Abstract
Metabolism alteration is one of the hallmarks of cancer cells. Although several studies have demonstrated that glycolysis played important roles in the progression of nasopharyngeal carcinoma (NPC), the functions of specific metabolism-associated genes remain largely unknown. In this study, it was found that Pyruvate dehydrogenase B (PDHB), which catalyzed the conversion of pyruvate to Acetyl-CoA, was downregulated in NPC cells. Forced expression of PDHB in NPC cells inhibited cell growth and migration, while knocking down the expression of PDHB promoted the growth, migration, and tumorigenesis of NPC cells. Mechanism study showed that PDHB inhibited ERK signaling and cell growth driven by RasV12. Collectively, our study demonstrated the suppressive roles of PDHB in the progression of NPC, and restoring the function of PDHB might be a promising strategy for NPC therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is a very common malignancy in the south of China, Southeast Asian and north of Africa [1]. Genetic alteration, virus infection, and exposure to the environmental factors are the major factors of NPC [2]. Although radiotherapy and chemotherapy are very effective for most of NPC, 30–40 % of NPC still progress into the distant metastasis, and the metastasis is the major cause for the NPC-related death [3]. Therefore, identifying the novel regulators for the growth and metastasis of NPC would benefit the clinical treatment.
It has been a belief that the glycolysis is one of the characteristics of the cancer cells, which supported the rapid growth and metastasis of the cancer cells [4]. Pyruvate dehydrogenase B (PDHB) localizes in the mitochondrial and consists of multiple subunits [5]. PDHB catalyzes the glucose-derived pyruvate to the acetyl-CoA and plays important roles in oxidative phosphorylation [5]. In cancer cells, PDH is phosphorylated by pyruvate dehydrogenase kinase (PDK), which inhibits the activity of PDH [6].
Dysregulation of PDH has been reported in various cancer types [5, 7, 8]. The expression of PDH is an independent prognostic factor for gastric cancer, and the positive expression of PDH is predictive for the favorable outcome. Cai et al. have shown the downregulation of PDH in the cardiac cancer through the proteomics approach [9]. Although the metabolic alteration of NPC has been recognized, the functions of PDH in NPC still remain unknown.
The aim of this study is to investigate the functions of PDHB in the growth, migration, and tumorigenesis of the NPC cells using the in vitro and in vivo system.
Materials and methods
Cell culture
The NPC cell lines and NP69 cells were obtained from ATCC (American Type Culture Collection). Cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) containing 10 % fetal bovine serum (FBS, Sigma) and antibiotics (Sigma), and incubated in a humidified atmosphere containing 5 % CO2 at 37 °C.
Plasmids and stable cell lines
The coding sequence of PDHB was amplified by PCR and inserted into the expression vector pcDNA 3.1-myc using EcoRI and EcoRV. The PDHB expression vector and empty pcDNA3.1 were transfected into CNE-1 and HNE-1 cells using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s instructions. After the selection with G418, the resistant cells were pooled and further confirmed the expression of exogenous PDHB using anti-myc antibody.
qPCR analysis
Total RNA was extracted from NPC cells and NP69 cells using TRIzol reagent (Invitrogen). The isolated RNA was used to cDNA synthesis using the reverse transcription kit (Promega, Madison, WI), following the manufacturer’s instructions. Real-time PCR was performed using a Stratagene MAXP3000 PCR system and Brilliant Q-PCR Master Mixture. The primer pair used for amplification of the human PDHB gene was as follows: forward primer, 5′-GATGAGAAGGTATTTCTGCT-3′, and reverse primer, 5′-GAGAAATTGAAGGTCATAAA-3′. As an internal standard, a fragment of human 18S was amplified by PCR using the following primers: forward primer, 5′-GATCATTGCTCCTCCTGAGC-3′, and reverse primer, 5′-ACTCCT GCTTGCTGATCCAC-3′.
Western blotting
Cells were harvested and lysed using RIPA buffer containing protease inhibitors and phosphotase inhibitors, proteins were separated by 10 % SDS-PAGE, transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA) and probed with specific antibodies. The immunoreactive protein bands were visualized by ECL kit (Pierce). Antibody to PDHB (1:1000) was purchased from Abcam, antibodies to ERK, phosphorylated ERK, and c-Myc were purchased from Cell Signaling Technology, and antibody to GAPDH (1:5000) was purchased from Santa Cruz Biotechnology. Secondary antibodies, mouse IgG, and Rabbit IgG, were obtained from Cell Signaling Technology.
Knocking down the expression of PDHB in NPC cells
RNAi lenti-virus particles (ShRNA con and ShPDHB) were purchased from GeneChem (China). Cells were incubated with the lenti-virus particles for 24 h and then selected with the medium containing puromycine.
