Abstract
Hepatocellular carcinoma (HCC) is a major cause of cancer-related deaths owing to its high rate of postoperative recurrence and metastasis. New research is continuously identifying novel metastasis-associated oncogenes and tumor suppressor genes. miRNAs are noncoding RNAs that regulate protein synthesis post-translationally. miR-130b is one of several miRNAs involved in tumor metastasis. However, the role of miR-130b in HCC remains controversial. Here, we demonstrate that miR-130b is highly expressed in HCC and that it correlates with tumor number, vascular invasion, and TNM stage—important predictors of postoperative recurrence and metastases. Moreover, high levels of miR-130b predicted poor overall and disease-free survival of HCC patients, and in vitro and in vivo research revealed that knockdown or overexpression of miR-130b inhibited and promoted proliferation and metastasis of HCC cells, respectively. We identified PTEN as a direct functional target of miR-130b using miRNA databases and a dual luciferase report assay. Next, using a gain and loss assay and epithelial-mesenchymal transition (EMT) relative assays, we show that miR-130b may promote proliferation and EMT-induced metastasis via PTEN/p-AKT/HIF-1α signaling. Collectively, our data suggests that miR-130b may have prognostic value in HCC. Additionally, the miR-130b/PTEN/p-AKT/HIF-1α axis identified in this study provides novel insight into the mechanisms of HCC metastasis, which may facilitate the development of new therapeutics against HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy with more than 780,000 new HCC cases and around 745,000 HCC-related deaths annually, and the second most common cause of cancer-related deaths [1]. Liver transplantation remains the optimal therapeutic strategy, with a 5-year survival rate of more than 80 % [2]. However, in countries with severe shortages of liver donors, liver resection is the main therapeutic avenue for most patients [3]. Importantly, long-time survival remains unsatisfactory because of high rates of HCC recurrence and metastasis after liver resection [4]. An increasing number of researchers are therefore devoted to studying HCC metastasis. Recent research suggests that PTPRS [5], JARID1B [6], and Sox12 [7] are important regulators of HCC metastasis. However, the molecular mechanisms of HCC metastasis remain largely unclear.
MicroRNAs (miRNAs) are a class of small endogenously expressed noncoding RNAs that can induce RNA degradation or repress protein translation by directly binding to the 3′ untranslated region (3′-UTR) of target mRNA [4]. Indeed, miRNAs have been implicated in almost every biological process, particularly in cancer progression and metastasis. For instance, miR-1269 [8], miR-494 [9], and miR-422a [10] play key roles in HCC metastasis by downregulating mRNA targets involved in the metastasis. Moreover, miR-29 [11] and miR-26a [12] are associated with better or worse prognosis, respectively, which illustrates the importance of miRNAs as clinical biomarkers in addition to being important regulators of HCC.
To gain a better understanding of miRNAs deregulated in HCC, we previously analyzed miRNA expression patterns in solitary large HCC—a subtype with unique and pathological characteristics firstly described by our group [13]. We previously showed that while miR-140-5p [14] and miR-188-5p [13] function as important tumor suppressors in HCC, but miR-331-3p [15] may promote HCC metastasis by targeting PHLPP. Here, we focus on miR-130b, which is significantly upregulated in HCC compared with adjacent nontumorous liver tissues (ANLT) by miRNA array (6.799 vs. 1). miR-130b is highly expressed and functions as an oncogene in various malignant tumors [16–23]. However, the mechanism and prognostic value of miR-130b in HCC warrant further investigation.
Here, we comprehensively investigate the expression, prognostic value, biological functions, and molecular mechanism of miR-130b in HCC proliferation and metastasis both in vitro and in vivo.
Materials and methods
Patients and tissue specimens
A total of 150 pairs of HCC and ANLTs collected between January 2006 and December 2013 were randomly selected from patients who underwent liver resection at the Department of Surgery, Xiangya Hospital of Central South University. The 150 HCC patients were divided into two cohorts: patients who underwent liver resection after January 2009 were used as the training cohort, while the rest were used as the validation cohort (Supplementary Fig. 1). The samples were snap-frozen in liquid nitrogen and stored at −80 °C for later RNA extraction or formalin-fixed and paraffin-embedded for immunohistochemistry. Histopathological analyses were performed by two certified pathologists. The clinical and pathological features of these patients are described in Supplementary Table 1. All research protocols strictly complied with REMARK guidelines for reporting prognostic biomarkers in cancer [24]. Prior informed consent was obtained from all patients and the study was approved by the Ethics Committee of Xiangya Hospital of CSU.
More details are described in the Supplementary Materials and Methods.
