Abstract
MicroRNAs (miRNAs) are a class of short, noncoding RNAs that act a crucial role in tumor development. Previous studies showed that miR-448 expression was deregulated in many tumors. However, the role of miR-448 in gastric cancer (GC) remains unknown. In our study, we demonstrated that miR-448 expression was downregulated in GC tissues compared with the corresponding nontumor tissues. We also showed that miR-448 expression was downregulated in GC cell lines. Ectopic expression of miR-448 suppressed GC cell proliferation, colony formation, and invasion. Moreover, we identified A Disintegrin And Metalloproteinases 10 (ADAM10) as a direct target gene of miR-448 in GC cell. ADAM10 expression was upregulated in GC tissues and cells. Furthermore, the expression level of miR-448 was negatively correlated with the expression level of ADAM10 in GC tissues. Moreover, ADAM10 overexpression rescued the effect of miR-448-mediated GC cell proliferation, colony formation, and invasion. These results demonstrated that miR-448 might play as a tumor suppressor miRNA partly through targeting ADAM10 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is one of the most common tumors and the second leading death-related tumor worldwide [1–4]. Despite recent advances in therapeutic strategies such as radiotherapy, surgery, and adjuvant chemotherapy, the prognosis of GC remains poor [2, 5–8]. Most GC patients are diagnosed with the late stage [9–12]. Therefore, it is crucial to identify novel biomarkers for diagnosis and therapy in GC patients.
MicroRNAs (miRNAs) are a group of short, small, noncoding RNAs that typically regulate gene expression through binding to the 3’-untranslated region (3′-UTR) of the target genes [13–15]. MiRNA plays crucial roles in many biological processes such as development, inflammation, differentiation, cell proliferation, apoptosis, and invasion [16–20]. Emerging evidences have demonstrated that miRNA are deregulated in various cancers including breast cancer, gladder cancer, bladder cancer, cutaneous squamous cell carcinoma, laryngeal cancer, rhabdomyosarcoma, and GC [21–27]. miRNA can act as a tumor suppressor gene or oncogene in the initiation and development of tumors [19, 28, 29]. Recently, increasing studies have demonstrated that miR-448 plays an important role in various tumors [30–32]. However, the expression and functional role of miR-448 in GC is still unknown.
In this study, we demonstrated that miR-448 expression was downregulated in the GC tissues and cell lines and overexpression of miR-448 suppressed GC cell proliferation, colony formation, and invasion. We identified A Disintegrin And Metalloproteinases (ADAM 10) as a direct target gene of miR-448 in the GC cell.
Materials and methods
Tissue samples
Primary GC tissues and their corresponding non-tumor tissues were collected from our department between 2010 and 2014. Written informed consent was collected from all patients, and this study was approved by the Ethics Committee of The Second Affiliated Hospital of Kunming Medical University. These samples were immediately frozen and stored in liquid nitrogen.
Cell lines cultured and transfection
Human GC cell lines (SGC-7901, MGC-803, HGC-27, and BGC-823) and normal gastric mucosa cells line (GES-1) were purchased from Cell Bank of Chinese Academy of Sciences (Beijing, China). The cell lines were cultured in RPMI 1640 medium (Gibco, USA) containing 10 % fetal bovine serum (FBS). miR-448 mimic and scramble, ADAM10 vector, and control were synthesized by RiboBio (Guangzhou, China). Cell transfection was performed by using Lipofectamine 2000 kit (Invitrogen, USA) following to the manufacturer’s information.
Quantitative real-time RT-PCR
Total RNA from cell or tissue collected was extracted by using TRIzol (Invitrogen, USA). Quantitative real-time PCR (qRT-PCR) analysis was performed to measure the mRNA and miRNA expression. PCR primers used were as follows: ADAM10, forward 5′-TCGAACCATCACCCTGCAACCT-3′ and reverse 5′-GCCCACCAATGAGCCACAATCC-3′; GAPDH, forward 5′-AATGGGCAGCCGTTAGGAAA-3′ and reverse 5′-TGAAGGGGTCATTGATGGCA-3′. The relative miR-448 expression was normalized to U6 SnRNA and the expression of ADAM10 was normalized to GAPDH.