Cell growth assay
Crystal violet assay was performed to examine the effects of PDHB on the growth of NPC cells. Equal number of control cells and experimental cells were seeded in 12-well plates and cultured in medium supplemented with 10 % FBS at a density of 1000 cells/well. Medium was changed every other day. After 10 days of culture under the standard condition, the medium was removed and the cells were stained with 0.5 % crystal violet solution in 20 % methanol. After staining for 10 min, the fixed cells were washed with phosphate-buffered saline (PBS) and photographed. The colonies were solved using 1 % SDS solution. OD 600 nm was measured.
Cell migration assay
Cell migration assay was performed using a modified Boyden chamber. Cells (2 × 105) suspended in 0.05 ml medium containing 1 % FBS were placed in the upper chamber, and the lower chamber was loaded with 0.152 ml medium containing 10 % FBS. Six hours later, cells migrated to the lower surface of the filters was detected with traditional H&E staining. The migrated cells were counted under the inverted microscope. The experiments were repeated for thrice.
Soft agar assay
The soft agar assay was performed to evaluate the effect of PDHB on the tumorigenesis in vitro. Briefly, cells (1 × 104) were resuspended in medium containing 10 % FBS with 0.3 % agarose. And layered on the top of 0.6 % agar in medium supplemented with 20 % FBS on 60-mm plates. After 14 days of culture at 37 °C, plates were stained with 0.005 % crystal violet for 1 h. Colonies were photographed and the relative colony sizes were measured.
Tumorigenesis assay
Four 4-week-old nude mice were used in this study. CNE-1 cells were labeled with luciferase and then knocked down the expression of PDHB. 1 × 106 PDHB knockdown cells (labeled with luciferase) and their control cells were subcutaneously injected into the nude mice. The growth of the tumors was monitored with the in vivo imaging system after administration of luciferin. Tumors were harvested 6 weeks after injection and individually weighted. Data were presented as tumor weight (mean ± SD). Statistical analysis was performed using the Student’s t test. All procedures performed in this study involving mouse were in accordance with the ethical standards of the hospital.
Results
PDHB was downregulated in nasopharyngeal carcinoma (NPC) cell lines
The expression pattern of PDHB in NPC remains unknown. To study the expression of PDHB in NPC, we first examined the mRNA level of PDHB in a panel of NPC cell lines (CNE-1, CNE-2, HNE-1, HNE-2) and normal nasopharyngeal epithelial cells NP69 by real-time PCR. Compared with the NP69 cells, the mRNA level of PDHB was downregulated in NPC cells (Fig. 1a). Consistent with the results form the real-time PCR, Western blot analysis showed the downregulation of PDHB protein level in NPC cell lines (Fig. 1b). Taken together, these results confirmed the decreased expression level of PDHB in NPC cells, indicating its suppressive roles in the progression of NPC.
The mRNA and protein level of PDHB was decreased in NPC cell lines. a The expression of PDHB mRNA level in NPC cell lines and normal nasopharyngeal epithelial cells was examined using real-time PCR. b The expression of PDHB protein level in NPC cell lines and normal nasopharyngeal epithelial cells was examined using Western blot analysis
Overexpression of PDHB inhibited the growth and migration of the NPC cells
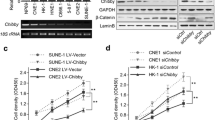
To study the functions of PDHB in the progression of NPC, myc-tagged PDHB was forced expressed in CNE-1 and HNE-1 cells (Fig. 2a). Next, we examined the effects of PDHB on the growth and migration of NPC cells using crystal violet assay, Boyden chamber assay and wound healing assay. Overexpression of PDHB inhibited the growth of CNE-1 and HNE-1 cells in the crystal violet assay (Fig. 2b). Moreover, PDHB inhibited the motility of NPC cells shown in the Boyden chamber assay and wound healing assay (Fig. 2c–d). Next, we examined the effects of PDHB on the expression of some apoptosis and cell cycle-related regulators. Overexpression of PDHB upregulated the expression of cleaved PARP and downregulated the expression of PCNA as well as CyclinD1, which was consistent with the inhibition of cell growth by PDHB in CNE-1 cells (Fig. 2e). Diisopropylamine dichloroacetate (DADA) was an agonist of PDHB. Treating CNE-1 and HNE-1 cells with DADA (10−4M) inhibited the colony formation on soft agar (Fig. 2f). Collectively, these observations suggested that PDHB impaired the growth and migration of the NPC cells.
