Abstract
Cancer stem cells (CSCs) have attracted much attention of the research community in the recent years. Due to their highly tumorigenic and drug-resistant properties, CSCs represent important targets for developing novel anticancer agents and therapeutic strategies. CSCs were first described in hematopoietic malignancies and subsequently identified in various types of solid tumors including brain, breast, lung, colon, melanoma, and ovarian cancer. CSCs possess special biological properties including long-term self-renewal capacity, multi-lineage differentiation, and resistance to conventional chemotherapy and radiotherapy. As such, CSCs are considered as a major source of residual disease after therapy leading to disease occurrence. Thus, it is very important to understand the cellular survival mechanisms specific to CSCs and accordingly develop effective therapeutic approaches to eliminate this subpopulation of cancer cells in order to improve the treatment outcome of cancer patients. Possible therapeutic strategies against CSCs include targeting the self-renewal pathways of CSCs, interrupting the interaction between CSCs and their microenvironment, and exploiting the unique metabolic properties of CSCs. In this review article, we will provide an overview of the biological characteristics of CSCs, with a particular focus on their metabolic properties and potential therapeutic strategies to eliminate CSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer stem cells (CSCs) are a small subgroup of special cancer cells capable of long-term self-renewal with the potential to differentiate into multiple cell lineages. Since the concept of CSCs was put forward decades ago, a growing body of studies suggests that the heterogeneous cancers could indeed be derived from a small fraction of special cancer cells with stem cell properties [1–4]. Such CSCs could be isolated using flow cytometry based on their specific cell-surface markers or their ability to exclude certain compounds including the DNA-binding dye Hoechst 33342 [5, 6]. The high expression of ATP-dependent efflux pump ABCG2 in CSCs confers them the ability to effectively export Hoechst 33342 out of the cells and leads to a low retention of the fluorescent signal in these cells, so they just appear at the low-left corner in flow cytometry analysis and thus are known as “side population” or SP cells [1, 7]. CSCs play a very important role in sustaining tumor growth and mediating tumor metastasis [1]. The subpopulation of cancer cells with such biological properties is rare in tumor tissues. In long-term cultured cancer cell lines, the fraction of these special cells may be in the range of no more than 0.1–2 % [8].
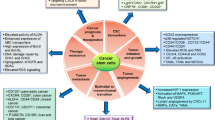
The high ability of CSCs to survive and resist conventional chemotherapy and radiotherapy has been observed in various cancer types. For instance, Kang et al. showed that only a small proportion of human glioblastoma cells survived and proliferated after they were exposed to lethal doses of 1,3-bis(2-chlorethyl)-1-nitrosourea (BCNU). The residual cells expressed CD133, CD117, CD90, CD71, and CD45 surface markers and could initiate tumors when transplanted orthotopically into severe combined immunodeficient mouse. They described these BCNU resistant cells as stem-like cancer cells [9]. This subset of cancer cells often exhibits the following main characteristics: (1) tumorigenic capacity; (2) specific molecular markers; (3) responsible for the maintenance of tumor’s growth; and (4) resistant to chemo- or radiation therapy [10]. Investigations of CSCs’ biologic behaviors and distinct metabolic features are of high clinical importance.
Conventional anticancer drugs are able to kill the bulk of “regular” cancer cells in the tumor mass and induce tumor shrinkage, but seem unable to kill CSCs, which are thought to be responsible for the persistence of residual disease, drug resistance, and eventually therapeutic failure. In addition to the above characteristics, CSCs seem to exhibit the metabolic alterations. Recent studies suggest that certain types of CSCs exhibit low mitochondrial respiration and high glycolytic activity, and such metabolic properties may render CSC more sensitive to glycolytic inhibitors [11, 12]. Since cancer stem cells play a major role in metastasis and drug resistance, it is essential to fully understand their biological properties and develop new therapies capable of effectively targeting the CSC subpopulation, leading to their elimination and potentially cure the malignant diseases.