Dual-luciferase reporter assay
Cells were cultured in the 96-well plate and were tranfected with pLuc-3’-UTR, renilla, and miR-448 mimic or scramble by using Lipofectamine 2000 kit (Invitrogen, USA) following to the manufacturer’s information. After 48 h, luciferase activities were analyzed by using a dual-luciferase reporter analysis system (Promega, USA). The relative luciferase activity was normalized to renilla luciferase activity.
Western blot
Protein was isolated from cell or tissue using RIPA buffer. Total protein was electrophoresed by 12 % SDS and transferred to PVDF membranes (Millipore, USA). After blocking with 10 % nonfat milk, the membrane was incubated with the primary antibody. The antibody was shown as the following: ADAM10, GAPDH (Abcam, England). Blots were measured by using the ECL system.
Cell growth and colony formation
Cell proliferation was detected using the Cell Counting Kit-8 kit (CCK-8 kit, Dojindo, Japan). The cell was cultured in the 96-well plate, and cell proliferation was detected on 0, 24, 48, and 72 h. The absorbance was detected at 450 nm. For cell colony formation, cells were seeded in the 6-well plate and kept in RPMI 1640 medium for 2 weeks. Colonies were stained with crystal violet and counted.
Cell invasion
Cell invasion was performed using transwell chambers. Cells were plated in the upper of the membranes coated with Matrigel (BD Biosciences, MD). The upper chamber was not supplemented with FBS, and the 10 % FBS was added to the lower membrane. After incubating for 48 h, the cells in the lower membrane were stained with 1 % crystal violet (Sigma, USA) and then counted.
Statistical analysis
Results presented are expressed as mean ± SD (standard deviation). Student’s t test was performed to measure the difference between two groups, and differences between more than two groups were assessed using one-way ANOVA. P < 0.05 was considered significant.
Results
miR-448 expression was downregulated in the GC cell and tissue
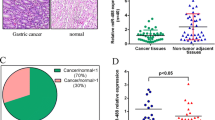
We firstly measured the expression of miR-448 in the 40 pairs of GC tissues. miR-448 expression was downregulated in GC tissues compared with the corresponding nontumor tissues (Fig. 1a). Moreover, miR-448 expression was downregulated in 28 cases (28/40; 70 %) compared with adjacent tissues (Fig. 1b). We also demonstrated that miR-448 expression was decreased in the GC cell lines (SGC-7901, MGC-803, HGC-27, and BGC-823) compared with normal gastric mucosa cells line (GES-1) (Fig. 1c).
miR-448 was downregulated in the GC cell and tissue. a The expression of miR-448 was measured by qRT-PCR. b miR-448 was downregulated in 28 cases (28/40; 70 %) compared to adjacent tissues. c The expression of miR-448 was decreased in the GC cell lines (SGC-7901, MGC-803, HGC-27, and BGC-823) compared with normal gastric mucosa cells line (GES-1). **p < 0.01
ADAM10 expression was upregulated in the GC cell and tissue
ADAM10 expression was upregulated in the GC tissues compared with the corresponding nontumor tissues (Fig. 2a). Moreover, ADAM10 expression was upregulated in 27 cases (27/40; 67 %) compared with the adjacent tissues (Fig. 2b). There was a negative relationship between the expression level of miR-448 and ADAM10 in the GC tissues (Fig. 2c). We also showed that the expression level of ADAM10 was increased in the GC cell lines (SGC-7901, MGC-803, HGC-27, and BGC-823) compared with GES-1 (Fig. 2d).
