Abstract
Transforming growth factor (TGF)-β1 is a significant stimulator of tumor invasion and metastasis. More recently, it has been found that TGF-β1 acts through microRNAs to regulate their target genes to promote cancer progresses. However, such similar regulation is rarely reported in colorectal cancer (CRC). Here, we observed a decrease in TGF-β1 expression in CRC specimens, compared with matched adjacent normal tissues. In parallel, there was an increase in miR-130b characterized in the same samples by microarray assay. Further, treatment of CRC cells with TGF-β1 caused a significant decrease in the expression of miR-130b and an increased CRC cell migration. Luciferase reporter assay revealed that miR-130b directly targeted the 3′ untranslated region (3′UTR) region of integrin α5 gene, which encodes a key molecule involved in cell motility. Subsequently, in the overexpression of miR-130b CRC cells, we observed a decreased level of integrin α5 protein. The regulation of integrin α5 by miR-130b was further shown using the miR-130b mimics and inhibitor of miR-130b. And, knockdown miR-130b with inhibitor in the overexpression of miR-130b CRC cells recovered integrin α5 expression and integrin α5-mediated cell motility. Moreover, the inverse relevance between miR-130b and integrin α5 was also observed in CRC specimens. At last, the enhancement of integrin α5 in TGF-β1-treated cells can be reversed partly when rescuing miR-130b expression. Together, our findings suggested that TGF-β1 acted through miR-130b to promote integrin α5 expression, resulting in the enhanced migration of CRC cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is one of the most common diagnosed cancers and the fourth leading cause of cancer death worldwide [1]. Metastasis causes death in many cancers, including CRC [2–4]. Therefore, a better understanding of the underlying molecular mechanisms causing metastasis would be beneficial to the development of effective therapeutic strategies for CRC. Transforming growth factor (TGF)-β1 is a polypeptide with multifunction and regulates various biological processes including proliferation, apoptosis, and differentiation [5, 6]. It is well known that TGF-β1 can strongly stimulate metastasis of tumor cells [7]. Cancer cells secrete abundant TGF-β1 then respond to it, resulting in an enhanced metastasis both in vitro and in vivo [8, 9]. Previous studies suggested that the expression of TGF-β1 is excess in CRC and is associated with cancer progression [10]. However, the mechanism of TGF-β1 in CRC is not well understood.
MicroRNAs (miRNAs) are endogenous single-stranded, small (22 nucleotides on average), non-coding RNAs. It is well known that miRNAs play crucial roles in regulation of gene expression by binding to the 3′ untranslated region (3′UTR) of their target messenger RNA (mRNAs) to inhibit protein translation or to induce mRNA degradation [11, 12]. Accumulating evidence shows that miRNAs influence a lot of cancer-related processes such as metastasis. More importantly, microRNAs have been already utilized as diagnostic biomarkers and therapeutic targets in the clinic trial [13]. Recently, a systemic meta-analysis of 33 comparisons, containing 4000 tumor and matched normal tissues, reported that miR-130b is a new tumor-related miRNA and is significantly dysregulated in cancers [14]. Inhibition of miR-130b by deficiency of TAp63 leads to increased metastasis in the TAp63 knockout mice [15]. In addition, the suppression of miR-130b by p53 mutant can enhance the ability of invasion mediated with ZEB1 in endometrial cancer cells [16]. These data suggested that miR-130b has a significant inhibitory impact on motility of cancer cells.
Recently, studies from independent laboratories demonstrated that TGF-β1 can regulate some microRNAs and their target genes in various diseases [17–19]. The latest report suggested that miR-130b modulated by TGF-β1 plays key roles in the pathogenesis of diabetic nephropathy (DN) [17]. Interestingly, a study reported that gastric cancer cells treated with TGF-β1 express 847 human miRNAs, only three microRNAs are significantly downregulated, and miR-130b is one of them [20]. Moreover, we previously investigated the microRNA expression using microRNA microarray profiling of colorectal tumors and matched adjacent normal tissue samples and characterized that miR-130b is ranked on the top four position of dysregulated miRNAs in CRC [21]. However, the relation of TGF-β1 and miR-130b in colorectal cancer is rarely reported. In the study, we observed decreased miR-130b and increased integrin α5 in TGF-β1-treated CRC cells. To gain insight on the relations of TGF-β1, miR-130b, and integrin α5 in CRC, we investigated these three important functional molecules in CRC cells and CRC specimens. Our data suggested that integrin α5 was a new target gene of miR-130b and TGF-β1 acted, at least in part, through miR-130b to promote integrin α5 expression, resulting in the enhanced migration of CRC cells.
Materials and methods
Clinical specimens
Colorectal cancer and matched adjacent normal tissue specimens were obtained from 39 patients at Beijing Hospital, Ministry of Health (Beijing, China) after surgical resection. The tumor tissues and matched adjacent normal tissues were frozen in liquid nitrogen after resection. No patient in the current study received chemotherapy or radiation therapy before the surgery. The clinicopathologic features of 39 patients were shown in Supplementary Table 1. All patients provided informed consent form for using their tissues, according to the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Beijing Institute of Geriatrics, Ministry of Health.