Identification and isolation of CSCs
Cancer cells with stem-like properties have been found in a large cohort of malignancies with different tissue origins. Currently, the commonly recognized features or characteristics of CSCs include the ability to self-renew for long-term, to form tumor in vivo, and to differentiate and generate down-stream progenitor cells of multi-lineages. As such, CSCs can regenerate heterogeneous tumor cells when serially transplanted into recipients. However, it is still unknown whether CSCs come from normal stem cells or more differentiated cells [13]. CSCs are often isolated by their expression of specific molecular markers, especially surface markers such as CD133, CD34, CD24, CD44, CD166, and epithelial cell adhesion molecule (EpCAM), which are also used to identify corresponding normal stem cells [14]. For example, Dou J et al. used cell-surface markers CD133+, CD44+, and CD24+ to identify cancer stem-like cells in the murine melanoma B16F10 cells. They also showed that CD44+CD133+CD24+ cells exhibited biological properties of cancer stem-like cells and behaved similarly to CSCs [15]. CD133 is also a popular CSC marker for gastrointestinal tract (GIT) cancer and hepatocellular carcinoma (HCC) [16, 17]. CD133+ HCC cells exhibited an increase in tumorigenicity in xenografted mice [16]. Interestingly, some CSCs do not express the typical stem cell markers. Besides, certain membrane proteins and receptors have been identified as potential markers for CSCs of certain tumors [18].
Although the commonly accepted CSC cell-surface markers can be used to identify and purify CSCs using proper assays such as flow cytometry and special beads coated with antibodies specific for CSC surface markers, these methods may not be able to detect/isolate certain CSCs that do not express such specific markers. The SP analysis is another method to identify and isolate CSCs based on their ability to exclude the fluorescent dye Hoechst 33342 via the membrane-bond proteins of ATP-binding cassette (ABC) transporter family. ABC transporters can efflux various endogenous and exogenous compounds including xenobiotics and also the fluorescent dye Hoechst 33342 out of the cells. This efflux process is an energy-driven process using ATP to drive the pump. CSCs express high levels of ABC transporters especially ABCG2, which actively exclude the dye out of the cell so the dim “tail” with low fluorescent signal is considered as CSCs. Goodell et al. developed this functional assay for enriching CSCs from mouse bone marrow cells in 1996. Since then, the SP analysis technique has been commonly used to determine and quantify stem-like cells in different types of cancers. In many cases, SP cells have been shown to possess high capacity of self-renewal with expression of stem cell markers and have high ability to form tumors when transplanted into immunocompromised mice [1, 19].
CSCs share a variety of biological properties with normal stem cells such as self-renewal, multi-lineage differentiation and ability to form sphere-like cell clusters. Colony or sphere formation assay is another method to assay CSCs on the basis of their functional properties. Under proper culture conditions, stem cells with differentiating capacity could proliferate and form clonogenic spheres. As such, cancer cells capable of sphere formation are sometimes considered as tumorigenic cells. Tang et al. has utilized this strategy to isolate prostate cancer cells and confirmed their ability to form tumor in mice [8].
CSCs can also be identified and isolated based on their expression of functional aldehyde dehydrogenase (ALDH) [20]. ALDH is positively associated with cell proliferation and differentiation as it can catalyze retinaldehyde irreversibly to retinoic acid [18]. Cancer stem cells isolated based on high ALDH activity has been reported in various tumors. Ginestier et al. isolated cells with high ALDH activity from human breast normal tissues and showed that these tumor cells had the characteristics of CSCs. Increased ALDH activity was observed in breast CSCs, and this increased activity was associated with enhanced capacity of self-renewal and tumorigenesis. Fewer ALDH1-positive cells than CD44+/CD24− tumor cells were needed to initiate breast cancer in immunodeficient mice, suggesting that this functional assay based on ALDH activity could be used to isolate highly tumorigenic CSCs [21].