Overexpression of PDHB inhibited the growth and migration of CNE-1 and HNE-1 cells. a Forced expression of PDHB in CNE-1 and HNE-1 cells. CNE-1 and HNE-1 cells were transfected with pcDNA3.1 vector or the PDHB expression vector and selected with G418. G418-resistant cells were pooled and confirmed the overexpression of PDHB by Western blot analysis. b Crystal violet assay to analyze the effects of PDHB on the growth of CNE-1 and HNE-1 cells. c Forced expression of PDHB inhibited the migration of CNE-1 and HNE-1 cells in the migration assay using a Boyden chamber. d Forced expression of PDHB inhibited the migration of CNE-1 and HNE-1 cells in the wound healing assay. d Forced expression of PDHB inhibited the expression of PCNA, CyclinD1 and promoted the expression of cleaved PARP in CNE-1 cells. f DADA, the agonist of PDHB, inhibited the colony formation of CNE-1 and HNE-1 cells in the soft agar. **P < 0.01
Knocking down the expression of PDHB promoted the growth and migration of the NPC cells
Next, we examined the functions of endogenously expressed PDHB in the progression of NPC. Two target sequences effectively downregulated the expression of PDHB in CNE-1 and HNE-1 cells (Fig. 3a). Consistent with the observations in the gain-of-function assay, knocking down the expression of PDHB promoted the growth of NPC cells significantly in the crystal violet assay (Fig. 3b). In addition, downregulation of PDHB enhanced the migration of CNE-1 and HNE-1 cells in the Boyden chamber assay (Fig. 3c). In summary, downregulation of PDHB promoted the growth and migration of the NPC cells.
Knocking down the expression of PDHB promoted the growth and migration of CNE-1 and HNE-1 cells. a Knocking down the expression of PDHB in CNE-1 and HNE-1 cells. b Crystal violet assay to examine the effects of knocking down PDHB on the growth of CNE-1 and HNE-1 cells. c Knocking down the expression of PDHB promoted the migration of CNE-1 and HNE-1 cells. **P < 0.01
PDHB inhibited the transformation of NPC cells driven by Ras
It has been reported that Ras-ERK signaling was activated in NPC [10]. We next tested whether overexpression of PDHB affected the oncogenic activity of Ras in NPC cells. It was found that overexpression of a constitutively active Ras, RasV12, dramatically promoted the growth of CNE-1 and HNE-1 cells (Fig. 4a, b). However, PDHB inhibited the growth of CNE-1 and HNE-1 cells driven by RasV12 (Fig. 4a-b), suggesting that the expression of PDHB impaired the oncogenic activity of RasV12. ERK was widely recognized as the downstream mediator of as signaling, and the activity of ERK was regulated by its phosphorylation. Therefore, we next examine whether PDHB affected the phosphorylation of ERK. As shown in Fig. 4c, overexpression of PDHB inhibited the phosphorylation of ERK in CNE-1 cells, while knocking down the expression of PDHB promoted the phosphorylation of ERK (Fig. 4c). Taken together, these data indicated that PDHB might negatively regulate the activity of ERK and thus inhibited the Ras-driven transformation.
Knocking down the expression of PDHB promoted the tumorigenesis of CNE-1 cells
We next studied the roles of PDHB in vivo by tumorigenesis assay. CNE-1 cells were labeled with luciferase, which enabled the visualization of tumor cells by the administration of luciferin, a substrate for luciferase. The labeled CNE-1 cells were knocked down the expression of PDHB and injected subcutaneously into the nude mice. The growth of the tumor cells were monitored using the in vivo imaging system. As shown in Fig. 5a, the signals for the activity of luciferase were dramatically stronger on day 42 than the control group (Fig. 5a), suggested the growth advantage of PDHB knocking down cells. Moreover, tumors formed by the PDHB knocking down cells were bigger than those formed by the control cells, which was demonstrated by the tumor weight (Fig. 5b).
Knocking down the expression of PDHB promoted the tumorigenesis of CNE-1 cells in vivo. a Monitoring tumorigenesis of CNE-1i/si con and CNE-1/si PDHB cells on day 42. Images were obtained at the indicated time point after injection, respectively. b Tumor weight. Data were represented as the mean ± SD. **P < 0.01
Discussion
In this study, we have shown that the expression of PDHB was downregulated in NPC cell lines. In the functional study, it was found that overexpression of PDHB inhibited the growth and migration of NPC cells, while knocking down the expression of PDHB promoted the growth, migration, and tumorigenecity of the NPC cells. The molecular mechanism study showed that PDHB inhibited the activity of ERK and the growth of NPC cells driven by the oncogenic Ras. These studies clearly demonstrated that PDHB showed suppressive roles in the progression of PDHB.