ADAM10 was upregulated in the GC cell and tissue. a The expression of ADAM10 was measured by qRT-PCR. b ADAM10 was upregulated in 27 cases (27/40; 67 %) compared to adjacent tissues. c There is a negative relation between the expression of miR-448 and the expression of ADAM10 in the GC tissues. d The expression of ADAM10 was upregulated in the GC cell lines (SGC-7901, MGC-803, HGC-27, and BGC-823) compared with normal gastric mucosa cells line (GES-1). **p < 0.01
miR-448 suppressed the GC cell proliferation, colony formation, and invasion
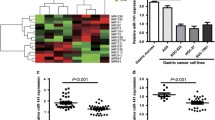
miR-448 expression was upregulated in the GC cell HGC-27 after being treated with miR-448 mimic, and this result confirmed that the efficiency of miR-448 mimic was high (Fig. 3a). Overexpression of miR-448 suppressed the GC cell HGC-27 proliferation (Fig. 3b). Moreover, ectopic expression of miR-448 inhibited the HGC-27 cell colony formation and invasion (Fig. 3c, d).
miR-448 suppressed the GC cell proliferation, colony formation, and invasion. a The expression of miR-448 in the HGC-27 cell after tranfected with miR-448 mimic using qRT-PCR. b Overexpression of miR-448 suppressed the HGC-27 cell proliferation. c Ectopic expression of miR-448 inhibited the HGC-27 cell colony information. d miR-448 overexpression suppressed the HGC-27 cell invasion. *p < 0.05,**p < 0.01,***p < 0.001
ADAM10 was a direct target gene of miR-448 in GC cell
Bioinformatics assay with TargetScan algorithms predicted ADAM10 as a hypothetical target gene of miR-448 (Fig. 4a). In HGC-27 cells co-transfected with miR-448 mimic or scramble and the reporter plasmid, the relative luciferase activity of this reporter with WT ADAM10 3′-UTR was inhibited by miR-448 mimic; however, this luciferase activity of MT reporter was unaffected (Fig. 4b). Furthermore, overexpression of miR-448 suppressed the ADAM10 protein expression in the HGC-27 cell (Fig. 4c).
ADAM10 was a direct target gene of miR-448 in GC cell. a Bioinformatics assay with TargetScan algorithms predicted ADAM10 as a hypothetical target gene of miR-448. b Luciferase activity assays of luciferase vectors with wild-type or mutant ADAM10 3′-UTR were performed after co-transfection with miR-448 mimic or scramble. c Overexpression of miR-448 suppressed the ADAM10 protein expression in the HGC-27 cell
ADAM10 rescued the effect of miR-448-mediated GC cell proliferation, colony formation, and invasion
ADAM10 expression was upregulated in the pCDNA-ADAM10-treated HGC-27 cell (Fig. 5a, b). We rescued ADAM10 expression in the miR-448-overexpressing HGC-27 cell. CCK8 analysis demonstrated that ADAM10 overexpression promoted the miR-448-overexpressing HGC-27 cell proliferation (Fig. 5c). Colony formation analysis showed that ADAM10 overexpression increased the miR-448-overexpressing HGC-27 cell colony formation (Fig. 5d). Invasion analysis demonstrated that ectopic expression of ADAM10 promoted the miR-448-overexpressing HGC-27 cell invasion (Fig. 5e).
ADAM10 rescued the effect of miR-448-mediated GC cell proliferation, colony formation, and invasion. a The protein expression of ADAM10 was measured by western blot. b The mRNA expression of ADAM10 was measured by qRT-PCR. c The cell proliferation was measured by CCK-8 analysis. d Colony formation analysis showed that ADAM10 overexpression increased the miR-448-overexpressing HGC-27 cell colony formation. e Invasion analysis demonstrated that ectopic expression of ADAM10 promoted the miR-448-overexpressing HGC-27 cell invasion. *p < 0.05,**p < 0.01,***p < 0.001
Discussion
In this study, we demonstrated that miR-448 expression was downregulated in the GC tissues compared with the corresponding nontumor tissues. We also showed that miR-448 expression was downregulated in GC cell lines compared with normal gastric mucosa cell line. Ectopic expression of miR-448 suppressed GC cell proliferation, colony formation, and invasion. Moreover, we identified ADAM10 as a direct target gene of miR-448 in GC cell, and ADAM10 expression was upregulated in GC tissues and cells. Furthermore, the expression level of miR-448 was negatively correlated with the expression level of ADAM10 in the GC tissues. ADAM10 overexpression rescued the effect of miR-448-mediated GC cell proliferation, colony formation, and invasion. These results demonstrated that miR-448 played as a tumor suppressor miRNA partly through targeting ADAM10 expression.