Characteristics of CSCs
In recent years, studies based on the concept of CSCs have provided plausible explanations for tumor initiation, proliferation, metastasis, and tumor heterogeneity. According to the CSC theory, tumors are originated from cancer stem cells, which produce the down-stream progeny cancer cells to proliferate and generate the bulk of the tumors. The ability of CSCs to differentiate into multi cell lineages further provides the capacity to generate heterogeneous cells within a tumor mass. In 1971, Park and his colleagues established that a single myeloma cell from a mouse tumor could initiate a new tumor in a recipient mouse [22]. Subsequently, CSCs have been identified in leukemia and multiple solid tumors and believed to be a common feature in oncogenesis. The presence of cancer stem cells were first demonstrated in human hematopoietic cancers about 20 years ago by Dick and colleagues. They found that the human acute myeloid leukemia (AML) cells with CD34+CD38- antigen profile could initiate AML in NOD/SCID mice. These cells were considered as leukemic stem cells with high capacity of proliferation and long-term self-renewal. Since then, CSCs have been found in many solid tumors including glioma, prostate cancer, colon cancer, liver cancer, gastric cancer, and many other malignant tumors [16, 23–25]. The term cancer stem cell is used to describe cancer cells capable of self-renewal and differentiation. Like normal stem cells, only a small subset of undifferentiated cells can generate tumors and maintain their growth. For example, one million human melanoma cells may only contain one cancer-initiating cell that could generate tumor in NOD/SCID mice [26]. However, another report by Quintana et al. revealed that the number of cells with tumorigenic potential in human cancers seems more common than previously thought if more careful optimization of xenotransplantation assays were used. Interestingly, the authors showed that a single human melanoma cell could be sufficient to initiate melanoma in vivo [27].
With the advance in CSCs research, increasing evidence suggests that cancer stem cells may play a key role not only in the tumor initiation but also in the invasiveness, progression, and metastasis. Researchers showed that cancer metastatic ability was closely associated with cancer stem cell phenotype, especially the process known as epithelial-mesenchymal transition (EMT) [28, 29]. Interestingly, Hermann et al. demonstrated that CD133+CXCR4+ cancer stem cells were necessary for tumor metastasis in pancreatic cancer. CXCR4, as the specific receptor of stromal cell-derived factor 1 (SDF-1), is a vital mediator during cell migration. They found that CD133+ cells which were considered as cancer stem cells in the invasive front strongly expressed CXCR4 [30]. Thus, new strategies aiming at regulating the SDF-1/CXCR4 axis may have potential applications in clinical treatment of the highly aggressive and therapy-resistant pancreatic cancer. In view of that CSCs are highly associated with metastasis, there is evidence suggesting that more aggressive tumors may contain a relatively higher percentage of CSCs [25]. Thus, elimination of this subpopulation of migrating CSC would be an important strategy to abrogate the metastatic activity of malignant tumors.
Resistance to therapy and mechanisms
Resistance of CSCs to standard chemotherapy or radiotherapy may lead persistence of residual diseases and cancer recurrence and thus constitutes a major challenge in clinical treatment of cancer. CSCs often exhibit resistance to many conventional chemotherapeutic agents due to multiple mechanisms. Hermann et al. uncovered that CSCs expressing CD133 in human pancreatic cancer tissue were highly resistant to standard chemotherapy and that new strategies such as multimodal treatment seemed to be more effective in eliminating CSCs, resulting in a better therapeutic outcome in mice bearing pancreatic cancer xenografts [31]. It is now clear that the failure of standard therapeutic regiments to kill cancer stem cells is a major problem in cancer treatment since the regrowth of the residual CSCs would lead to tumor recurrence [32, 33].
However, the exact mechanisms by which CSCs resist chemotherapy and radiotherapy are not fully understood. Recent studies suggest that multiple mechanisms are likely involved. CSCs seem able to preferentially activate the DNA damage checkpoint in response to DNA damage induced by either radiation or DNA-damaging drugs and thus increase their ability to repair DNA. Indeed, Bao et al. discovered that radiated CD133+ glioma cells activated the DNA damage checkpoint and repaired radiation-induced DNA damage more efficiently than the CD133- counterparts. Interestingly, specific inhibitors of cell cycle checkpoint kinase 1 (Chk1) and cell cycle checkpoint kinase 2 (Chk2) could partially reverse the radiation-resistance phenotype in stem-like glioma cells [6].
