Abstract
Several phase III clinical trials had authenticated that the addition of bevacizumab to paclitaxel plus carboplatin or gemcitabine plus cisplatin showed encouraging efficacy as first-line therapy for advanced NSCLC patients. However, the benefits of adding bevacizumab to other chemotherapy regimens in first- or second-line therapy have not been reported. To compare the clinical efficacy and safety of bevacizumab concomitant with chemotherapy regimens in patients with advanced NSCLC as first- or second-line therapy, we retrospectively reviewed the effects of adding bevacizumab to chemotherapy regimens in naive-chemotherapy and pre-chemotherapy patients with advanced non-squamous NSCLC. A total of 79 patients with advanced non-squamous NSCLC received at least two cycles of bevacizumab with chemotherapy between October 2010 and December 2013 were selected. Our primary end points were overall response rate (ORR) and disease control rate (DCR). The secondary objective was overall survival (OS) and safety. Seventy-nine patients were included in this study. Overall response rates at first evaluation (after 2 cycles) were 23.1 % (9/39) and 5.0 % (2/40) in first- and second-line therapy (P = 0.020), respectively. And disease control rates were 84.6 % (33/39) and 50 % (20/40), respectively (P = 0.001). The median OS were 27.2 months (95 % CI 13.3–41.1 months) and 29.6 months (95 % CI 6.7–52.5 months), respectively (P = 0.740). Grade 3–4 adverse events included leukopenia (2/39), and neutropenia (3/39) in first-line therapy versus neutropenia (1/40) and thrombocytopenia (2/40) in second-line treatment. In our experience, combination of bevacizumab and chemotherapy had encouraging anti-tumor efficacy as both first- and second-line therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer has become the primary cause of cancer-related death whether in developing or developed countries recently [1]. Probably 80 % lung cancer cases were diagnosed as non-small-cell lung cancer (NSCLC). For the last decade, adding bevacizumab to platinum-based chemotherapy regimens as first-line treatments had dramatically prolonged OS and DFS, with a median PFS of 6 to 7 months and median OS of 12 to 24 months [2–4]. Markedly, the BEYOND trial reveals that bevacizumab plus paclitaxel and carboplatin led to a significant treatment outcome in first-line treatment with a median survival surpassing 24 months in Chinese patient population. These results suggest that bevacizumab in combination with platinum-based chemotherapy obtains significant survival benefits for bevacizumab-eligible patients with advanced non-squamous NSCLC.
However, patients will finally undergo disease progression and have to accept further treatment, even though obtained temporary disease control with first-line treatment firstly. In chemotherapy-experienced patients with NSCLC, erlotinib, gefitinib, gemcitabine, docetaxel, and pemetrexed are recommended in second-line therapy as single antitumor agents [5–9]. Meantime, several studies demonstrated that bevacizumab combined with chemotherapy would benefit patients with previously treated NSCLC who obtained a median overall survival of approximately 9 months [10–12]. The phase II trial SWOG Study N0426 [11] to evaluate the efficacy of toxicity of pemetrexed combined with bevacizumab as second-line treatment for patients with advanced NSCLC had reported a median overall survival and disease-free survival of 8.6 months and 4.0 months, respectively. Moreover, another phase II study of oxaliplatin, pemetrexed, and bevacizumab in previously treated advanced NSCLC reported a median OS of 12.5 months and a median PFS of 5.8 months [12]. These studies showed that adding bevacizumab to second-line treatment increased overall response rate and enhanced disease-free survival.
Currently, pemetrexed or docetaxel plus bevacizumab as second-line treatment were mostly common because of their low adverse effects. However, the trials were only researched in first- or second-line setting, respectively. There was lack of evidence to investigate the efficacy and safety of them between first- and second-line treatment. Herein, we retrospectively analyzed these regimens for NSCLC patients in our Cancer Center.