Results
miR-130b is commonly upregulated in HCC tissues
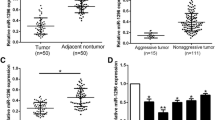
miRNA array analysis was performed to compare miRNA expression profiles in solitary large HCC (SLHCC), small HCC (SHCC), and nodular HCC (NHCC) as described in the “Supplementary material and methods.” miR-130b was upregulated more than threefolds in HCC compared with ANLTs (Fig. 1a). To validate this miRNA array-based result, miR-130b expression was measured in 150 pairs of HCC tissues and their corresponding ANLTs and these cases were divided into a training and validation cohort as described in the “Materials and methods” section. miR-130b expression was higher in 90.6 % of HCC tissues than in matched ANLT tissues in the training cohort (Fig. 1b). Similarly, in the validation cohort, miR-130b was upregulated in 86.2 % of HCC tissues (Fig. 1c).
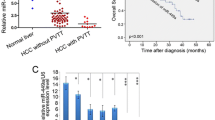
miR-130b is upregulated in HCC and is a promised biomarker for poor prognosis of HCC. a Cluster analysis of miRNAs expression profiles in HCC and ANLT. b, c Expression of miR-130b of HCC tissues and the corresponding ANLTs in training cohort (85 cases) and validation cohort (65 cases). Expression levels of miR-130b were normalized to the corresponding levels of U6 snRNA. Fold change were analyzed using the formula 2−(ΔΔCT[HCC/ANLT]). Red line indicates fold change of miR-130b equal to 2, which is cut off line to divide relative high miR-130b group and relative low miR-130b group. d, e miR-130b expression in HCC subtypes including SHCC, SLHCC, and NHCC in training cohort and validation cohort. The expression level was compared with ANOVA. f, g Overall survival and disease-free survival of HCC patients with different miR-130b expression. According to the data of qRT-PCR in (a, b), HCC cases in training cohort and validation cohort was classified into low-expression group and high-expression group. Survival curves were constructed using the Kaplan-Meier method and evaluated using the log-rank test
We previously identified a unique subtype of HCC named solitary large hepatocellular carcinoma (SLHCC), which was >5 cm in diameter, consisted of a single lesion, and always grew expansively within an intact capsule or pseudocapsule [25]. After hepatic resection, SLHCC had a similar long-term overall and disease-free survival to SLHCC (solitary nodular, diameter ≤ 5 cm), but much better than that of NHCC (node number ≥ 2) [25, 26]. In this study, these subtypes were analyzed individually within training and the validation cohorts. miR-130b expression was significantly higher in NHCC than in SLHCC or SHCC in both the training cohort (Fig. 1d) and the validation cohort (Fig. 1e), which is in agreement with the metastatic potential of SLHCC, SHCC, and NHCC.
Correlations between miR-130b expression and clinicopathologic characteristics
Considering that NHCC has the greatest metastatic potential among the three HCC subtypes [25], we investigated whether miR-130b correlates with metastasis-associated HCC clinicopathologic characteristics in the two cohorts. To evaluate the correlations between miR-130b and clinicopathological variables, patients were stratified according to their HCC miR-130b expression with those having higher than twofold increase relative to the corresponding ANLT, classified as the relative high-expression group and the rest as the relative low-expression group. First, we compared all clinicopathologic variables between the training and validation cohorts and found no significant differences (Table S1). We then analyzed the correlation between miR-130b expression and HCC clinicopathologic characteristics. miR-130b expression levels were significantly correlated with tumor number, vascular invasion, and TNM stage in both the training cohort (Table 1) and in the validation cohort (Table S2).
High miR-130b expression is associated with poor prognosis of HCC patients
Kaplan-Meier analysis showed that HCC patients with high miR-130b expression had poorer overall survival and disease-free survival than patients with low miR-130b expression in both the training (Fig. 1f) and validation cohorts (Fig. 1g).
Univariate and multivariate survival analyses were also performed to identify independent risk factors of overall survival and disease-free survival. Tumor number, vascular invasion, TNM stage, BCLC stage, and miR-130b expression were independent risk factors for overall survival in both the training cohort (Table S3) and the validation cohort (Table S5). Vascular invasion, BCLC stage, and miR-130b expression were independent risk factors for disease-free survival in both the training cohort (Table S4) and validation cohort (Table S6), while tumor number was also an independent risk factors for disease-free survival in the validation cohort (Table S6). Overexpression of miR-130b therefore appears to be a predictor of poor prognosis in HCC patients.