Another potentially important mechanism contributing to drug resistance in CSCs is the high expression of ABC transporters, the ATP-binding cassettes capable of exporting certain chemicals and drugs out of the cells leading to multidrug resistance [34, 35]. Liu and colleagues recently found that the expression of ABCG2 in SP cells was higher than that in the non-SP cells, and the glucose in the microenvironment could further up-regulate the expression of ABCG2 [19]. Since high expression of ABC transporters would enable the cells to effectively pump traditional chemotherapeutic drugs out of the cells, the high level of ABCG2 in SP cells would render them resistance to multiple chemotherapeutic agents. As such, inhibition of ABC pump function would be a potential strategy to overcome drug resistance in cancer stem cells. Interestingly, Xu et al. showed that depletion of cellular ATP by inhibition of glycolysis could overcome drug resistance due to over expression of the multidrug resistant molecules [36].
CSCs seem to have a lower level of ROS due in part to the higher expression of free radical-scavenging molecules such as glutathione (GSH). The increase in cellular GSH may provide another mechanism of drug resistance, since GSH is involved in cellular detoxification by conjugation with toxic chemicals and certain chemotherapeutic agents such as cisplatin and promote their export from the cells. Indeed, Diehn et al. showed that breast CSCs are more resistant to radiation, and such radioresistance was closely associated with the increased capacity of breast CSCs to synthesize glutathione (GSH) [37]. Importantly, the same study showed that inhibition of GSH synthesis by a chemical inhibitor buthionine sulfoximine (BSO) significantly suppressed the clonogenic potential of breast CSCs and enhanced their sensitivity to radiotherapy.
Another factor that contributes to the drug-resistant phenotype of CSCs is their relatively quiescent or dormant state. Recent studies revealed the presence of prostate cancer stem cells as subsets of slow-cycling and long-term BrdU-retaining cells [8, 38]. Since many chemotherapeutic drugs and radiation preferentially kill fast-growing cells which are active in DNA replication and thus highly sensitive to DNA-damaging agents, the relatively dormant or slow-cycling CSCs would be less vulnerable to DNA-damaging agents for the reason that the DNA damage induced in non-cycling cells is unlikely to cause catastrophic events without DNA replication. This situation would allow CSCs more time to repair the DNA damage and stay viable. Indeed, recent studies suggest that after radiation killed the bulk of cycling cancer cells, the residual CSCs were able to re-enter the cell cycle leading to tumor recurrence [39, 40].
Other mechanisms were also involved in chemo- and radioresistance of CSCs. Previous studies reported that the preferential expression of the survival proteins involved in the Akt/PKB and Bcl-2 pathway in CD133+ HCC CSCs rendered them to resistant to doxorubicin and 5-fluorouracil or to radiation therapy [41, 42]. In addition, a recent study reported that CD133+ HCC CSCs exhibited a resistance to IFN-γ-induced autophagy, which might be an important immune-resistant mechanism of CSCs [43].
Taken together, CSCs are relatively insensitive to standard chemotherapy and radiotherapy due to multiple mechanisms, including high capacity in DNA damage repair and drug export, high antioxidant and detoxification capacity, and quiescent or slow-cycling state. It is important to develop new therapeutic strategies and agents capable of effective killing CSCs based on their special characteristics.
Metabolic alterations in CSCs and therapeutic implications
More than 80 years ago, Warburg first observed that cancer cells exhibited an increase in glycolytic activity, even in the presence of abundant oxygen [44]. This phenomenon (aerobic glycolysis in cancer cells) is known as the Warburg effect, which is subsequently observed in many cancer types [45]. Recent studies suggest that compared to the bulk of “regular” cancer cells, CSCs seem to exhibit a lower rate of mitochondrial respiration, are highly active in glycolysis, and largely depend on this metabolic pathway to generate ATP for survival (Fig. 1).