Cell culture
The human colorectal cancer cell line SW480 was purchased from the Cell Resource Center, IBMS, CAMS/PUMS and passed in less than 6 months. SW480 cells that stably overexpressed miR-130b (Lenti-miR-130b cells) and the respective control cells (Lenti-vector cells) were constructed as described previously [21]. Cells were cultured in RPMI-1640 (Gibco, Paisley, UK) with 10 % FBS (Gibco, Paisley, UK), 2 mmol/L L-glutamine (Gibco, Paisley, UK), 100 U/mL of penicillin (Gibco, Paisley, UK), and 100 μg/mL of streptomycin sulfate (Gibco, Paisley, UK). The lentiviral vector that we generated expressed primary miR-130b, and it requires microRNA biogenesis to generate mature microRNAs in cells. The stable cell lines were maintained in complete growth medium in the presence of 1 mg/mL hygromycin (Roche Applied Science, Mannheim, Germany). Recombinant human TGF-β1 was purchased from Invitrogen (Carlsbad, USA) and used at a final concentration of 10 or 20 ng/mL.
RNA reversed transcription and quantitative real-time PCR assays
The total RNA, including small RNA, was extracted from the clinical specimens or from the CRC cells and subjected to reverse transcription. Quantitative real-time PCR (qRT-PCR) was performed using the SYBR Premix Ex Taq mix (TaKaRa, Dalian, China) according to the manufacturer’s instructions, and the samples were run on an iQ5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, USA). Thermal reaction cycles of 95 °C for 30s and 45 repetitions of 95 °C for 5 s and 60 °C for 20s were used. The primers used for qRT-PCR were as follows:
TGF-β1 forward 5′-GGACACCAACTATTGCTTCAG-3′; TGF-β1 reverse 5′-TCCAGGCTCCAAATGTAGG-3′; integrin α5 gene (ITGA5) forward 5′-AATTTGACAGCAAAGGCTCTCGGC-3′; ITGA5 reverse 5′-ACCACTGCAAGGACTTGTACTCCA-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) forward 5′-CAACAGCCTCAAGATCATCAGCA-3′; GAPDH reverse 5′-TGGCATGGTCTGTGGTCATGAGT-3′; hsa-miR-130b forward 5′-GCCGCCAGTGCAATGATGAA-3′; hsa-miR-130b reverse 5′-GTGCAGGGTCCGAGGT-3′; U6 forward 5′-CGCTTCGGCAGCACATATACTA-3′; U6 reverse 5′-CGCTTCACGAATTTGCGTGTCA-3′.
3′UTR reporter vector and Luciferase reporter assay
The wild-type full-length 3′UTR fragment of the ITGA5 was amplified by PCR from human genomic DNA. The seed sequences of miR-130b on 3′UTR of ITGA5 were predicted by PICTAR5 (http://pictar.mdc-berlin.de/). The predicted seed sequences were deleted from 3′UTR of ITGA5 using overlapping PCR method. The primers are as follows: ITGA5 forward 5′-GCTCAGCTCAAGCCTCCAGCCACC-3′; ITGA5 overlapping reverse 5′-GTAAACAAGGGTCCTTCTGGGGGGGAGGGAT-3′; ITGA5 overlapping forward 5′-ATCCCTCCCCCCCTGAAGGACCCTTGTTTAC-3′; ITGA5 reverse 5′-ATGAGGGGCAGCATGCTGGCAGGCAGA-3′.
The SacI-XhoI-digested products were cloned into a pmirGlo Dual-Luciferase miRNA Target Expression Vector (Promega, Madison, USA) to form 3′UTR-luciferase reporter vector. The SW480 cells were cotransfected in 24-well plates using Lipofectamine 2000 (Invitrogen, Carlsbad, USA) with 3′UTR-luciferase reporter vector and the indicated miRNAs. Twenty-four hours after transfection, firefly and Renilla luciferase activities were measured consecutively using Dual-Luciferase Reporter Assay System (Promega, Madison, USA) with INFINITE 200 Pro multimode reader (TECAN, Mannedorf, Switzerland). The firefly luciferase activity was normalized by Renilla luciferase activity for transfection efficiency.
Cell transfection
The hsa-miR-130b mimics, hsa-miR-130b inhibitor (anti-miR-130b), and control mimics were synthesized by Ribobio (Guangzhou, China). The sequences used were as follows: hsa-miR-130b mimics 5′-CAGUGCAAUGAUGAAAGGGCAU-3′, hsa-miR-130b inhibitor 5′-AUGCCCUUUCAUCAUUGCACUG-3′. siRNA against integrin α5 was purchased from Santa Cruz Biotechnology (Santa Cruz, USA). The SW480 cells were transfected using RNAiMax (Invitrogen, Carlsbad, USA) or Lipofectamine 2000 (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions.