Metabolism alteration is one of the hallmarks of cancer cells [11]. Multiple metabolic enzymes have shown important functions in the carcinogenesis [11]. Lactate dehydrogenase A (LDHA) has been reported to be upregulated in several cancer types and show oncogenic roles in the carcinogenesis [8]. Also, the expression of pyruvate kinase PKM2, Hexokinase HK, and phosphofructokinase PFK was reported to be upregulated in lung cancer [8]. These studies emphasized the pivotal roles of glycolysis enzymes in the tumorgenesis. It has been reported that EBV-miR-BART1 was involved in regulating metabolism-associated genes in nasopharyngeal carcinoma [8]. Also, several studies have shown that Epstein-Barr virus oncoprotein LMP1 promoted the glycolysis in NPC cells, and targeting LMP1 mediated glycolysis sensitized NPC cells to the radiotherapy [12, 13]. With regard to the specific functions of the glycolysis-associated genes in the progression of NPC, inhibition of LDHA induced cell cycle arrest, apoptosis and increased radiosensitivity [12]. Our study revealed the suppressive roles of PDHB in NPC, indicating the functions of glycolysis in the progression of NPC.
ERK signaling has been reported to be involved in the tumorigenesis of NPC [14, 15]. Inhibition of ERK signaling by dasatinib suppressed the invasion of the NPC cells [16]. In this study, overexpression of PDHB inhibited the phosphorylation of ERK and the growth driven by RasV12, further demonstrating the suppressive roles of PDHB in NPC cells. Moreover, it has been reported that Ras modulated the activity of PDH and promoted the tumorigenesis o glioblastoma. Combining with this study, it seemed that Ras and PDH formed a positive feedback.
Although our study is very indicative, further investigations using agonist of PDHB would provide novel insights into the therapy of NPC.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Lourembam DS et al. Evaluation of risk factors for nasopharyngeal carcinoma in a high-risk area of India, the northeastern region. Asian Pac J Cancer Prev. 2015;16(12):4927–35.
Isobe K et al. Advanced nasopharyngeal carcinoma treated with chemotherapy and radiotherapy: distant metastasis and local recurrence. Int J Oncol. 1998;12(5):1183–7.
Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134(5):703–7.
Saunier E, Benelli C, Bortoli S. The pyruvate dehydrogenase complex in cancer: an old metabolic gatekeeper regulated by new pathways and pharmacological agents. Int J Cancer. 2015;138:809–17.
Kerbey AL, Richardson LJ, Randle PJ. The roles of intrinsic kinase and of kinase/activator protein in the enhanced phosphorylation of pyruvate dehydrogenase complex in starvation. FEBS Lett. 1984;176(1):115–9.
Goh WQ et al. DLAT subunit of the pyruvate dehydrogenase complex is upregulated in gastric cancer-implications in cancer therapy. Am J Transl Res. 2015;7(6):1140–51.
Sun XR et al. Expression of pyruvate dehydrogenase is an independent prognostic marker in gastric cancer. World J Gastroenterol. 2015;21(17):5336–44.
Cai Z et al. A combined proteomics and metabolomics profiling of gastric cardia cancer reveals characteristic dysregulations in glucose metabolism. Mol Cell Proteomics. 2010;9(12):2617–28.
Zuo Q et al. The Ras signaling pathway mediates cetuximab resistance in nasopharyngeal carcinoma. Biomed Pharmacother. 2011;65(3):168–74.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Lo AK et al. Activation of the FGFR1 signalling pathway by the Epstein-Barr virus-encoded LMP1 promotes aerobic glycolysis and transformation of human nasopharyngeal epithelial cells. J Pathol. 2015. doi:10.1002/path.4575.
Xiao L et al. Targeting Epstein-Barr virus oncoprotein LMP1-mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene. 2014;33(37):4568–78.
Lan YY et al. Epstein-Barr virus latent membrane protein 2A promotes invasion of nasopharyngeal carcinoma cells through ERK/Fra-1-mediated induction of matrix metalloproteinase 9. J Virol. 2012;86(12):6656–67.
Peng C et al. BRD7 suppresses the growth of nasopharyngeal carcinoma cells (HNE1) through negatively regulating beta-catenin and ERK pathways. Mol Cell Biochem. 2007;303(1–2):141–9.
Li YJ et al. Dasatinib suppresses invasion and induces apoptosis in nasopharyngeal carcinoma. Int J Clin Exp Pathol. 2015;8(7):7818–24.
Acknowledgments
This work was supported by the grants of Chen T. from the Medical Research Foundation of Guangdong Provincial Health and Family Planning Commission (A2015005), the National Natural Science Foundation of China (81402963), and the First Affiliated Hospital of Guangzhou Medical University (the “Young Elite Talents Program” and the “Science and Technology Support Projects”).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures performed in this study involving mouse were in accordance with the ethical standards of the hospital.
Conflicts of interest
None
Additional information
Hongbo Tang, Xinggu Luo, Juan Li and Yi Zhou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tang, H., Luo, X., Li, J. et al. Pyruvate dehydrogenase B promoted the growth and migration of the nasopharyngeal carcinoma cells. Tumor Biol. 37, 10563–10569 (2016). https://doi.org/10.1007/s13277-016-4922-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4922-4