Zhou et al. isolated highly tumorigenic cancer cells from the tumor xenografts of human glioblastoma U87 cells in mice. These cells exhibited stem-like properties, low mitochondrial respiration, increased glycolysis for ATP generation, and preference for hypoxia to maintain their stemness [11]. Vincent et al. performed metabolome analysis of colon cancer and reported that compared with CD133- cells, CD133+ colon cancer colo205 cells displayed a significant down regulation of protein synthesis with a decrease of some nucleotides like acetyl-CoA, cholesterol, and glucose-dependent lipid synthesis. This unique metabolic profile in colon CSCs endowed them with a quiescent or slow-cycling nature which was responsible for chemo- and radioresistance [46].
In addition, Yuan and his colleagues also showed that glioblastoma stem cells exhibited a metabolic shift to more glycolytic phenotype. These stem-like glioblastoma cells were highly resistant to standard drugs such as carmustine and temozolomide but highly sensitive to glycolytic inhibitor 3-BrOP. The combination of glycolytic inhibitor with conventional drug carmustine could effectively eradicate glioblastoma cells through synergistic mechanisms by carmustine-mediated DNA damage by 3-BrOP-induced rapid depletion of cellular ATP that abolished ability of cells to repair DNA damage [12]. Consistently, Liu et al. showed that SP cells with stem-like properties were more dependent on glucose compared with non-SP cells [19]. The same study also demonstrated that the glycolytic inhibitor 3-BrOP could significantly decrease the percentage of SP cells in vitro and impair their ability to form tumor in vivo. Several lines of evidence also showed that metformin, a biguanide class of antidiabetic drugs, may selectively kill CSCs through modulation of glucose homeostasis [47, 48]. However, the detailed mechanism by which CSCs are more sensitive to metformin has not been fully understood. Researchers also confirmed that therapy combined metformin with standard chemotherapeutic drug doxorubicin could achieved a synergistic effect in breast cancer [49]. Together, targeting glycolysis may be an effective strategy to eliminate CSCs and thus may have significant implications in cancer treatment (Fig. 2). Therefore, the potential metabolic targeting strategies for eradicating CSCs could help to develop a more effective therapeutic approach for cancer treatment. The possibility of combining specific CSC metabolism inhibitors with existing therapeutic approaches could have profound anticancer effects.
Targeting glycolysis will be a new strategy to eliminate CSCs in cancer treatment. a The persistence of viable stem-cell like cancer cells after conventional therapy is a major reason for development of drug resistance and disease recurrence. b Based on the metabolic alterations in CSCs, therapeutic strategies using proper combination of standard chemotherapeutic agents and glycolytic inhibitors that target CSC preferentially target CSCs will effectively eradicate both cancer cells and CSCs and would potentially improve the outcome of clinical cancer treatment
As described previously, high expression of chemical exporter ABCG2 in CSCs plays a major role in drug resistance. Given the fact that the function of ABC transporter is highly dependent on ATP and that glycolysis seems to be the main metabolic pathway for ATP generation in CSCs, thus it is possible to overcome drug resistance by inhibition of glycolysis leading to depletion of cellular ATP and thus inactivation of the ABC transporters. This therapeutic strategy, as illustrated in Fig. 3, is expected to restore drug sensitivity of CSCs, especially in those with high expression of ABC transporters such skin cancer stem cells [50]. Nakano et al. showed that glycolysis inhibitor 3-BrPA could inactivate the ABC transporters in SP cells isolated from RPMI8226. Such glycolytic inhibition rendered the cancer cells unable to export Hoechst 33342, suggesting inhibition would significantly reduce the ability of CSCs to export chemotherapeutic drugs. Similarly, 3-BrPA restored cytotoxic effects of daunorubicin and doxorubicin on KG-1 and RPMI8226 cells and markedly suppressed subcutaneous tumor growth when RPMI8226 cells were implanted in mice [51]. Similarly hematopoietic stem cells were shown to use aerobic glycolysis as a preferred pathway to generate ATP [52]. Thus, combination of glycolytic inhibition and conventional chemotherapeutic agents will likely applicable to various cancer types. It should be noted that since most non-stem cancer cells also exhibited higher glycolytic activity compared to normal cells, this drug combination strategy could also be effective in causing a complete destruction to the entire tumor.