Cell migration assay
Cell migration assay was evaluated using transwell chambers (8 μm, BD Bioscience, San Jose, USA). Cells (5 × 105) were placed into the upper chamber of each insert coated with 20 μg/mL fibronectin (FN) (Invitrogen, Carlsbad, USA), and 500 μL of complete medium was added to the bottom well. The cells that had not migrated were removed from the upper surfaces of the filters using cotton swabs, and the cells that had migrated to the lower surfaces of the filters were fixed with 4 % paraformaldehyde solution and stained with 0.1 % crystal violet. Images of three random fields were captured from each membrane, and the number of migratory cells was counted.
Western blotting
Proteins were separated by SDS-PAGE and transferred to a PVDF membrane (Millipore, Billerica, USA). The membrane was blocked with 5 % non-fat milk and incubated with rabbit anti-integrin α5 (1/1000, Santa Cruz Biotechnology, Santa Cruz, USA) and mouse anti-GAPDH (1/10000, Sigma, St. Louis, USA) antibodies.
Immunofluorescent staining
The cells were grown on cover glasses, fixed with 4 % paraformaldehyde for 10 min. After incubation with 5 % BSA for 1 h, the cells were stained with an antibody against integrin α5 (1/200, Santa Cruz Biotechnology, Santa Cruz, USA) overnight at 4 °C followed by incubation with Alexa Fluor 488 donkey anti-rabbit antibody (1/1000, Invitrogen, Carlsbad, USA) for 1 h. DNA was stained using 1 μg/mL DAPI (Invitrogen, Carlsbad, USA). The fluorescent signal was detected by a confocal microscopy (A1 Confocal Laser Microscope System, Nikon, Japan).
Statistical analysis
Data were processed using SPSS 16.0. Data that follow normal distribution were denoted as the mean ± SD. A comparison between data of the two groups was performed using Student’s t test (two-tailed distribution). The expression data of TGF-β1, miR-130b, and integrin α5 of 39 human colorectal cancers and matched adjacent normal samples which do not follow normal distribution were processed by Wilcoxon signed-rank test. Spearman’s rho was used to measure the correlation between the expression of TGF-β1, miR130b and integrin α5 because they may not be normally distributed. Two-tailed P value <0.05 was defined as statistic significant.
Results
TGF-β1 decreased the expression of miR-130b and promoted migration of CRC cells
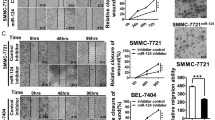
Based on the microRNA expression profiles in three colorectal tumors and adjacent normal tissue samples, we reported using the Affymetrix’s GeneChip microRNA array (GEO accession number GSE53592); we found that miR-130b was consistently upregulated in all three CRC specimens, compared to matched adjacent normal tissues (Fig. 1a). We then used qRT-PCR to confirm the miR-130b expression in these CRC specimens. Consistent with the microarray readouts shown in Fig. 1a, the qRT-PCR results showed the enhanced expression of miR-130b in the CRC specimens (Fig. 1b). Because recent studies have indicated that miR-130b can be modulated by TGF-β1 in diabetic nephropathy [17] as well as gastric cancer [20], we further examined TGF-β1 expression in these CRC specimens and observed the decreased TGF-β1 in the same tumor tissues (Fig. 1b). Next, we tested whether miR-130b can be regulated by TGF-β1 in CRC cells (the human colon cancer cell line SW480 cells). The levels of miR-130b from the SW480 cells treated with TGF-β1 were analyzed with qRT-PCR (Fig. 1c). We found that in contrast to controls, TGF-β1 (10 and 20 ng/mL) for 24 h caused about 0.4-fold decrease and about 0.36-fold decrease in miR-130b expression levels, respectively. To further test the relationship between TGF-β1 and miR-130b, we extended our analysis in 39 human colorectal tumor tissues through examining the expression levels of miR-130b and TGF-β1 by qRT-PCR. We found that an inverse relevance between miR-130b and TGF-β1 expression existed in 36 of 39 (92 %) tumor tissues (Fig. 1d).
TGF-β1 reduced the expression of miR-130b and promoted cell migration. a Relative expressions of miR-130b (normalized to U6) in tumors over matched adjacent normal tissues (T/N), using the Affymetrix’s GeneChip miRNA array chip V1.0. The tumors were referred to T1, T2 and T3. The matched adjacent normal tissues were referred to N1, N2 and N3. b Relative expressions of miR-130b (normalized to U6) and TGF-β1 (normalized to GAPDH) in tumors over matched adjacent normal tissues (T/N) determined by qRT-PCR (mean ± SD; n = 3). c qRT-PCR analysis of relative expressions of miR-130b (normalized to U6) in SW480 cells treated without or with 10 and 20 ng/mL TGF-β1 for 24 h (mean ± SD; n = 3; *, P < 0.05). d Heat map diagram indicated the expression of TGF-β1 and miR-130b in 39 colorectal cancer tissues detected by qRT-PCR. The tumors were referred to T1 to T39 and were shown in columns. The expression of TGF-β1 and miR-130b were shown in rows. Expression data were adjusted by mean center and natural logarithm using Cluster 3.0. Red and green color represented a high and low expression, respectively. The relative levels of expression were indicated using a color scale. The Spearman’s rho of negative correlation was −0.47 (P value = 0.008). e Transwell migration assays of SW480 cells treated without or with 10 ng/mL TGF-β1 for 24 h (scale bar = 100 μm; mean ± SD; n = 3; *P < 0.05). Representative images of migrated cells were shown (a–b). qRT-PCR analysis of relative expressions of miR-130b (normalized to U6) in SW480 cells from the same pool of cells utilized in a (mean ± SD; n = 3; *P < 0.05) (c)
It is well known that TGF-β1 can strongly stimulate the metastasis of tumor cells [8]. We next subjected SW480 cells to migration assay to examine the effect of TGF-β1 on cell migration of CRC cells. An evident increase in cell migration was observed after 24 h of 10 ng/mL TGF-β1 treatment (Fig. 1e (a–b)) and 20 ng/mL TGF-β1 treatment (Supplementary Fig. 1). As shown in Fig. 1e (c), a decrease in miR-130b was also observed in the same pool of cells subjected for migration assay in Fig. 1e (a). Moreover, previous studies have reported that miR-130b has a notably inhibitory effect on migration of various types of cancer cells [15, 16, 21]. Taken together, we hypothesized that miR-130b and its target might involve cell migration process stimulated by TGF-β1 in CRC cells.