miR-130b promotes proliferation and metastasis in HCC cells in vitro
To study the role of miR-130b in HCC cells, we first measured miR-130b expression in these cells. Real-time PCR showed that, compared with a liver cell line, miR-130b was highly expressed in HCC cell lines (Fig. 2a). Interestingly, the HCCLM3 cell line, which has the highest metastatic potential among the five HCC lines, expressed the highest level of miR-130b. Of the five HCC cell lines, HCCLM3 and HepG2 cells were chosen for further in vitro study. We subsequently suppressed miR-130b expression in HCCLM3 and restored miR-130b expression in HepG2 by lentiviral-mediated inhibition and overexpression, respectively. Real-time PCR was used to confirm differential miR-130b expression in cells infected by lentivirus (Fig. 2b).
miR-130b promotes proliferation and metastasis of HCC cell in vitro. a miR-130b expression in liver cell line (L02) and five HCC cell lines (HepG2, SMMC7721, MHCC97-L, MHCC97-H, HCCLM3). miR-130b expression in L02 is set as 1. b HepG2 and HCCLM3 are infected by miR-130b overexpression lentivirus or anti-miR-130b lentivirus or their corresponding control lentivirus. qRT-PCR were used to validate the miR-130b expression in these cells infected by lentivirus. The wound-healing assay (c), transwell assay (d), growth curve assay (e), and colony formation assay (f) are performed as described in “Materials and methods” with HepG2NC, HepG2miR-130b, HCCLM3NC, and HCCLM3Anti-miR-130b cells to determine the role of miR-130b
A wound-healing assay and transwell assay were used to assess the role of miR-130b in HCC cell metastasis. miR-130b overexpression accelerated wound closure in HepG2 cells and miR-130b inhibition suppressed wound closure of HCCLM3 cells (Fig. 2c). Transwell assays with Matrigel showed that increased expression of miR-130b in HepG2 cells was associated with increased invasive activity (Fig. 2d), whereas reduced miR-130b expression in HCCLM3 cells resulted in reduced invasive activity (Fig. 2d). The role of miR-130b in HCC cell proliferation was assessed using cell growth curves and colony formation assays. The cell growth curve clearly showed increased proliferation of HepG2miR-130b compared with HepG2NC cells and decreased proliferation of HCCLM3Anti-miR-130b compared with HCCLM3NC cells (Fig. 2e). Similarly, colony formation assays showed significantly more colonies in HepG2miR-130b than in HepG2NC cells and significantly less colonies in HCCLM3Anti-miR-130b than in HCCLM3NC cells (Fig. 2f). These results indicate that miR-130b promotes proliferation and metastasis of HCC in vitro.
miR-130b promotes growth and metastasis of HCC in vivo
HepG2miR-130b, HepG2NC, HCCLM3Anti-miR-130b, and HCCLM3NC cells were implanted into Balb/c nude mice as described in the “Supplementary Materials and Methods” section. Tumors were significantly larger in HepG2miR-130b mice compared with HepG2NC mice (Fig. 3a) and smaller in HCCLM3Anti-miR-130b mice than HCCLM3NC mice (Fig. 3b). Moreover, rates of intrahepatic and pulmonary metastasis in HepG2miR-130b mice were significantly higher than in HepG2NC mice. Similarly, rates of intrahepatic and pulmonary metastasis (Fig. 3c–e) were significantly lower in HCCLM3Anti-miR-130b mice than in HCCLM3NC mice. Taken together, these results indicate that miR-130b promotes HCC growth and metastasis in vivo.
miR-130b promote growth and metastasis of HCC in vivo. The HCC xenograft mouse model was constructed by using HepG2NC and HepG2miR-130b cells (a) or HCCLM3NC and HCCLM3Anti-miR-130b (b) as described in “Materials and methods.” The tumor volume was measured and compared. c Representative figures for lung metastases of each group (hematoxylin and eosin stain; original magnification ×100 or ×400). The mice with intrahepatic (d) or lung metastatic nodules (e) were counted under microscope and analyzed
Phosphatase and tensin homolog (PTEN) is a direct target of miR-130b
We identified potential miR-130b target genes using the miRNA databases TargetScan, PicTar, and miRanda. PTEN was a predicted target of miR-130b based on their complementary sequences. Wild-type and mutant 3′-UTR PTEN target sequences were cloned into the pGL3 luciferase reporter vector (Fig. 4a). Using a dual luciferase reporter assay, we showed that miR-130b significantly inhibits the activity of PTEN wild-type 3′-UTR but not PTEN mutant 3′-UTR in both HCCLM3 and HepG2 cells (Fig. 4b, c). Western blot analysis showed that endogenous PTEN protein levels were indeed suppressed in HCC cells with relatively high miR-130b expression (Fig. 4d). We next evaluated the expression of potential downstream targets of PTEN by western blot analysis. The results showed that p-AKT and HIF-1α expression was positively associated with miR-130b expression in HepG2 and HCCLM3 cells, but that AKT expression was not affected by miR-130b expression (Fig. 4e). Taken together, these results indicated that miR-130b may inhibit p-AKT/HIF-1α signaling by directly targeting PTEN.