ATP generated from glycolysis contributes to the function of ABCG2. The ATP-dependent drug transporter ABCG2 is highly dependent on ATP for proper function. The ability of CSCs to export anticancer drugs out of the cells can be inhibited when the main ATP-generating pathway (glycolysis) is suppressed
In contrast to general cancer cells in which ROS levels are increased, CSCs exhibited lower levels of ROS. The maintenance of low ROS levels is essential to preserve CSC self-renewal and stemness [37]. The lower ROS in CSCs compared to non-tumorigenic cells seems due in part to high expression of ROS scavenging molecules such as GSH and to high activity of pentose phosphate pathway where NADPH is generated. Ishimoto et al. found that CD44+ gastrointestinal CSCs showed an enhanced capacity of GSH synthesis and defense against ROS by activation of cystine-glutamate exchange transporter [53]. These properties contribute to the radioresistance of stem cells, since radiation exerts cytotoxic effect through generation of free radicals and the critical mediator ROS is decreased in stem cells [54]. Low level of ROS in CSCs appears to support cell viability. In contrast, an excessive increase in ROS level might reach a critical point or threshold that triggers CSCs’ death. Therefore, the use of pro-oxidant agents such as PEITC, which depletes GSH and increases ROS in cancer cells, may be a potentially effective to eliminate CSCs that are highly dependent on the antioxidant system (Fig. 4) [55–57].
Cancer stem cells are highly dependent on antioxidant system to maintain a low level of cellular ROS. Under the normal conditions, CSCs have a low level of ROS compared with non-stem cancer cells, due in part to the increase of antioxidants GSH and NADPH, which promote cell survival and resistance to chemotherapy/radiotherapy. Pro-oxidants such as PEITC can increase ROS in cancer cells and would be effective in eliminating CSCs. MRC mitochondrial respiratory
Conclusions
CSCs possess the ability to self-renew, differentiate to multiple lineages of cancer cells, and initiate tumor formation in vivo. This subpopulation of cancer cells also plays an important role in cancer metastasis and resistance to chemotherapy and radiotherapy. The current methods for identification and enrichment of CSCs mainly based on stem cell markers or the on the ability of CSCs to export Hoechst 33342. However, each method has its shortcomings. Currently there is not an accepted set of surface markers that can be used to definitely isolate a pure population of CSCs [58]. The SP technique is also imperfect due to the toxicity of nucleic acid binding dye Hoechst 33342 and its inability to differentiate the function of ABCG2 from other ABC transporters [59]. As such, developing more efficient methods to identify and isolate CSCs is in urgent need. The ability of CSCs to resist conventional chemotherapy and radiotherapy poses a great challenge to cancer treatment. Thus, a promising anticancer therapy must target not only the bulk tumor population but also the CSCs. The recent findings that CSCs exhibit certain metabolic feature such as highly active in glycolysis and dependent on this metabolic pathway provide a biochemical basis to develop promising new therapeutic strategies to effectively eliminate CSCs. Combination of glycolysis inhibitors and standard chemotherapeutics appears highly affective. However, recent studies also show that certain CSCs seems highly active in mitochondrial respiration and seem to depend on oxidative phosphorylation for energy generation [12, 19]. Thus, it is important to investigate the underlying mechanisms responsible for active OXPHOS in this subset of CSCs. Obviously, inhibition of glycolysis would not be effective against this type of CSCs, and different metabolic intervention strategies such as inhibition of mitochondrial respiratory chain function seem reasonable therapeutic approaches to be tested in the future. Since CSCs seem to require active synthesis of GSH to counteract oxidative stress and maintain their stemness, this unique property provide yet another possible way to preferentially impact CSCs by modulation of ROS and redox states, aiming to selectively kill CSCs or induce differentiation and ultimately increase therapeutic activity and selectivity. To successfully accomplish the goals of metabolic intervention, it is extremely important to fully understand the metabolic properties of CSCs and the underlying regulatory mechanisms. This would enable us to design new strategies specifically targeting the critical metabolic pathway in CSCs and to test their therapeutic activity in vitro and in vivo and eventually translate the new findings into clinical/therapeutic applications.