miR-130b targeted integrin α5 3′UTR
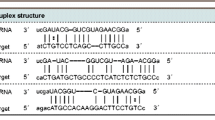
To test our hypothesis, we firstly predicated miR-130b potential targets using PicTar; the result showed that integrin α5, a key molecule involved in cell motility, was a putative target of miR-130b (Fig. 2a). The integrin family members are heterodimeric transmembrane receptors for ECM and have many important functions, such as development, cell proliferation, apoptosis, and malignant transformation [22]. The FN receptor integrin α5 is one of representative receptors and has key roles in tumor metastasis [23]. Our previous studies have shown that the post-translational modification has extensive implications in integrin α5-mediated cell migration [24]. In this study, we found that integrin α5 has conserved binding sites for miR-130b (Fig. 2a). As shown in Supplementary Fig. 2, the putative binding sites with the seed region of miR-130b were at 187–193 and 902–908 bp of integrin α5 3′UTR, respectively. To determine whether miR-130b directly targets integrin α5 in CRC cells, we conducted luciferase reporter assays. The pmirGlo Dual-Luciferase Reporter Vector containing the full-length wild-type 3′UTR of integrin α5 gene was shown in Fig. 2b. We co-transfected the cells with pmirGlo vector carrying the wild-type integrin α5 3′UTR and miR-130b mimics. The result exhibited significantly lower activity of luciferase than that of the cells transfected with the same luciferase reporter plasmid and control microRNA mimics (NC) (Fig. 2c). To further confirm the target binding between integrin α5 3′UTR and miR-130b, mutagenesis assays were performed. The effect of miR-130b on luciferase activity was eliminated when the predicated binding sites of miR-130b were deleted from the 3′UTR of integrin α5 (Fig. 2b, c). Together, these data showed that miR-130b targeted integrin α5.
miR-130b directly targeted integrin α5. a Predicted duplex formation between hsa-miR-130b (top) and integrin α5 3′UTRs (bottom). Potential complementary residues were shown in bold. Sequence of the miR-130b-binding sites within the integrin α5 3′UTR of Homo sapiens (Human), Mus musculus (Mouse), Pan troglodytes (Chimpanzee), and Canis lupus familiaris (Dog) are highly conserved. b The potential binding sites of integrin α5 3′UTR with the seed region of miR-130b (an exact 7-nt match underlined). The WT integrin α5 3′UTR contained a putative binding site with the seed region of miR-130b, which was deleted in Mut integrin α5 3′UTR (upper panel). Schematic diagram showing pmirGlo Dual-Luciferase Reporter Vector with a 1049 bp fragment of the WT integrin α5 3′UTR, which contains two miR-130b binding sites (position 187–193 bp and position 902–908 bp) (middle panel). Mutant integrin α5 3′UTR where two putative miR-130b binding sites were deleted (lower panel). “X” represented deletion. c The pmirGlo Dual-Luciferase Reporter Vector containing either a WT 3′UTR or a mutant 3′UTR was co-transfected with miR-130b mimics (miR-130b) or control miR mimics (NC), respectively. The firefly luciferase activity was measured and normalized to Renilla luciferase activity (mean ± SD; n = 12; **P < 0.01)
Downregulation of integrin α5 expression by miR-130b
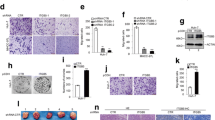
Next, to further confirm the regulation of miR-130b to integrin α5, we tested the integrin α5 protein level in the CRC cells transfected with miR-130b mimics and miR-130b inhibitor (anti-miR-130b), respectively (Fig. 3a, b). miR-130b mimics are chemically synthesized and designed to mimic endogenous mature miR-130b. The results showed that the expression of integrin α5 was suppressed after transfection with miR-130b mimics (Fig. 3a). Knockdown endogenous miR-130b using miR-130b inhibitor (anti-miR-130b) boosted integrin α5 expression (Fig. 3b). We further examined the regulation of miR-130b to integrin α5 expression in SW480 cells, which stably overexpresses miR-130b (Lenti-miR-130b cells) and the respective control cells (Lenti-vector cells) (Fig. 3d). Results showed that endogenous integrin α5 protein levels in two Lenti-miR-130b cell lines were decreased by approximate 64 and 42 %, respectively, compared with that of the Lenti-vector cells (Fig. 3c). As integrin family is transmembrane proteins, we then performed immunofluorescence staining to show the expression of integrin α5 with confocal microscopy. Consistent with data in Fig. 3a, b, c, reduced integrin α5 expression was observed in Lenti-miR-130b cells compared to the Lenti-vector cells (Fig. 3e (a)), and increased integrin α5 expression was observed in miR-130b inhibitor-treated cells compared to the control cells (Fig. 3f (a)). Moreover, as shown in Fig. 3e (b), f (b), similar changes in integrin α5 expression also exhibited in the same pool of cells subjected for immunofluorescence staining in Fig. 3e (a), f (a), respectively. Together, our data suggested that integrin α5 was a novel target gene of miR-130b and miR-130b can directly downregulate integrin α5 expression in CRC cells. To further test the relationship between miR-130b and integrin α5, we performed qRT-PCR to examine the expressions of miR-130b and integrin α5 in 39 colorectal tumor tissues. As shown in Fig. 3g, an inverse relevance between miR-130b and integrin α5 expression was found in 35 of 39 (90%) clinical specimens.
