Abstract
The aim of this study was to evaluate the potential prognostic significance of interleukin-33 (IL-33) in patients with clear-cell renal cell carcinoma (ccRCC) after surgical resection. In this retrospective research, we enrolled 203 patients with ccRCC undergoing nephrectomy between 2003 and 2004 in a single institution. We recorded clinicopathologic features, overall survival (OS), and recurrence-free survival (RFS) and assessed IL-33 expression by immunohistochemical staining. On such bases, the correlations between IL-33 expression and clinicopathologic features and prognosis were evaluated. A high expression of IL-33 was significantly associated with advanced TNM stage and Fuhrman grade (p = 0.017 and p < 0.001, respectively) in patients with ccRCC. Moreover, multivariate analysis identified IL-33 as an independent prognostic factor of OS for patients with ccRCC after surgery (hazard ratio = 2.050; 95 % CI 1.223–3.447; p = 0.006). The incorporation of IL-33 into the TNM stage and Fuhrman grade might help to refine individual risk stratification. The IL-33 expression may serve as an independent negative predictor of survival for patients with ccRCC after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) accounts for almost 90 % of all renal malignancies [1], and clear-cell renal cell carcinoma (ccRCC) comprises the major histological subtype of RCC [2]. Despite the advanced diagnostic technologies, such as the improved abdominal imaging, 20–30 % patients still present metastatic disease at diagnoses [3]. Besides, another 20 % of patients, having received surgical resection, are found during follow-up with relapses and metastatic RCC [1], which has an extremely poor prognosis. Currently, TNM stage, Fuhrman grade, and Eastern Cooperative Oncology Group performance status (ECOG-PS) have been proven to be independent prognostic factors for patients with ccRCC [4]. Based on these independent factors, several integrated prognostic systems have been established to predict patients’ outcome, such as the University of California Integrated Staging System (UISS) [5] and Mayo Clinic stage, size, grade, and necrosis (SSIGN) score [6]. However, precise prediction of clinical outcomes remains difficult and improved predictors are still much needed. Previous studies have demonstrated that some specific RCC biomarkers have the potential to improve the prognostic ability when combined with traditional clinicopathologic variables [7].
IL-33, which is a member of IL-1 family, is constitutively expressed in endothelial and epithelial cells of mucous membranes, fibroblasts, and keratinocytes [8]. It can lead to the activation of nuclear factor κB (NF-κB) and mitogen-activated protein kinase signaling pathways through binding to its receptor interleukin-1 receptor-like 1 (ST2) [9], which is expressed on most cells especially on mast cells and activated type 2 helper T (Th2) cells [10]. IL-33 mainly promotes Th2 immune responses [11], and it is believed to have a close relationship with some chronic inflammatory diseases, such as asthma and rheumatoid arthritis [12]. Upon cell necrosis, IL-33 is released from the nucleus, and thus, it may serve as an endogenous danger signal to alert the immune system after endothelial or epithelial cell damage [13]. By far, the role of IL-33 in carcinogenesis has not been well understood, but there is increasing evidence that IL-33 can induce angiogenesis [14] and alternative macrophage activation [15], suggesting that it may play an important role in tumor growth and progression. Previous studies have demonstrated that abundant expression of IL-33 can be observed in human tumors [16], and it is of prognostic value in hepatocellular carcinoma [17], breast cancer [18], and lung cancer [19]. However, few studies have been designed to investigate the prognostic value of IL-33 in RCC, especially in ccRCC.
In this study, the expression of IL-33 was assessed by immunohistochemistry in ccRCC tumor tissues, and its associations with clinicopathologic features and prognosis were evaluated. Moreover, we explore the hypothesis that incorporation of TNM stage and UISS with IL-33 expression could refine these prognostic models.
Patients and methods
Patients
In this retrospective study, we enrolled 203 patients with ccRCC who received nephrectomy at Zhongshan Hospital of Fudan University (Shanghai, China) between 2003 and 2004. The inclusion criteria were as follows: no history of previous anticancer therapy, acceptance of radical or partial nephrectomy, and histopathologically proven ccRCC. Patients who suffered from other types of renal cancer, had tumors with necrosis >80 %, or died within the first month after surgery due to surgical complications were excluded. For each patient, the following clinicopathologic information was collected: age at the time of surgery, gender, tumor size, TNM stage, Fuhrman grade, presence of tumor necrosis, and ECOG-PS. All the patients were staged based on radiographic reports and postoperative pathologic data and were reassigned in accordance to the 2010 American Joint Committee on Cancer TNM classification [20]. A total of nine patients with N1- or M1-stage tumors were considered to have metastasis and were excluded while analyzed for RFS. Tumor size was recorded as the longest diameter described in pathologic reports. Tumor necrosis was defined as the presence of microscopic coagulative necrosis. The UISS predictive models were applied to classify patients into three risk groups (low risk [LR], intermediate risk [IR], and high risk [HR]). We calculated OS and RFS from the date of surgery to the date of death and recurrence, respectively, or to the date of last follow-up. This study was approved by the hospital’s Ethics Committee, and informed consent was obtained from each patient.