PTEN is direct downstream target for miR-130b. a miR-130b and its putative binding sequence in the 3′-UTR of PTEN. The mutant miR-130b binding site was generated in the complementary site for the seed region of miR-130b. Relative luciferase activity was analyzed in HepG2NC and HepG2miR-130b cells (b) or HCCLM3NC and HCCLM3Anti-miR-130b cells (c). Firefly luciferase reporter containing either a wild type or a mutant 3′-UTR (indicated as WT or Mut on the X axis). The normalized luciferase activity of wild type was set as relative luciferase activity. Western blot results of PTEN (d) or its potential downstream protein such as p-AKT, AKT, and HIF-1α (e) in HepG2NC, HepG2miR-130b, HCCLM3NC, and HCCLM3Anti-miR-130b cells. P < 0.05
miR-130b promotes cell proliferation and metastasis by suppressing PTEN expression
To examine whether miR-130b exerts its function via PTEN, we restored PTEN expression in HepG2miR-130b cells with a PTEN expression vector without the PTEN 3′-UTR and silenced PTEN expression in HCCLM3Anti-miR-130b cells with PTEN-shRNA. Restoration of PTEN in HepG2miR-130b cells suppressed miR-130b-induced proliferation and colony formation (Fig. 5a, b). In contrast, silencing PTEN in HCCLM3Anti-miR-130b cells promoted proliferation and colony formation suppressed by miR-130b silencing (Fig. 5a, b). Similarly, wound-healing and transwell assays showed that PTEN expression blocked miR-130b-mediated migration and metastasis of HepG2 cells (Fig. 5c, d). Conversely, silencing PTEN abrogated the function of anti-miR-130b in migration and metastasis (Fig. 5c, d). These results suggest that PTEN is a direct functional target of miR-130b, whereby miR-130b promotes HCC cell proliferation and metastasis.
miR-130b promotes proliferation and metastasis of hepatocellular carcinoma through PTEN/AKT/HIF-1α signaling. Cell proliferation analysis (a), colony formation assay (b), wound-closure assay (c), and transwell assay (d) in HCCLM3Anti-miR-130b and HepG2miR-130b cells infected with PTEN-shRNA vector or PTEN expression vector or their control vector. e Western blot results of p-AKT, AKT, and HIF-1α as well as EMT associated markers, Snail, N-cadherin, E-cadherin, and vimentin in HCCLM3Anti-miR-130b and HepG2miR-130b cells infected with or without PTEN-shRNA plasmids
miR-130b promotes metastasis via epithelial-mesenchymal transition
Epithelial-mesenchymal transition (EMT) is an important step during metastasis and migration [5, 27] and AKT and HIF-1α promote EMT in HCC [28]. In our study, we found that miR-130b and PTEN affects HCC cell morphology (S-Fig. 3). We therefore investigated whether miR-130b induces EMT in HCC. Western blot showed that miR-130b induced Snail, N-cadherin, and vimentin and suppressed E-cadherin via PTEN/p-AKT/HIF-1α (Fig. 5e). Moreover, p-AKT, HIF-1α, Snail, N-cadherin, and vimentin expression was higher in an HCC sample with high miR-130b expression (D107) than in another HCC sample with low miR-130b expression (D165); E-cadherin expression was also lower in the HCC sample with high miR-130b expression; and there was no significant difference in AKT expression between the two samples (Supplementary Fig. 4a). Similarly, in HCC tissues from the xenograft mouse model, HepG2miR-130b mice had higher p-AKT, HIF-1α, Snail, N-cadherin, and vimentin expression and lower E-cadherin expression than HepG2NC mice. HCCLM3Anti-miR-130b mice had lower p-AKT, HIF-1α, Snail, N-cadherin, and vimentin expression and higher E-cadherin expression than HCCLM3NC mice (Supplementary Fig. 4b). These results were consistent with the in vitro results. Taken together, these findings provide evidence that miR-130b may promote EMT through PTEN/p-AKT/HIF-1α signaling.