Materials and methods
Patients characteristic
A retrospective screening of the medical records in Sun Yat-Sen University Cancer Center was conducted to identify advanced non-squamous NSCLC patients treated with bevacizumab and chemotherapy as first- or second-line therapy. A total of 79 patients between October 2010 and December 2013 were enrolled. To eligible for this study, all patients were ≥18 years old, with histologically or cytologically diagnosed with advanced non-squamous. The stage of disease was stage IIIB (T4N3M0) or IV based on the AJCC Cancer Staging Manual (7th Edition). Clinic variables including age, sex, ECOG PS, smoking history, smoking index, stage, EGFR status, ALK status, and chemotherapy regimens were recorded by an electronic medical record system. Blood sample at baseline and before each cycle of treatment should be collected for the measurement of white blood cell count, absolute neutrophil count, hematoglobin, and platelet count. We also recorded the changes of transaminase levels.
Assessments
Tumor response was evaluated by computed tomography scans according to Response Evaluation Criteria in Solid Tumor (RECIST) criteria 1.1 [13]. Disease control was defined as complete remission (CR), partial remission (PR), or stable disease (SD). Patients who had a progression disease after two cycles of treatment were defined as progression disease (PD). PFS was defined as time between the start of the treatment and disease progression or death. OS was defined as time between the diagnosis of advanced NSCLC and last contact or death. Toxicity was graded according to the United States National Cancer Institute’s common toxicity criteria (version 2.0).
Statistics analysis
All of the statistics analyses were performed using SPSS version 13.0 (SPSS Institute, Inc). All of the tests were two-sided, and P < 0.05 was considered statistically significant. The categorical variables and the response rates were expressed as the number of patients and compared using the chi-square test or Fisher exact test. Because chi-square test or Fisher exact test could be used to compare rates or proportions between to two independent samples. Estimates of OS and PFS were calculated using the Kaplan-Meier method and two-sided 95 % confidence interval were obtained. Significant differences between groups were identified using the log-rank test by calculating the P value.
Results
Patients characteristics
From October 2010 to December 2013, 79 patients are available in our study. The clinical characteristics of these patients are listed in Table 1. All the patients received at least two cycles of bevacizumab plus chemotherapy. Data was frozen on May 1st, 2015. The median age was 54.0 years (ranging from 31 to 79 years), with 64.1 % of male patients, 84.6 % of patients having PS of 0 or 1 at baseline, 51.3 % of nonsmokers, 94 % of patients having stage IV disease and 5.1 % being stage IIIB (T4N3M0), 20.5 % of patients being EGFR mutant type and 7.7 % of patients being ALK mutant type in first-line therapy. Meanwhile, the median age was 55.0 years (ranging from 29 to 72 years), with 57.5 % of male patients, 97.5 % of patients having PS of 0 or 1 at baseline, 60.0 % of nonsmokers, 95 % of patients having stage IV disease and 5.0 % being stage IIIB (T4N3M0), 27.5 % of patients being EGFR mutant type and 5.0 % of patients being ALK mutant type in first-line therapy.
Treatment
The median number of treatment cycles delivered was 4 (range 1–8) and 3 (range 1–10) in first- and second-line therapy, respectively. All the chemotherapy regimens were offered in Table 2 and all the chemotherapy regimens were combined with bevacizumab. In first-line therapy, 33 patients had received pemetrexed plus platinum regimens (18:cisplatin; 15:carboplatin) and 6 patients had received pemetrexed single agent. In second-line therapy, 12 patients had received pemetrexed plus platinum regimens (8:cisplatin; 4:carboplatin) and 17 patients had received pemetrexed monotherapy. In addition, 9 patients accepted docetaxel with platinum (1:nedaplatin; 1:cisplatin; 3:carboplatin) and 4 patients had received docetaxel monotherapy. Two patients followed the gemcitabine + cisplatin protocol.
Treatment consisted in bevacizumab (7.5 mg/kg), pemetrexed (500 mg/m2) with or without cisplatin (75 mg/m2), or bevacizumab (7.5 mg/kg), docetaxel (75 mg/m2) with or without cisplatin (75 mg/m2), or carboplatin (AUC = 6) as an alternative of cisplatin, and bevacizumab (7.5 mg/kg), gemcitabine (1250 mg/m2) with cisplatin (75 mg/m2). All the regimens were 21-day cycles.
Follow-up
The follow-up time was the beginning of diagnoses to end of loss to follow-up or May 2015. The median follow-up time was 20.5 months (range 3.2–52.3 months) and 21.3 months (range 5.4–64.8 months) in first- and second-line therapy, respectively.