Reference
Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11.
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–61.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401.
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60.
Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33.
Tang DG, Patrawala L, Calhoun T, Bhatia B, Choy G, Schneider-Broussard R, et al. Prostate cancer stem/progenitor cells: identification, characterization, and implications. Mol Carcinog. 2007;46:1–14.
Kang MK, Kang SK. Tumorigenesis of chemotherapeutic drug-resistant cancer stem-like cells in brain glioma. Stem Cells Dev. 2007;16:837–47.
Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell. 2007;1:241–2.
Zhou Y, Zhou Y, Shingu T, Feng L, Chen Z, Ogasawara M, et al. Metabolic alterations in highly tumorigenic glioblastoma cells: preference for hypoxia and high dependency on glycolysis. J Biol Chem. 2011;286:32843–53.
Yuan S, Wang F, Chen G, Zhang H, Feng L, Wang L, et al. Effective elimination of cancer stem cells by a novel drug combination strategy. Stem Cells. 2013;31:23–34.
Puglisi MA, Tesori V, Lattanzi W, Gasbarrini GB, Gasbarrini A. Colon cancer stem cells: controversies and perspectives. World J Gastroenterol. 2013;19:2997–3006.
Fatima F, Nawaz M. Stem cell-derived exosomes: roles in stromal remodeling, tumor progression, and cancer immunotherapy. Chin J Cancer. 2015;34:46.
Dou J, Pan M, Wen P, Li Y, Tang Q, Chu L, et al. Isolation and identification of cancer stem-like cells from murine melanoma cell lines. Cell Mol Immunol. 2007;4:467–72.
Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of cd133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–4.
Zhang X, Hua R, Wang X, Huang M, Gan L, Wu Z, et al. Identification of stem-like cells and clinical significance of candidate stem cell markers in gastric cancer. Oncotarget. 2016.
Sugihara E, Saya H. Complexity of cancer stem cells. Int J Cancer. 2013;132:1249–59.
Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL, et al. Metabolic regulation of cancer cell side population by glucose through activation of the akt pathway. Cell Death Differ. 2014;21:124–35.
Hong IS, Jang GB, Lee HY, Nam JS. Targeting cancer stem cells by using the nanoparticles. Int J Nanomedicine. 2015;10:251–60.
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. Aldh1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67.
Park CH, Bergsagel DE, McCulloch EA. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971;46:411–22.
Takiguchi G, Nishita M, Kurita K, Kakeji Y, Minami Y: Wnt5a-Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16-CXCR6 axis. Cancer Sci 2015 doi: 10.1111/cas.12871
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7.
Regenbrecht CR, Lehrach H, Adjaye J. Stemming cancer: functional genomics of cancer stem cells in solid tumors. Stem Cell Rev. 2008;4:319–28.
Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–9.
Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8.
Taube JH, Malouf GG, Lu E, Sphyris N, Vijay V, Ramachandran PP, et al. Epigenetic silencing of microRNA-203 is required for EMT and cancer stem cell properties. Sci Rep. 2013;3:2687.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15.
Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–23.
Hermann PC, Trabulo SM, Sainz Jr B, Balic A, Garcia E, Hahn SA, et al. Multimodal treatment eliminates cancer stem cells and leads to long-term survival in primary human pancreatic cancer tissue xenografts. PLoS One. 2013;8:e66371.