Discussion
Numerous studies indicate the critical role of miRNAs in tumorigenesis and progression in various human cancers [29]. Studies on cancer-specific miRNAs and their direct targets provide mechanistic insight into the process of tumorigenesis and enable identification of novel biomarkers and therapeutic targets for human cancers. miR-130b is commonly expressed in cancer with diverse roles depending on cancer type. In 2008, miR-130b was identified as one of the regulators of cell growth in T cell leukemia via its target protein TP53INP1 [30]. Several studies subsequently showed that miR-130b is overexpressed in different cancers [18–23]. miR-130b likely has specific functions depending on the type of malignancy. miR-130b was recently identified as a potential prognostic biomarker for HCC [31]. However, the prognostic value of miR-130b requires further validation with longer follow-up times. Our study included 150 HCC patients who underwent liver resection between January 2006 and December 2013. We showed that miR-130b was correlated with tumor number, vascular invasion, and TNM stage in the training and validation cohorts. We also demonstrated that miR-130b promotes HCC proliferation in vitro but that miR-130b expression was not significantly correlated with tumor size in HCC clinical samples. One possible explanation for this is that tumor size does not fully represent proliferative potential of HCC cells. For example, a tumor with low proliferative potential can reach the same size as tumor with high proliferative potential given enough time. We aim to evaluate this inference in future studies by determining whether miR-130b expression correlates with Ki67 expression, which is a marker of proliferation. Kaplan-Meier analysis showed that overexpression of miR-130b might indicate poor prognosis in HCC patients. In the validation cohort, the 7-year overall survival and disease-free survival rates in the high miR-130b-expression group was 11.8 and 8.2 %, and 38.4 and 24.8 % in the low-miR-130b expression group. Further study showed that miR-130b is an independent risk factor for overall survival and disease-free survival of HCC patients. These findings further support the prognostic value of miR-130b in HCC.
To further understand the underlying mechanisms of miR-130b, its downstream functional targets need to be identified. Tu et al. reported that miR-130b is elevated in HCC tissues and may promote HCC cell migration and invasion by inhibiting PPAR-γ [16]. However, using in silico analyses, we predicted another important downstream target of miR-130b, the tumor suppressor gene, PTEN. To validate whether miR-130b is able to inhibit PTEN expression, we used a dual luciferase reporter assay and western blot to confirm that miR-130b directly binds to the 3′-UTR sequence of PTEN and inhibits its expression in HCC cells. Complementary gain and loss of function assays revealed that PTEN recovery significantly diminished the effect of miR-130b on proliferation and metastasis. Our study provides the first concrete evidence that miR-130b suppresses HCC proliferation and metastasis by directly inhibiting PTEN expression.
TP53INP1 and PPAR-γ were previously reported to be targets of miR-130b [16, 32]. We therefore determined their expression in HepG2NC, HepG2miR-130b, HCCLM3NC, and HCCLM3Anti-miR-130b cell lines. TP53INP1 expression was similar across cell lines (Supplementary Fig. 5a), leading us to speculate that miR-130b may regulate TP53INP expression only in cancer stem cells [32]. PPAR-γ was, however, highly expressed in HCC cells with relatively low miR-130b expression (HepG2NC and HCCLM3Anti-miR-130b cells) indicating that PPAR-γ was also a miR-130b target (Supplementary Fig. 5a). We also determined PPAR-γ expression in HepG2miR-130b+PTEN, HepG2miR-130b+Vector, HCCLM3Anti-miR-130b+Vector, and HCCLM3Anti-miR-130b+PTEN-shRNA cell lines (Supplementary Fig. 5b), but PTEN mostly mimicked or eliminated the role of miR-130b without affecting PPAR-γ expression (Fig. 5; Supplementary Fig. 5b). Colangelo et al. reported that miR-130b promotes colorectal cancer development via PPARγ suppression, which in turn deregulates PTEN, E-cadherin, Snail, and vascular endothelial growth factor [33]. We therefore suggest that miR-130b promotes HCC proliferation and metastasis by two ways (Supplementary Fig. 6): by directly regulating PTEN expression and promoting tumor development and by regulating PPAR-γ expression, which then regulates PTEN expression to promote tumor development. The relationship between miR-130b, PTEN, and PPARγ, however, requires further validation.
EMT is an important step of tumor metastasis [34]. There is ample evidence that major signaling pathways such as TGF-β, Wnt, and AKT pathways involved in the regulation of transcription factors repress the transcription of E-cadherin and lead to EMT [35]. Although numerous factors participate in EMT, the role of miR-130b in EMT and cancer metastasis remains unclear. PTEN is a key inhibitor of the PI3K/AKT pathway [36], and emerging evidence suggests that PTEN regulates EMT of HCC cells via the AKT pathway [36, 37]. Interestingly, we also found that suppression or overexpression of miR-130b may significantly affect HCC cell morphology. We therefore speculated that miR-130b may induce HCC EMT through the PTEN/p-AKT pathway. We found that miR-130b expression was positively associated with vimentin and N-cadherin expression and negatively associated with E-cadherin expression in HCC cells or tissues. Taken together, these results suggest that miR-130b may regulate EMT via PTEN/p-AKT/HIF-1α signaling in HCC.