Efficacy
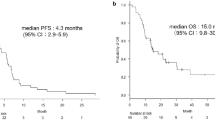
All the 79 patients included in the study were analyzed for efficacy. Among the 79 patients, assessments of response at first evaluation (after 2 cycles) were PR in 9 patients (23.1 %) and 2 patients (5.0 %), SD in 24 patients (61.5 %) and 18 patients (45.0 %), and PD in 0 patients (0 %) and 14 patients (35.0 %) in first- and second-line therapy, respectively (Table 3). Moreover, there were 3 patients (3/39) obtained confirmed partial response rate in first-line therapy. Equivalently, overall response rate was 23.1 % (9/39) and 5.0 % (2/40) and disease control rate was 84.6 % (33/39) and 50.0 % (20/40) in first- and second-line therapy, with both P value lower than 0.05, respectively (Table 3). The median OS were 27.2 months (95 % CI 13.3–41.1 months) and 29.6 months (95 % CI 6.7–52.5 months), respectively (P = 0.740) (Fig. 1). Records of follow-up were not sufficient for PFS analysis in both first- and second-line therapy.
Adverse events
Main hematologic toxicities possibly related to therapy were listed in Table 4. Adverse events of these chemotherapeutical regimens were generally mild, mainly ranging from grade 1 to grade 2. Grade 3–4 adverse events included leukopenia (2/39; 5.2 %), and neutropenia (3/39; 7.7 %) in first-line therapy and neutropenia (1/40; 2.5 %) versus thrombocytopenia (2/40; 5.0 %) in second-line treatment. There was not a great influence on hypopatia. No patients experienced drug-related deaths during our records.
Discussion
It was no incontrovertible that the efficacy of bevacizumab in combination with chemotherapy significantly prolonged both OS and PFS, compared with chemotherapy alone, in chemotherapy naive patients with non-squamous NSCLC. However, there was no robust evidence certifying the differences about bevacizumab adhere to chemotherapy between first-line and second-line treatment. In our study, we found that pemetrexed-containing chemotherapy plus bevacizumab as first-line regimen yielded a 23.1 % partial response rate, a 7.7 % confirmed partial response rate. And the median OS was 27.2 months (95 % CI 3.3 to 41.1 months,). Meanwhile, in second-line treatment, chemotherapy combined with bevacizumab obtained a 5.0 % partial response rate, with a median OS of 29.6 months (95 % CI 6.7 to 52.5 months). These data showed that the addition of bevacizumab to second-line treatment also provided significant advantages of treatment for patients with advanced non-squamous NSCLC, consistent with that seen in conducted trials [11, 12, 14]. In addition, bevacizumab showed increased efficacy in previously treated advanced NSCLC when combined with erlotinib. It was illustrated that the response rates of bevacizumab plus erlotinib were 51.3 %, and the median PFS and OS was 4.4 and 13.7 months, respectively [15]. The patients of our study in second-line setting were mostly accepted EGFR-TKIs treatment. So, the longer OS possibly was attributed to the reason that more than 20 % patients who harbor activating mutations in EGFR had been treated with EGFR-TKIs in their lifetime. Moreover, the PR rate in second-line therapy was low, which maybe ascribed the larger proportion of regimens were monotherapy plus bevacizumab.
Standard second-line treatments for patients with advanced NSCLC were mainly single agents. A phase III trials revealed that therapy with pemetrexed led to isovalent treatment effect compared with docetaxel in the second-line treatment of patients with advanced NSCLC and the latter should be considered a standard treatment option [16, 17]. So our results would not be affected by the inconsistent regimens in second-line treatment. Moreover, it was noticed that 10 patients also obtained stable disease after first evaluation in second-line treatment of bevacizumab in combination with pemetrexed who receipted pemetrexed plus platinum in first-line treatment. Hence, maybe patients who were intractable to pemetrexed still achieve the treatment effect from bevacizumab in combination with pemetrexed. These studies suggested that bevacizumab made patients with advanced non-squamous NSCLC in second-line or beyond settings enjoy benefits. In multivariable analysis, there were no distinction in OS when stratified by gender, PS, smoking history, EGFR mutation status, and ALK mutation status. But the analysis revealed that age effected overall survival, that was to say, younger people could gain most benefits from treatment, in line with many phase III trials. It is so regretful that the follow-up was not sufficient for PFS analysis because the censored data of progression-free survival. A majority of patients’ status were kept track by telephone. The exact time of death could be offered, but not the time of relapse, as imaging information was incomplete.