Yoshida GJ, Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2015;107(1):5–11.
Prost S, Relouzat F, Spentchian M, Ouzegdouh Y, Saliba J, Massonnet G, et al. Erosion of the chronic myeloid leukaemia stem cell pool by PPARgamma agonists. Nature. 2015;525:380–3.
Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and Abcg2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–19.
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–26.
Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, et al. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 2005;65:613–21.
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3.
Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–9.
Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–45.
Skvortsova I, Debbage P, Kumar V, Skvortsov S. Radiation resistance: cancer stem cells (CSCs) and their enigmatic pro-survival signaling. Semin Cancer Biol. 2015.
Piao LS, Hur W, Kim TK, Hong SW, Kim SW, Choi JE, et al. Cd133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012;315:129–37.
Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. Cd133+ HCC cancer stem cells confer chemoresistance by preferential expression of the akt/pkb survival pathway. Oncogene. 2008;27:1749–58.
Li J, Chen JN, Zeng TT, He F, Chen SP, Ma S, et al. Cd133+ liver cancer stem cells resist interferon-gamma-induced autophagy. BMC Cancer. 2016;16:15.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39:267–74.
Vincent Z, Urakami K, Maruyama K, Yamaguchi K, Kusuhara M. Cd133-positive cancer stem cells from colo205 human colon adenocarcinoma cell line show resistance to chemotherapy and display a specific metabolomic profile. Genes Cancer. 2014;5:250–60.
Chen X, Hu C, Zhang W, Shen Y, Wang J, Hu F, et al. Metformin inhibits the proliferation, metastasis, and cancer stem-like sphere formation in osteosarcoma mg63 cells in vitro. Tumour Biol. 2015;36:9873–83.
Janzer A, German NJ, Gonzalez-Herrera KN, Asara JM, Haigis MC, Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPS in cancer stem cells. Proc Natl Acad Sci U S A. 2014;111:10574–9.
Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11.
Fukunaga-Kalabis M, Herlyn M. Beyond ABC: another mechanism of drug resistance in melanoma side population. J Invest Dermatol. 2012;132:2317–9.
Nakano A, Tsuji D, Miki H, Cui Q, El Sayed SM, Ikegame A, et al. Glycolysis inhibition inactivates ABC transporters to restore drug sensitivity in malignant cells. PLoS One. 2011;6:e27222.
Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–90.
Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. Cd44 variant regulates redox status in cancer cells by stabilizing the XCT subunit of system XC(-) and thereby promotes tumor growth. Cancer Cell. 2011;19:387–400.
Schieber MS, Chandel NS. ROS links glucose metabolism to breast cancer stem cell and EMT phenotype. Cancer Cell. 2013;23:265–7.
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–52.
Wu WJ, Zhang Y, Zeng ZL, Li XB, Hu KS, Luo HY, et al. Beta-phenylethyl isothiocyanate reverses platinum resistance by a GSH-dependent mechanism in cancer cells with epithelial-mesenchymal transition phenotype. Biochem Pharmacol. 2013;85:486–96.
Trachootham D, Zhang H, Zhang W, Feng L, Du M, Zhou Y, et al. Effective elimination of fludarabine-resistant CLL cells by PEITC through a redox-mediated mechanism. Blood. 2008;112:1912–22.
Gilbert CA, Ross AH. Cancer stem cells: cell culture, markers, and targets for new therapies. J Cell Biochem. 2009;108:1031–8.
Hambardzumyan D, Becher OJ, Holland EC. Cancer stem cells and survival pathways. Cell Cycle. 2008;7:1371–8.
Acknowledgments
This work was supported by research grants from the National Natural Science Foundation of China (no. 81502573) and Natural Science Foundation of Guangdong Province (no. 2014A030310421).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Mengqi Yang and Panpan Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, M., Liu, P. & Huang, P. Cancer stem cells, metabolism, and therapeutic significance. Tumor Biol. 37, 5735–5742 (2016). https://doi.org/10.1007/s13277-016-4945-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4945-x