In summary, we found miR-130b to be highly expressed in HCC via miRNA arrays in various HCC subtypes. Our study demonstrates that miR-130b is an independent prognostic factor associated with aggressive tumor phenotypes in two cohorts. We are the first to show that miR-130b promotes proliferation and EMT-mediated metastasis of HCC via PTEN/AKT/HIF-1α. This implies that miR-130b may promote proliferation and metastasis by directly binding to PTEN mRNA or by inhibiting PPAR-γ expression, which then suppresses PTEN. We uncovered a novel function and molecular mechanism for miR-130b in HCC, which improves our understanding of proliferation and metastasis.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- SLHCC:
-
Solitary large hepatocellular carcinoma
- SHCC:
-
Small hepatocellular carcinoma
- NHCC:
-
Nodular hepatocellular carcinoma
- ANLT:
-
Adjacent nontumorous liver tissues
- miRNA:
-
MicroRNA
- 3′-UTR:
-
3′ Untranslated region
- mRNA:
-
Messenger RNA
- qRT-PCR:
-
Quantitative reverse-transcription polymerase chain reaction
- PTEN:
-
Phosphatase and tensin homolog
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262.
Otto G, Schuchmann M, Hoppe-Lotichius M, Heise M, Weinmann A, Hansen T, et al. How to decide about liver transplantation in patients with hepatocellular carcinoma: size and number of lesions or response to TACE? J Hepatol. 2013;59(2):279–84. doi:10.1016/j.jhep.2013.04.006.
Yang LY, Chang RM, Lau WY, Ou DP, Wu W, Zeng ZJ. Mesohepatectomy for centrally located large hepatocellular carcinoma: indications, techniques, and outcomes. Surgery. 2014;156(5):1177–87. doi:10.1016/j.surg.2014.05.012.
Giordano S, Columbano A. MicroRNAs: new tools for diagnosis, prognosis, and therapy in hepatocellular carcinoma? Hepatology. 2013;57(2):840–7. doi:10.1002/hep.26095.
Wang ZC, Gao Q, Shi JY, Guo WJ, Yang LX, Liu XY, et al. PTPRS acts as a metastatic suppressor in hepatocellular carcinoma by control of EGFR induced epithelial-mesenchymal transition. Hepatology. 2015. doi:10.1002/hep.27911.
Tang B, Qi G, Tang F, Yuan S, Wang Z, Liang X, Li B, Yu S, Liu J, Huang Q, Wei Y, Zhai R, Lei B, Yu H, Jiao X, He S. JARID1B promotes metastasis and epithelial-mesenchymal transition via PTEN/AKT signaling in hepatocellular carcinoma cells. Oncotarget. 2015;6(14):12723-12739. doi:10.18632/oncotarget.3713
Huang W, Chen Z, Shang X, Tian D, Wang D, Wu K, et al. Sox12, a direct target of FoxQ1, promotes hepatocellular carcinoma metastasis through up-regulating Twist1 and FGFBP1. Hepatology. 2015;61(6):1920–33. doi:10.1002/hep.27756.
Bu P, Wang L, Chen KY, Rakhilin N, Sun J, Closa A, et al. miR-1269 promotes metastasis and forms a positive feedback loop with TGF-beta. Nat Commun. 2015;6:6879. doi:10.1038/ncomms7879.
Chuang KH, Whitney-Miller CL, Chu CY, Zhou Z, Dokus MK, Schmit S, et al. MicroRNA-494 is a master epigenetic regulator of multiple invasion-suppressor microRNAs by targeting ten eleven translocation 1 in invasive human hepatocellular carcinoma tumors. Hepatology. 2015. doi:10.1002/hep.27816.
Zhang J, Yang Y, Yang T, Yuan S, Wang R, Pan Z, et al. Double-negative feedback loop between microRNA-422a and forkhead box (FOX)G1/Q1/E1 regulates hepatocellular carcinoma tumor growth and metastasis. Hepatology. 2015;61(2):561–73. doi:10.1002/hep.27491.
Parpart S, Roessler S, Dong F, Rao V, Takai A, Ji J, et al. Modulation of miR-29 expression by alpha-fetoprotein is linked to the hepatocellular carcinoma epigenome. Hepatology. 2014;60(3):872–83. doi:10.1002/hep.27200.