Previous studies demonstrated that miR-448 played an important role in the development of tumors [30–32]. For example, Lv et al. showed that miR-448 acted as a tumor suppressor gene through inhibiting the CXCL12 expression in ovarian cancer [30]. Zhu et al. demonstrated that miR-448 expression was downregulated in hepatocellular carcinoma (HCC) tissues and inhibition of miR-448 increased HCC cell invasion and epithelial-mesenchymal transition (EMT) through targeting ROCK2 expression [31]. Li et al. showed that miR-448 suppression promoted EMT by inhibiting special AT-rich sequence-binding protein-1 (SATB1) expression in breast cancer. However, the role of miR-448 in GC remains still unknown. In our study, we firstly measured the expression of miR-448 in the GC tissues [32]. Our results showed that miR-448 expression was downregulated in the GC tissues compared with the corresponding nontumor tissues. Moreover, miR-448 was downregulated in 28 cases (28/40; 70 %) compared with adjacent tissues. Furthermore, overexpression of miR-448 suppressed the GC cell proliferation, colony formation, and invasion.
ADAM10 is one member of the ADAM family, and ADAM-mediated shedding was essential for many cell processes including cell development, determination, invasion, migration, proliferation, and wound healing [33–36]. Recent studies have found that ADAM10 plays important roles in the GC development. For example, Yoshimura et al. demonstrated that H. pylori infection was associated with high expression of ADAM10 and high expression of ADAM10 was present in gastric tumor [37]. Tanida et al. showed that IL-8 induced EGFR ligand shedding through regulating the ADAM10-dependent pathway in GC cells [38]. High ADAM10 expression was associated with the location and size of tumor, as well as vessel invasion, depth of invasion, TNM stage, and lymph node. However, the underlying mechanisms about deregulated expression of ADAM10 remain unclear. In line with previous data, we also demonstrated that ADAM10 expression was upregulated in GC tissues and cell lines. Moreover, we identified ADAM10 as a direct target gene of miR-448 in GC cells. ADAM10 overexpression rescued the effect of miR-448-mediated GC cell proliferation, colony formation, and invasion. These results suggested might provide one potential mechanism of post-transcriptional regulation of ADAM10.
In conclusion, our results suggested that miR-448 expression was upregulated in GC tissues and miR-448 acted as a tumor suppressor miRNA in the development of GC through inhibiting ADAM10 expression. Restoration of miR-448 may be a potential therapeutic strategy for GC patients in the future.
References
Liang J, Liu X, Xue H, Qiu B, Wei B, Sun K. Microrna-103a inhibits gastric cancer cell proliferation, migration and invasion by targeting c-myb. Cell Prolif. 2015;48:78–85.
Duan JH, Fang L. MicroRNA-92 promotes gastric cancer cell proliferation and invasion through targeting FXR. Tumour Biol. 2014;35(11):11013–9.
Liu G, Jiang C, Li D, Wang R, Wang W. MiRNA-34a inhibits EGFR-signaling-dependent MMP7 activation in gastric cancer. Tumour Biol. 2014;35(10):9801–6.
Wan X, Ding X, Chen S, Song H, Jiang H, Fang Y, et al. The functional sites of miRNAs and lncRNAs in gastric carcinogenesis. Tumour Biol. 2015;36:521–32.