miR-130b suppressed the expression of integrin α5. a, b Western blot analyses of integrin α5 expression in the SW480 cells transfected with NC or miR-130b mimics (a) and miR inhibitor control (anti-NC) or miR-130b inhibitor (anti-miR-130b) (b). Densitometry analysis of the Western blot data normalized with GAPDH (mean ± SD; n = 4; *P < 0.05). c Western blot analyses of integrin α5 protein expression in Lenti-control cells and two Lenti-miR-130b cell lines (referred to 1, 2). Densitometry analysis of the Western blot data normalized with GAPDH (mean ± SD; n = 4; *P < 0.05, **P < 0.01). d qRT-PCR analyses of relative expressions of miR-130b (normalized to U6) in Lenti-control cells and two Lenti-miR-130b cell lines (referred to 1, 2) from the same pool of the cells utilized in panel c (mean ± SD; n = 4; *P < 0.05, **P < 0.01). e, f Immunofluorescence staining of integrin α5 (green) in Lenti-control cells and Lenti-miR-130b cells (e (a)) and immunofluorescence staining of integrin α5 (green) in miR-130b inhibitor-treated SW480 cells (anti-miR-130) and control-treated cells (anti-NC) (f (a)) by confocal microscopy demonstrated expression of integrin α5. Overlay of integrin α5 (green) and nuclear 4,6-diamidino-2-phenylindole (DAPI; blue) staining of the same filed. Scale bar = 50 μm. e (b) and f (b), Western blot analysis of integrin α5 expressions in the corresponding cells from the same pool of cells utilized in e (a) or f (a). g Heat map diagram indicated the expression of miR-130b and integrin α5 in 39 colorectal cancer tissues detected by qRT-PCR. The tumors were referred to T1 to T39 and were shown in columns. The expressions of miR-130b and integrin α5 were shown in rows. Expression data are adjusted by mean center and natural logarithm using Cluster 3.0. Red in the color bar indicated higher expression, and green indicated lower expression. The Spearman’s rho of negative correlation was −0.42 (P value = 0.021)
TGF-β1 increased the expression of integrin α5 through inhibition of miR-130b in CRC cells
Given that TGF-β1 induced a decrease in miR-130b and miR-130b suppressed its target-integrin α5 expression in CRC cells, we then test whether TGF-β1 can promote integrin α5 expression in CRC cells. As shown in Fig. 4a, TGF-β1 (10 and 20 ng/mL) for 24 h caused approximately 0.57-fold increase and 0.3-fold increase in integrin α5 expression levels in SW480 cells, respectively. Next, enhancement of integrin α5 by TGF-β1 can be reversed by about 30 % after transfection of miR-130b mimics (Fig. 4b), suggesting that TGF-β1 increased the expression of integrin α5 through inhibition of miR-130b. Moreover, integrin α5 expression was recovered by about 72% when treated the overexpression of miR-130b CRC cells with miR-130b inhibitors (Fig. 4c). Overexpression of miR-130b led to a decrease in cell migration of two CRC cell lines (Fig. 4d), and such decrease can be rescued after treatment of miR-130b inhibitors (Fig. 4e). Moreover, the knockdown of integrin α5 also led to a decrease in cell migration of SW480 cells (Fig. 4f, g). All these data above indicated that miR-130b suppressed its target-integrin α5 expression, resulting in inhibition of integrin α5-mediated migration of CRC cells.