Yang X, Zhang XF, Lu X, Jia HL, Liang L, Dong QZ, et al. MicroRNA-26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor-cMet pathway. Hepatology. 2014;59(5):1874–85. doi:10.1002/hep.26941.
Fang F, Chang RM, Yu L, Lei X, Xiao S, Yang H, et al. MicroRNA-188-5p suppresses tumor cell proliferation and metastasis by directly targeting FGF5 in hepatocellular carcinoma. J Hepatol. 2015. doi:10.1016/j.jhep.2015.05.008.
Yang H, Fang F, Chang R, Yang L. MicroRNA-140-5p suppresses tumor growth and metastasis by targeting transforming growth factor beta receptor 1 and fibroblast growth factor 9 in hepatocellular carcinoma. Hepatology. 2013;58(1):205–17. doi:10.1002/hep.26315.
Chang RM, Yang H, Fang F, Xu JF, Yang LY. MicroRNA-331-3p promotes proliferation and metastasis of hepatocellular carcinoma by targeting PH domain and leucine-rich repeat protein phosphatase. Hepatology. 2014;60(4):1251–63. doi:10.1002/hep.27221.
Tu K, Zheng X, Dou C, Li C, Yang W, Yao Y, et al. MicroRNA-130b promotes cell aggressiveness by inhibiting peroxisome proliferator-activated receptor gamma in human hepatocellular carcinoma. Int J Mol Sci. 2014;15(11):20486–99. doi:10.3390/ijms151120486.
Lin YH, Wu MH, Liao CJ, Huang YH, Chi HC, Wu SM, et al. Repression of microRNA-130b by thyroid hormone enhances cell motility. J Hepatol. 2015;62(6):1328–40. doi:10.1016/j.jhep.2014.12.035.
Lai KW, Koh KX, Loh M, Tada K, Subramaniam MM, Lim XY, et al. MicroRNA-130b regulates the tumour suppressor RUNX3 in gastric cancer. Eur J Cancer. 2010;46(8):1456–63. doi:10.1016/j.ejca.2010.01.036.
Yang C, Cai J, Wang Q, Tang H, Cao J, Wu L, et al. Epigenetic silencing of miR-130b in ovarian cancer promotes the development of multidrug resistance by targeting colony-stimulating factor 1. Gynecol Oncol. 2012;124(2):325–34. doi:10.1016/j.ygyno.2011.10.013.
Li BL, Lu C, Lu W, Yang TT, Qu J, Hong X, et al. miR-130b is an EMT-related microRNA that targets DICER1 for aggression in endometrial cancer. Med Oncol. 2013;30(1):484. doi:10.1007/s12032-013-0484-0.
Zhao G, Zhang JG, Shi Y, Qin Q, Liu Y, Wang B, et al. MiR-130b is a prognostic marker and inhibits cell proliferation and invasion in pancreatic cancer through targeting STAT3. PLoS One. 2013;8(9):e73803. doi:10.1371/journal.pone.0073803.
Chen Q, Zhao X, Zhang H, Yuan H, Zhu M, Sun Q, et al. MiR-130b suppresses prostate cancer metastasis through down-regulation of MMP2. Mol Carcinog. 2014. doi:10.1002/mc.22204.
Yu T, Cao R, Li S, Fu M, Ren L, Chen W, et al. MiR-130b plays an oncogenic role by repressing PTEN expression in esophageal squamous cell carcinoma cells. BMC Cancer. 2015;15:29. doi:10.1186/s12885-015-1031-5.
Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012;9(5):e1001216. doi:10.1371/journal.pmed.1001216.
Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg. 2009;249(1):118–23. doi:10.1097/SLA.0b013e3181904988.
Wang W, Yang LY, Huang GW, Lu WQ, Yang ZL, Yang JQ, et al. Genomic analysis reveals RhoC as a potential marker in hepatocellular carcinoma with poor prognosis. Br J Cancer. 2004;90(12):2349–55. doi:10.1038/sj.bjc.6601749.
Xia H, Jianxiang C, Shi M, Gao H, Karthik S, Pratap SV, et al. EDIL3 is a novel regulator of epithelial mesenchymal transition controlling early recurrence of hepatocellular carcinoma. J Hepatol. 2015. doi:10.1016/j.jhep.2015.05.005.
Tang B, Qi G, Tang F, Yuan S, Wang Z, Liang X, et al. JARID1B promotes metastasis and epithelial-mesenchymal transition via PTEN/AKT signaling in hepatocellular carcinoma cells. Oncotarget. 2015;6(14):12723–39.