Wu Y, Tao Y, Chen Y, Xu W. RhoC regulates the proliferation of gastric cancer cells through interaction with IQGAP1. PLoS One. 2012;7:e48917.
Shen J, Niu W, Zhou M, Zhang H, Ma J, Wang L. MicroRNA-410 suppresses migration and invasion by targeting MDM2 in gastric cancer. PLoS One. 2014;9:e104510.
Otsubo T, Akiyama Y, Hashimoto Y, Shimada S, Goto K, Yuasa Y. MicroRNA-126 inhibits SOX2 expression and contributes to gastric carcinogenesis. PLoS One. 2011;6:e16617.
Yasui W, Sentani K, Sakamoto N, Anami K, Naito Y, Oue N. Molecular pathology of gastric cancer: research and practice. Pathol Res Pract. 2011;207:608–12.
Li X, Luo F, Li Q, Xu M, Feng D, Zhang G, et al. Identification of new aberrantly expressed miRNAs in intestinal-type gastric cancer and its clinical significance. Oncol Rep. 2011;26:1431–9.
Inoue T, Iinuma H, Ogawa E, Inaba T, Fukushima R. Clinicopathological and prognostic significance of microRNA-107 and its relationship to DICER1 mRNA expression in gastric cancer. Oncol Rep. 2012;27:1759–64.
Fesler A, Zhai H, Ju J. Mir-129 as a novel therapeutic target and biomarker in gastrointestinal cancer. OncoTargets Ther. 2014;7:1481–5.
Wang M, Gu H, Wang S, Qian H, Zhu W, Zhang L, et al. Circulating miR-17-5p and miR-20a: molecular markers for gastric cancer. Mol Med Report. 2012;5:1514–20.
Zhang X, Tang J, Zhi X, Xie K, Wang W, Li Z, et al. miR-874 functions as a tumor suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway in gastric cancer. Oncotarget. 2015;6:1605–17.
Li Z, Yu X, Wang Y, Shen J, Wu WK, Liang J, et al. By downregulating TIAM1 expression, microRNA-329 suppresses gastric cancer invasion and growth. Oncotarget. 2015;6:17559–69.
Li Z, Lei H, Luo M, Wang Y, Dong L, Ma Y, et al. DNA methylation downregulated miR-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer. 2015;18:43–54.
Yu X, Li Z, Chen G, Wu WK. MicroRNA-10b induces vascular muscle cell proliferation through Akt pathway by targeting TIP30. Curr Vasc Pharmacol. 2015;13:679–86.
Yu X, Li Z, Shen J, Wu WK, Liang J, Weng X, et al. MicroRNA-10b promotes nucleus pulposus cell proliferation through RhoC-Akt pathway by targeting HOXD10 in intervetebral disc degeneration. PLoS One. 2013;8:e83080.
Song JH, Meltzer SJ. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology. 2012;143:35–47. e32.
Deng H, Guo Y, Song H, Xiao B, Sun W, Liu Z, et al. MicroRNA-195 and microRNAa-378 mediate tumor growth suppression by epigenetical regulation in gastric cancer. Gene. 2013;518:351–9.
Bin Z, Dedong H, Xiangjie F, Hongwei X, Qinghui Y. The microRNA-367 inhibits the invasion and metastasis of gastric cancer by directly repressing Rab23. Genet Test Mol Biomarkers. 2015;19:69–74.
Yu X, Li Z: The role of mirnas in cutaneous squamous cell carcinoma. Journal of cellular and molecular medicine 2016;20:3–9.
Li Z, Yu X, Shen J, Law PT, Chan MT, Wu WK. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget. 2015;6:13914–24.
Yu X, Li Z. The role of microRNAs expression in laryngeal cancer. Oncotarget. 2015;6:23297–305.