TGF-β1 increased the expression of integrin α5 through decreasing miR-130b in CRC cells. a Western blot analyses of integrin α5 expression in SW480 cells treated without or with 10 and 20 ng/mL TGF-β1 for 24 h. Densitometry analysis of the Western blot data normalized with GAPDH (mean ± SD; n = 4; *P < 0.05). b Western blot analyses of integrin α5 expression in SW480 cells treated without or with 10 ng/mL TGF-β1 for 24 h then transfected with either NC or miR-130b mimics. Densitometry analysis of the Western blot data normalized with GAPDH (mean ± SD; n = 3; *P < 0.05). c Western blot analyses of integrin α5 expression in Lenti-miR-130b cells after transfection of miR inhibitor control (anti-NC) or miR-130b inhibitor (anti-miR-130b). Densitometry analysis of the Western blot data normalized with GAPDH (mean ± SD; n = 3; *P < 0.05). d Transwell migration assays of Lenti-control cells and Lenti-miR-130b cells (scale bar = 100 μm; mean ± SD; n = 4; *P < 0.05, **P < 0.01). Representative images of migrated cells were shown. e Transwell migration assays of Lenti-miR-130b cells after transfection of miR inhibitor control (anti-NC) or miR-130b inhibitor (anti-miR-130b) (scale bar = 100 μm; mean ± SD; n = 3; *P < 0.05). Representative images of migrated cells were shown. f Western blot analyses analyzed the expression of integrin α5 in SW480 cells transfected with a siRNA against integrin α5 (α5 siRNA) or negative control siRNA (scramble) at 100 nM. Densitometry analysis of the Western blot data normalized with GAPDH (mean ± SD; n = 4; **P < 0.01). g Transwell migration of SW480 cells transfected with α5 siRNA or scramble at 100 nM (scale bar = 100 μm; mean ± SD; n = 4; **P < 0.01). Representative images of migrated cells are shown
We further extended our analysis by comparing tumors to normal tissues to detect the relationship of these three molecules in 39 pairs of clinical specimens. The inverse relevance between TGF-β1 and miR-130b-integrin α5 axis was observed in 18 out of 39 pairs of these clinical specimens (Fig. 5a). Compared to the matched adjacent control tissues (N), ten colorectal cancer tissues (T) consistently showed increased TGF-β1 (P = 0.005), decreased miR-130b (P = 0.005), and increased integrin α5 (P = 0.005) (Fig. 5b). Conversely, eight colorectal cancer tissues (T) showed decreased TGF-β1 (P = 0.008), increased miR-130b (P = 0.018), and decreased integrin α5 (P = 0.008) (Fig. 5c).
Inverse relevance between TGF-β1 and miR-130b-integrin α5 axis in CRC specimens. a The relationship of TGF-β1, miR-130b, and integrin α5 in 39 pairs of clinical specimens were examined, and the inverse relevance between TGF-β1 and miR-130b-integrin α5 axis was observed in 18 out of 39 pairs of clinical specimens. The upward arrows referred to the increased expression level in tumor (T) compared to that of matched normal tissue (N). The downward arrows referred to the decreased expression level in tumor (T) compared to that of matched normal tissue (N). b, c Box plots indicated median, 25th, and 75th percentiles. Whiskers represented 5th and 95th percentiles. “o” represented outlier and “asterisk” represented extreme values. P values were from Wilcoxon signed-rank test. b Compared to the matched adjacent control tissues (N), ten colorectal cancer tissues (T) showed consistently increased TGF-β1, decreased miR-130b, and increased integrin α5. c Eight colorectal cancer tissues (T) showed decreased TGF-β1, increased miR-130b, and decreased integrin α5
Taken together, our findings suggested that TGF-β1 increased integrin α5, at least in part, through inhibition of miR-130b, resulting in enhanced motility of CRC cells.
Discussion
In this study, we observed that TGF-β1 decreased the expression of miR-130b in CRC cells and increased cell migration (Fig. 1). miR-130b has been revealed as an anti-metastatic miRNA and is significantly dysregulated in various types of cancers: gastric cancer [25, 26], cutaneous malignant melanoma [27], head and neck squamous cell carcinoma [28], and bladder cancer [29]. As mentioned before, in the TAp63 knockout mouse model, downregulation of miR-130b by the deficiency of TAp63 led to an increase in cancer metastasis [15]. The inhibition of miR-130b by a p53 mutant caused the enhanced invasion of endometrial cancer cells [16]. On the contrary, it is well known TGF-β1 acts as a significant stimulator of tumor invasion and metastasis underlying the tumor progresses [8]. Moreover, accumulating data suggested that TGF-β1 exerted the regulation of some microRNAs and their key target genes in some diseases, including cancers [30]. A latest study suggested that TGF-β1 seems to downregulate the post-transcriptional activity of miR-130b host gene RIK, therefore suppressed the expression of miR-130b during the pathogenesis of diabetic nephropathy [17]. miR-130b is located at the intron of the non-coding RNA, 2610318N02RIK (RIK), and its expression is dependent on the transcription of RIK gene. The study further suggested that TGF-β1 downregulated three functional NF-YCs, which bind to the promoter of RIK to regulate the post-transcriptional activity of the RIK gene [17]. Thus, understanding the mechanism by which TGF-β1 downregulates NF-YC, RIK, and miR-130b would be beneficial in the further elucidating the importance of such regulation in CRC.