Tissue microarray and immunohistochemistry
Primary anti-IL-33 antibody (1:600 dilution, Abcam, Cambridge, MA, USA) was used for immunohistochemical staining on tissue microarray (TMA), which was established with formalin-fixed, paraffin-embedded tissue blocks of 203 patients. All the cases were stained at once. The detailed procedure was carried out as previously described [21]. The staining intensity of each specimen was scored independently by two pathologists without the knowledge of the patient’s outcome. A semi-quantitative immunochemistry score for each specimen, ranging from 0 to 300, was calculated by multiplying the staining intensities (0, negative; 1, weak staining; 2, moderate staining; 3, strong staining) by the area distributions (0–100 %). Based on the association with the patients’ OS, we selected the optimum cutoff score (205) for the expression of IL-33 through using X-tile software version 3.6.1 (Yale University School of Medicine, New Haven, CT, USA).
Statistical analysis
SPSS 21.0 (IBM Corporation, Armonk, NY, USA), R software version 3.0.2 and the “rms” package (R Foundation for Statistical Computing, Vienna, Austria) were applied for statistical analysis. Categorical variables were compared by Pearson χ 2 test or Fisher’s exact test while continuous variables were analyzed by Wilcoxon rank-sum test or Kruskal–Wallis test. Survival curves were established using the Kaplan–Meier method, and the log-rank test was applied to compare the difference between curves. The Cox proportional hazards regression model was used to perform univariate and multivariate analyses. The Harrell’s Concordance index (C-index) was used to quantify the predictive accuracy of multivariate models. All statistical tests were two-sided and were performed at a significance level of 0.05.
Results
Correlations between patients’ characteristics and IL-33 expression
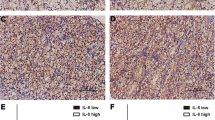
A total of 203 patients with ccRCC were included, and 69.5 % of them were men. As it is summarized in Table 1, the median age at surgery and tumor size were 54 years and 4.0 cm, respectively. Positive staining of IL-33 was mainly observed in cytoplasm and membrane (Fig. 1), and 74.4 % (151/203) of the tumor tissues were considered to have low IL-33 expression. As shown in Table 1, a high IL-33 expression was statistically associated with advanced TNM stage (P = 0.017), high Fuhrman grade (P < 0.001), presence of tumor necrosis (P = 0.003), and high UISS risk-level (P = 0.025).
Kaplan–Meier survival analysis and subgroup analysis
We applied Kaplan–Meier survival analysis to compare OS and RFS, respectively, in order to investigate the relations between IL-33 expression and patients’ outcomes. Patients with synchronous nodal (N1) or distant metastasis (M1) were excluded during the analyzing of RFS. Patients with high IL-33 expression tended to have poorer OS and RFS (P < 0.001 and P = 0.002, respectively; Fig. 2a, d).
Kaplan–Meier analysis of overall survival (OS) and recurrence-free survival (RFS) based on the expression of IL-33. Kaplan–Meier analysis of OS in all cases (a), patients with TNM stage I (b), and stage II (c). Kaplan–Meier analysis of RFS in all cases (d), patients with T1N0M0 (e), and T2N0M0 (f). P value was calculated by log-rank test
To investigate if IL-33 expression could stratify patients with different TNM stage, we performed a subgroup analysis by TNM stage. And, we found that IL-33 expression showed a strong relevance with OS in patients with TNM stage I and stage II (p < 0.001 for both; Fig. 2b, c) but not for patients with TNM stage III and TNM stage IV (P = 0.083 and P = 0.127, respectively; Fig. S1a, b). And, for nonmetastatic ccRCC, IL-33 expression could also be a prognostic factor for recurrence in patients with T1 stage and T2 stage (P = 0.011 and P < 0.001, respectively; Fig. 2d, e) whereas it failed in patients with T3–4 stage (P = 0.472; Fig. S1c)
We further performed a subgroup analysis by UISS groups. In UISS low-risk group, IL-33 expression was of prognostic value for both of OS and RFS (P = 0.002 for both; Fig. 3a, c). However, in UISS intermediate/high-risk group, IL-33 expression could only stratify patients for OS (P = 0.021; Fig. 3b), while it failed in terms of RFS (P = 0.074; Fig. 3d).