Our study showed encouraging findings. Moreover, treatment with the combination of pemetrexed or docetaxel and bevacizumab was well tolerated. Toxicities were also manageable, which rarely produced grade 3 or higher adverse events. These results were possibly due to the good performance status and young population (age ≤60 years and ECOG ≤1 accounts for 69.5 and 91.4 % respectively). Maybe overwhelming majority were never or light smokers contributed to the good OS. And we demonstrated an association between poor OS and pack-years in multivariate analysis. Toxicities of this combination treatment were generally tolerable, in keeping with the AVAPEAL study [18].
Nevertheless, our study has several limitations, including its single-center, retrospective design. The sample size is small, which limits the generalizability of our findings. However, this is the first study to investigate the differences of bevacizumab combined with chemotherapy between first- and second-line therapy in patients with advanced NSCLC, it may be helpful for clinical doctors in making decisions.
In conclusion, the combination of bevacizumab with monotherapy or doublets obtained better overall response rate as well as disease control rate for chemotherapy naive patients with non-squamous NSCLC when compared to those previously treated. Non-inferiority was confirmed for over survival when compared adding bevacizumab in first-line therapy with which in second-line therapy. More clinical trials are needed to further elaborate the relationship between these regimens and advanced NSCLC in second-line or beyond setting.
Clinical practice points
It is no incontrovertible that the use of bevacizumab in combination with chemotherapy significantly prolonged both OS and PFS in first-line therapy in patients with NSCLC, compared with chemotherapy alone. Our study demonstrated that bevacizumab in combination with chemotherapy in second-line therapy in NSCLC had also obtained a good disease control rate. It may be helpful for clinical doctors to make a decision.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33:2197–204.
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAiL. J Clin Oncol. 2009;27:1227–34.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50.
Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–18.
Shepherd FA, Rodrigues PJ, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32.
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O’Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103.
Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62.
Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, et al. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002;20:4285–91.
Liu KJ, Wu HY. A retrospective analysis of cisplatin, pemetrexed, and bevacizumab in previously treated non-small-cell lung cancer. Oncotarget 2015.
Adjei AA, Mandrekar SJ, Dy GK, Molina JR, Adjei AA, Gandara DR, et al. Phase II trial of pemetrexed plus bevacizumab for second-line therapy of patients with advanced non-small-cell lung cancer: NCCTG and SWOG study n0426. J Clin Oncol. 2010;28:614–9.
Heist RS, Fidias P, Huberman M, Ardman B, Sequist LV, Temel JS, et al. A phase II study of oxaliplatin, pemetrexed, and bevacizumab in previously treated advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:1153–8.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Ding L, Liu K, Jiang Z, Chen Q, Zhou N, Liang Y, et al. The efficacy and safety of pemetrexed plus bevacizumab in previously treated patients with advanced non-squamous non-small cell lung cancer (ns-NSCLC). Tumour Biol. 2015;36:2491–9.
Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25:4743–50.
Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97.
Weiss JM, Stinchcombe TE. Second-line therapy for advanced NSCLC. Oncologist. 2013;18:947–53.
Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer Tur N, Kim JH, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol. 2013;31:3004–11.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Microabstract
We performed the retrospective study to compare the clinical efficacy and safety of bevacizumab concomitant with chemotherapy regimens in patients with advanced NSCLC as first- or second-line therapy. In our experience, combination of bevacizumab and chemotherapy had encouraging anti-tumor efficacy as both first- and second-line therapy.
Rencui Quan and Jiaxing Huang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Quan, R., Huang, J., Chen, N. et al. A retrospective analysis of efficacy and safety of adding bevacizumab to chemotherapy as first- and second-line therapy in advanced non-small-cell lung cancer (NSCLC). Tumor Biol. 37, 11479–11484 (2016). https://doi.org/10.1007/s13277-016-5031-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5031-0