miRNAs exert important functions by targeting their target genes. Here, we identified that integrin α5 was a novel target of miR-130b. Integrin α5 has been known to play crucial roles in promoting tumor metastasis [23]. Our data showed a decreased level of integrin α5 protein due to overexpression of miR-130b in CRC cells; miRNA mimic and inhibitor experiments further indicated that miR-130b downregulated the expression of integrin α5 (Fig. 3). The luciferase reporter assays showed that the binding of miR-130b to the 3′UTR of integrin α5 was specific, as mutant 3′UTR vector lacking the miR-130b binding sites did not respond to miR-130b (Fig. 2). Moreover, knockdown miR-130b in miR-130b overexpression of CRC cells recovered integrin α5 expression and its mediated cell motility (Fig. 4). Thus, our data suggested that miR-130b targetly regulated integrin α5 and miR-130b suppressed cell migration of CRC cells, at least in part, through downregulation of integrin α5. The inhibition of CRC cell migration through directly targeting integrin α5 is consistent with the anti-metastatic role of miR-130b [15, 16]. Nonetheless, one miRNA may regulate over 200 target genes on average [31]; our data cannot exclude the existence of uncharacterized miR-130b targets that are implicated in the migration in a manner similar to the consequences of decreased integrin α5 expression.
TGF-β1 could strongly stimulate metastasis of cancer cells; one of the possible reasons is that TGF-β1 can increase expressions of some integrins including integrin α5 in hepatocellular carcinoma cells and lung cancer cells [32–36]. In the study, we found that TGF-β1 stimulated migration of CRC cells. Consistently, TGF-β1 increased the expression of integrin α5 (Fig. 4a). TGF-β1 appeared to stimulate a higher expression of integrin through Ets-dependent transcriptional regulation in HepG2 cells [35]. In our current study, we found that TGF-β1 suppressed miR-130b expression in CRC cells. And, miR-130b can negatively regulate its target-integrin α5. Further, the enhancement of integrin α5 expression in TGF-β1-treated CRC cells can be reversed partly when rescuing miR-130b expression by transfection of miR-130b mimics (Fig. 4b). Together, our findings suggested that TGF-β1 enhanced integrin α5 expression, at least in part, through decreasing miR-130b, by which to promote motility of CRC cells. Besides the stimulation of integrin α5 expression by TGF-β1, it was reported recently that TGF-β1 could phosphorylate integrin α5-mediated signal molecules through Smad-2 and Smad-3 in liver cancer, suggesting that TGF-β1 promoted tumor process by affecting the functional status of integrin α5 [37]. However, we cannot preclude the possibility that uncharacterized biological pathways may act the similar role. Mechanisms regarding regulation of TGF-β1 to integrin α5 need to be further explored.
In summary, our data suggested that integrin α5 was a novel target gene of miR-130b and TGF-β1 acted, at least in part, through miR-130b to promote integrin α5 expression, resulting in the enhanced migration of CRC cells.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917.
Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–8.
Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62:1315–26.
Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, et al. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006;131:830–40.
Derynck R, Feng XH. TGF-beta receptor signaling. Biochim Biophys Acta. 1997;1333:F105–50.
Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–7.
Welch DR, Fabra A, Nakajima M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc Natl Acad Sci U S A. 1990;87:7678–82.
Akhurst RJ, Derynck R. TGF-beta signaling in cancer—a double-edged sword. Trends Cell Biol. 2001;11:S44–51.
Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–29.
Tsushima H, Kawata S, Tamura S, Ito N, Shirai Y, Kiso S, et al. High levels of transforming growth factor beta 1 in patients with colorectal cancer: association with disease progression. Gastroenterology. 1996;110:375–82.
Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252–63.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33.
Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610.
Wang W, Peng B, Wang D, Ma X, Jiang D, Zhao J, et al. Human tumor microRNA signatures derived from large-scale oligonucleotide microarray datasets. Int J Cancer. 2011;129:1624–34.
Su X, Chakravarti D, Cho MS, Liu L, Gi YJ, Lin YL, et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature. 2010;467:986–90.
Dong P, Karaayvaz M, Jia N, Kaneuchi M, Hamada J, Watari H, et al. Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene. 2013;32:3286–95.
Castro NE, Kato M, Park JT, Natarajan R. Transforming growth factor beta1 (TGF-beta1) enhances expression of profibrotic genes through a novel signaling cascade and microRNAs in renal mesangial cells. J Biol Chem. 2014;289:29001–13.
Liu F, Kong X, Lv L, Gao J. TGF-beta1 acts through miR-155 to down-regulate TP53INP1 in promoting epithelial-mesenchymal transition and cancer stem cell phenotypes. Cancer Lett. 2015;359:288–98.
Sun MM, Li JF, Guo LL, Xiao HT, Dong L, Wang F, et al. TGF-beta1 suppression of microRNA-450b-5p expression: a novel mechanism for blocking myogenic differentiation of rhabdomyosarcoma. Oncogene. 2014;33:2075–86.