Li Z, Yu X, Shen J, Liu Y, Chan MT, Wu WK. MicroRNA dysregulation in rhabdomyosarcoma: a new player enters the game. Cell Prolif. 2015;48:511–6.
Itesako T, Seki N, Yoshino H, Chiyomaru T, Yamasaki T, Hidaka H, et al. The microRNA expression signature of bladder cancer by deep sequencing: the functional significance of the miR-195/497 cluster. PLoS One. 2014;9:e84311.
Vrba L, Munoz-Rodriguez JL, Stampfer MR, Futscher BW. Mirna gene promoters are frequent targets of aberrant DNA methylation in human breast cancer. PLoS One. 2013;8:e54398.
Xu X, Wang W, Su N, Zhu X, Yao J, Gao W, et al. miR-374a promotes cell proliferation, migration and invasion by targeting SRCIN1 in gastric cancer. FEBS Lett. 2015;589:407–13.
Kong WQ, Bai R, Liu T, Cai CL, Liu M, Li X, et al. MicroRNA-182 targets camp-responsive element-binding protein 1 and suppresses cell growth in human gastric adenocarcinoma. FEBS J. 2012;279:1252–60.
Han TS, Hur K, Xu G, Choi B, Okugawa Y, Toiyama Y, et al. MicroRNA-29c mediates initiation of gastric carcinogenesis by directly targeting ITGB1. Gut. 2015;64:203–14.
Lv Y, Lei Y, Hu Y, Ding W, Zhang C, Fang C. miR-448 negatively regulates ovarian cancer cell growth and metastasis by targeting CXCL12. Clin Transl Oncol. 2015;17:903–9.
Zhu H, Zhou X, Ma C, Chang H, Li H, Liu F, et al. Low expression of miR-448 induces EMT and promotes invasion by regulating ROCK2 in hepatocellular carcinoma. Cell Physiol Biochem. 2015;36:487–98.
Li QQ, Chen ZQ, Cao XX, Xu JD, Xu JW, Chen YY, et al. Involvement of Nf-kappab/miR-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells. Cell Death Differ. 2011;18:16–25.
Mullooly M, McGowan PM, Kennedy SA, Madden SF, Crown J, O’ Donovan N, et al. Adam10: a new player in breast cancer progression? Br J Cancer. 2015;113:945–51.
Jing P, Sa N, Liu X, Xu W. MicroR-140-5p suppresses tumor cell migration and invasion by targeting ADAM10-mediated Notch1 signaling pathway in hypopharyngeal squamous cell carcinoma. Exp Mol Pathol. 2015;100:132–8.
Saftig P, Lichtenthaler SF. The alpha secretase ADAM10: a metalloprotease with multiple functions in the brain. Prog Neurobiol. 2015;135:1–20.
Chang YC, Jan CI, Peng CY, Lai YC, Hu FW, Yu CC. Activation of microRNA-494-targeting Bmi1 and ADAM10 by silibinin ablates cancer stemness and predicts favourable prognostic value in head and neck squamous cell carcinomas. Oncotarget. 2015;6:24002–16.
Yoshimura T, Tomita T, Dixon MF, Axon AT, Robinson PA, Crabtree JE. ADAMs (a disintegrin and metalloproteinase) messenger RNA expression in helicobacter pylori-infected, normal, and neoplastic gastric mucosa. J Infect Dis. 2002;185:332–40.
Tanida S, Joh T, Itoh K, Kataoka H, Sasaki M, Ohara H, et al. The mechanism of cleavage of EGFR ligands induced by inflammatory cytokines in gastric cancer cells. Gastroenterology. 2004;127:559–69.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Xuesong Wu and Haoran Tang are co-first authors.
Rights and permissions
About this article
Cite this article
Wu, X., Tang, H., Liu, G. et al. miR-448 suppressed gastric cancer proliferation and invasion by regulating ADAM10. Tumor Biol. 37, 10545–10551 (2016). https://doi.org/10.1007/s13277-016-4942-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4942-0