ, Hackett C, Aldape K, Hill JR, Kuriyama H, Kuriyama N, Milshteyn N, Roberts T, Wendland MF, DePinho R, Israel MA. Genetic determinants of malignancy in a mouse model for oligodendroglioma. Cancer Res. 2003;63:1589–95.
Xiao A, Yin C, Yang C, Di Cristofano A, Pandolfi PP, Van Dyke T. Somatic induction of Pten loss in a preclinical astrocytoma model reveals major roles in disease progression and avenues for target discovery and validation. Cancer Res. 2005;65:5172–80.
Lassman AB, Dai C, Fuller GN, Vickers AJ, Holland EC. Overexpression of c-MYC promotes an undifferentiated phenotype in cultured astrocytes and allows elevated Ras and Akt signaling to induce gliomas from GFAP-expressing cells in mice. Neuron Glia Biol. 2004;1:157–63.
Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–25.
Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211–5.
Li J, Lam M. Reproducibility Project: Cancer Biology. Registered report: the microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Elife. 2015;4:e06434. doi:10.7554/eLife.06434.
Chang YL, Zhou PJ, Wei L, Li W, Ji Z, Fang YX, Gao WQ. MicroRNA-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21 pathway. Oncotarget. 2015;6(27):24017–31.
Zoni E, van der Horst G, van de Merbel AF, et al. miR-25 modulates invasiveness and dissemination of human prostate cancer cells via regulation of αv- and α6-integrin expression. Cancer Res. 2015;75:2326–36.
Yata K, Beder LB, Tamagawa S, Hotomi M, Hirohashi Y, Grenman R, Yamanaka N. MicroRNA expression profiles of cancer stem cells in head and neck squamous cell carcinoma. Int J Oncol. 2015. doi:10.3892/ijo.2015.3145.
Sun Z, Hu W, Xu J, Kaufmann AM, Albers AE. MicroRNA-34a regulates epithelial-mesenchymal transition and cancer stem cell phenotype of head and neck squamous cell carcinoma in vitro. Int J Oncol. 2015. doi:10.3892/ijo.2015.3142.
Shimono Y, Zabala M, Cho RW, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603. doi:10.1016/j.cell.2009.07.011.
Feng ZM, Qiu J, Chen XW, et al. Essential role of miR-200c in regulating self-renewal of breast cancer stem cells and their counterparts of mammary epithelium. BMC Cancer. 2015;15:645.
Hwang WL, Jiang JK, Yang SH, Huang TS, Lan HY, Teng HW, Yang CY, Tsai YP, Lin CH, Wang HW, Yang MH. MicroRNA-146a directs the symmetric division of Snail-dominant colorectal cancer stem cells. Nat Cell Biol. 2014;16:268–80. doi:10.1038/ncb2910.
Xu XT, Xu Q, Tong JL, Zhu MM, Nie F, Chen X, Xiao SD, Ran ZH. MicroRNA expression profiling identifies miR-328 regulates cancer stem cell-like SP cells in colorectal cancer. Br J Cancer. 2012;106:1320–30. doi:10.1038/bjc.2012.88.
Bitarte N, Bandres E, Boni V, et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells. 2011;29:1661–71. doi:10.1002/stem.741.
Chen DQ, Huang JY, Feng B, et al. Histone deacetylase 1/Sp1/microRNA-200b signaling accounts for maintenance of cancer stem-like cells in human lung adenocarcinoma. PLoS One. 2014;9(10):e109578. doi:10.1371/journal.pone.0109578. .eCollection 2014
Shi Y, Liu C, Liu X, Tang DG, Wang J. The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PLoS One. 2014;9(3):e90022. doi:10.1371/journal.pone.0090022. .eCollection 2014
Liu J, Ma L, Wang Z, Wang L, Liu C, Chen R, Zhang J. MicroRNA expression profile of gastric cancer stem cells in the MKN-45 cancer cell line. Acta Biochim Biophys Sin Shanghai. 2014;46(2):92–9. doi:10.1093/abbs/gmt135.
Ma C, Ding YC, Yu W, Wang Q, Meng B, Huang T. microRNA-200c overexpression plays an inhibitory role in human pancreatic cancer stem cells by regulating epithelial-mesenchymal transition. Minerva Med 2015;106(4):193–202.
Ma C, Huang T, Ding YC, Yu W, Wang Q, Meng B, Luo SX. microRNA-200c overexpression inhibits chemoresistance, invasion and colony formation of human pancreatic cancer stem cells. Int J Clin Exp Pathol. 2015;8:6533–9 .eCollection 2015
Babashah S, Sadeghizadeh M, Hajifathali A, Tavirani MR, Zomorod MS, Ghadiani M, Soleimani M. Targeting of the signal transducer Smo links microRNA-326 to the oncogenic Hedgehog pathway in CD34+ CML stem/progenitor cells. Int J Cancer. 2013;133:579–89. doi:10.1002/ijc.28043.
Zhang J, Luo N, Luo Y, Peng Z, Zhang T, Li S. microRNA-150 inhibits human CD133-positive liver cancer stem cells through negative regulation of the transcription factor c-Myb. Int J Oncol. 2012;40(3):747–56. doi:10.3892/ijo.2011.1242.
Liu F, Kong X, Lv L, Gao J. MiR-155 targets TP53INP1 to regulate liver cancer stem cell acquisition and self-renewal. FEBS Lett. 2015;589(4):500–6. doi:10.1016/j.febslet.2015.01.009.
Liu T, Qin W, Hou L, Huang Y. MicroRNA-17 promotes normal ovarian cancer cells to cancer stem cells development via suppression of the LKB1-p53-p21/WAF1 pathway. Tumour Biol. 2015;36(3):1881–93. doi:10.1007/s13277-014-2790-3.
Liu T, Hou L, Huang Y. EZH2-specific microRNA-98 inhibits human ovarian cancer stem cell proliferation via regulating the pRb-E2F pathway. Tumour Biol. 2014;35:7239–47. doi:10.1007/s13277-014-1950-9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Funding
UKM DLP grant-2014-009.
Rights and permissions
About this article
Cite this article
Chakraborty, C., Chin, KY. & Das, S. miRNA-regulated cancer stem cells: understanding the property and the role of miRNA in carcinogenesis. Tumor Biol. 37, 13039–13048 (2016). https://doi.org/10.1007/s13277-016-5156-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5156-1