Hall DP, Cost NG, Hegde S, Kellner E, Mikhaylova O, Stratton Y, et al. TRPM3 and miR-204 establish a regulatory circuit that controls oncogenic autophagy in clear cell renal cell carcinoma. Cancer Cell. 2014;26(5):738–53. doi:10.1016/j.ccell.2014.09.015.
Yeung ML, Yasunaga J, Bennasser Y, Dusetti N, Harris D, Ahmad N, et al. Roles for microRNAs, miR-93 and miR-130b, and tumor protein 53-induced nuclear protein 1 tumor suppressor in cell growth dysregulation by human T-cell lymphotrophic virus 1. Cancer Res. 2008;68(21):8976–85. doi:10.1158/0008-5472.CAN-08-0769.
Wang WY, Zhang HF, Wang L, Ma YP, Gao F, Zhang SJ, et al. High expression of microRNA-130b correlates with poor prognosis of patients with hepatocellular carcinoma. Diagn Pathol. 2014;9:160. doi:10.1186/s13000-014-0160-5.
Ma S, Tang KH, Chan YP, Lee TK, Kwan PS, Castilho A, et al. miR-130b promotes CD133(+) liver tumor-initiating cell growth and self-renewal via tumor protein 53-induced nuclear protein 1. Cell Stem Cell. 2010;7(6):694–707. doi:10.1016/j.stem.2010.11.010.
Colangelo T, Fucci A, Votino C, Sabatino L, Pancione M, Laudanna C, et al. MicroRNA-130b promotes tumor development and is associated with poor prognosis in colorectal cancer. Neoplasia. 2013;15(10):1218–31.
Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158(1):171–84. doi:10.1016/j.cell.2014.06.004.
Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139(19):3471–86. doi:10.1242/dev.071209.
Tian H, Ge C, Li H, Zhao F, Hou H, Chen T, et al. Ribonucleotide reductase M2B inhibits cell migration and spreading by early growth response protein 1-mediated phosphatase and tensin homolog/Akt1 pathway in hepatocellular carcinoma. Hepatology. 2014;59(4):1459–70. doi:10.1002/hep.26929.
Du R, Wu S, Lv X, Fang H, Wu S, Kang J. Overexpression of brachyury contributes to tumor metastasis by inducing epithelial-mesenchymal transition in hepatocellular carcinoma. J Exp Clin Cancer Res : CR. 2014;33(1):105. doi:10.1186/s13046-014-0105-6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Clinical Subjects’ Key Project of Ministry of Health (No. 2010439), National Science & Technology Major Projects (2009ZX09103-681, 2012ZX100020122011), National Nature Science Foundation of China (No. 81272395), Key Project of National Nature Science Foundation of China (No. 81330057), and The Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20130162130007).
Conflicts of interest
None
Ethical standards
Prior informed consent was obtained from all patients and the study was approved by the Ethics Committee of Xiangya Hospital of CSU.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 30 kb)
Supplementary Table 1
(DOCX 30 kb)
Supplementary Figure 1
Flow diagram of patients included in study. (GIF 97 kb)
Supplementary Figure 2
The infection efficiency of miR-130b expression lentivirus or anti-miR-130b lentivirus in HepG2 (a) and HCCLM3 cells (b). (GIF 212 kb)
Supplementary Figure 3
Representative immunofluorescence images of the cellular morphology of HCC cells infected with miR-130b. Cytoskeleton and cell nuclei were stained with TRITC Phalloidin and DAPI, respectively. Original magnification × 400. (GIF 74 kb)
Supplementary Figure 4
miR-130b promote EMT of hepatocellular carcinoma through AKT/HIF1-α signaling. Immunohistochemistry of p-AKT, AKT, HIF1-α, Snail, N-cadherin, E-cadherin, and vimentin in HCC tissue with differential miR-130b expression (a) and in the HCC metastatic mouse model constructed by using HepG2NC,, HepG2miR-130b, HCCLM3NC and HCCLM3Anti-miR-130b cells (b). (GIF 570 kb)
Supplementary Figure 5
(a) PPAR-γ and TP53INP1 expression in HCC cell lines with different miR-130b expression. (b) PPAR-γ expression in HCC cell lines transfected with PTEN vector or PTEN-shRNA vector10. (GIF 47 kb)
Supplementary Figure 6
A schematic and simplified representation of the role and mechanism of miR-130b in HCC progression. (GIF 77 kb)
Rights and permissions
About this article
Cite this article
Chang, RM., Xu, JF., Fang, F. et al. MicroRNA-130b promotes proliferation and EMT-induced metastasis via PTEN/p-AKT/HIF-1α signaling. Tumor Biol. 37, 10609–10619 (2016). https://doi.org/10.1007/s13277-016-4919-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4919-z