Zhou H, Wang K, Hu Z, Wen J. TGF-beta1 alters microRNA profile in human gastric cancer cells. Chin J Cancer Res. 2013;25:102–11.
Zhao Y, Miao G, Li Y, Isaji T, Gu J, Li J, et al. MicroRNA- 130b suppresses migration and invasion of colorectal cancer cells through downregulation of integrin beta1 [corrected]. PLoS One. 2014;9, e87938.
Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87.
Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–30.
Isaji T, Sato Y, Zhao Y, Miyoshi E, Wada Y, Taniguchi N, et al. N-glycosylation of the beta-propeller domain of the integrin alpha5 subunit is essential for alpha5beta1 heterodimerization, expression on the cell surface, and its biological function. J Biol Chem. 2006;281:33258–67.
Kim BH, Hong SW, Kim A, Choi SH, Yoon SO. Prognostic implications for high expression of oncogenic microRNAs in advanced gastric carcinoma. J Surg Oncol. 2013;107:505–10.
Lai KW, Koh KX, Loh M, Tada K, Subramaniam MM, Lim XY, et al. MicroRNA-130b regulates the tumour suppressor RUNX3 in gastric cancer. Eur J Cancer. 2010;46:1456–63.
Sand M, Skrygan M, Sand D, Georgas D, Gambichler T, Hahn SA, et al. Comparative microarray analysis of microRNA expression profiles in primary cutaneous malignant melanoma, cutaneous malignant melanoma metastases, and benign melanocytic nevi. Cell Tissue Res. 2013;351:85–98.
Chen Z, Jin Y, Yu D, Wang A, Mahjabeen I, Wang C, et al. Down-regulation of the microRNA-99 family members in head and neck squamous cell carcinoma. Oral Oncol. 2012;48:686–91.
Scheffer AR, Holdenrieder S, Kristiansen G, von Ruecker A, Muller SC, Ellinger J, et al. Circulating microRNAs in serum: novel biomarkers for patients with bladder cancer? World J Urol. 2014;32:353–8.
Bu P, Wang L, Chen KY, Rakhilin N, Sun J, Closa A, et al. miR-1269 promotes metastasis and forms a positive feedback loop with TGF-beta. Nat Commun. 2015;6:6879.
Li S, Fu H, Wang Y, Tie Y, Xing R, Zhu J, et al. MicroRNA-101 regulates expression of the v-fos FBJ murine osteosarcoma viral oncogene homolog (FOS) oncogene in human hepatocellular carcinoma. Hepatology. 2009;49:1194–202.
Cai T, Lei QY, Wang LY, Zha XL. TGF-beta 1 modulated the expression of alpha 5 beta 1 integrin and integrin-mediated signaling in human hepatocarcinoma cells. Biochem Biophys Res Commun. 2000;274:519–25.
Fong YC, Hsu SF, Wu CL, Li TM, Kao ST, Tsai FJ, et al. Transforming growth factor-beta1 increases cell migration and beta1 integrin up-regulation in human lung cancer cells. Lung Cancer. 2009;64:13–21.
Giannelli G, Fransvea E, Marinosci F, Bergamini C, Colucci S, Schiraldi O, et al. Transforming growth factor-beta1 triggers hepatocellular carcinoma invasiveness via alpha3beta1 integrin. Am J Pathol. 2002;161:183–93.
Katabami K, Mizuno H, Sano R, Saito Y, Ogura M, Itoh S, et al. Transforming growth factor-beta1 upregulates transcription of alpha3 integrin gene in hepatocellular carcinoma cells via Ets-transcription factor-binding motif in the promoter region. Clin Exp Metastasis. 2005;22:539–48.
Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11:97–105.
Fransvea E, Angelotti U, Antonaci S, Giannelli G. Blocking transforming growth factor-beta up-regulates E-cadherin and reduces migration and invasion of hepatocellular carcinoma cells. Hepatology. 2008;47:1557–66.
Acknowledgments
This work was supported by National Natural Science Foundation of China Grant 81101859, National Natural Science Foundation of China Grant 81000780, National Natural Science Foundation of China Grant 81472408, National Natural Science Foundation of China Grant 81541152, and Beijing Natural Science Foundation 5102039.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Transwell migration assays of SW480 cells treated without or with 20 ng/mL TGF-β1 for 24 hours (scale bar=100 μm; mean±s.d.; n=3; *, P <0.05). Representative images of migrated cells were shown. (JPG 529 kb)
Supplementary Figure 2
The putative binding sites with the seed region of miR-130b were at 187-193bp (underlined) and 902-908bp (underlined) of the full-length integrin α5 3′UTR. (JPG 1117 kb)
Supplementary Table 1
The clinicopathologic features of 39 patients. (XLSX 42 kb)
Rights and permissions
About this article
Cite this article
Yi, R., Li, Y., Wang, F. et al. Transforming growth factor (TGF) β1 acted through miR-130b to increase integrin α5 to promote migration of colorectal cancer cells. Tumor Biol. 37, 10763–10773 (2016). https://doi.org/10.1007/s13277-016-4965-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4965-6