Subgroup analysis to identify the prognostic value of IL-33 by UISS in ccRCC patients. Kaplan–Meier analysis of OS in patients classified into UISS low-risk group (a) and UISS intermediate/high-risk group (b), Kaplan–Meier analysis of RFS in patients classified into UISS low-risk group, (c) and UISS intermediate/high-risk group (d). P value was calculated by log-rank test
Next, we used univariate and multivariate Cox regression analysis to evaluate the prognostic value of IL-33. As shown in Table 2, IL-33 had strong relevance with OS and RFS (P < 0.001 and P = 0.003, respectively). Based on multivariate analysis, IL-33 remained an independent prognostic factor for OS (HR 2.050, 95 % CI 1.223–3.447, P = 0.006), as well as TNM stage (P < 0.001), Fuhrman grade (P = 0.001), and ECOG-PS (P = 0.026). However, IL-33 failed to be proven as an independent prognosticator of RFS (P = 0.159) (Table 2).
Extension of prognostic models with IL-33 expression
We combined IL-33 expression with TNM stage, UISS, and SSIGN scores, respectively, to assess whether the predictive accuracy could be improved for OS. The C-indices of the TNM stage, UISS, and SSIGN scores were originally 0.692, 0.666, and 0.732, respectively, and increased to 0.749, 0.730, and 0.760 when IL-33 expression was incorporated (Table 3).
Predictive nomogram for OS
Depending on the proven independent indicators consisting of TNM stage, Fuhrman grade, ECOG-PS, and IL-33 expression, we constructed a nomogram to further predict the outcome of the patients (Fig. 4a). The calibration plots are shown for 5- and 10-year survival (Fig. 4b, c). The C-index of the prognostic model based on TNM stage, Fuhrman stage, and ECOG-PS was 0.755, and it improved to 0.787 when the IL-33 expression was incorporated.
Discussion
To our knowledge, this is the first study to investigate the prognostic value of IL-33 expression in tumor tissues for patients with ccRCC after surgery. High level of IL-33 expression is associated with shortened survival time and increased risk of recurrence, especially for patients categorized into UISS low-risk group and TNM stage I and II. Furthermore, high IL-33 expression is proven to be an independent negative prognostic factor of OS. In addition, the incorporation of intratumoral IL-33 expression into TNM staging system, UISS, and SSIGN scores would refine the predictive accuracy of these models for OS. Thus, we consider the IL-33 expression as a potential prognostic biomarker for better individual risk stratification to select patients who need to receive adjuvant therapy and intense follow-up after surgery, even if they have a low-risk according to the traditional clinicopathologic analysis. However, IL-33 fails to demonstrate a statistically significant association with RFS after multivariate analysis in our study. Thus, it needs to be further investigated whether IL-33 was an independent prognostic biomarker for patients’ RFS in larger cohorts.
Recent studies have demonstrated that IL-33 could be involved in the development and progression of many tumor types, such as breast cancer [18], myeloproliferative neoplasms [22], and gastric cancer [23]. In BALB/c mice, repeated administration of IL-33 enhanced mammary tumors progression and metastases to lungs and livers could be observed [18]. Besides, serum IL-33 may serve as a diagnostic and prognostic marker in nonsmall cell lung cancer [19]. In line with these findings, our data shows that high levels of IL-33 were remarkably correlated with advanced tumor stages and poor clinical outcomes.
One of the possible mechanisms for these phenomena may be the ability of IL-33 to induce angiogenesis and vascular permeability, which is indispensible for the growth and metastatic potential of all solid tumors. In addition, IL-33 can lead to the activation of NF-κB by binding to its receptor ST2 [24]. An early report has revealed that NF-κB can be an activator of anti-apoptotic genes and induce proinflammatory cytokines which have negative effects on tumor development [25]. In this way, the downstream transduction of IL-33 signal pathway may promote certain kinds of malignancies. Moreover, IL-33 is believed to have the ability to promote Th2 immune response, which in turn can facilitate intratumoral accumulation of immunosuppressive cells, thus creating a microenvironment beneficial for tumor development and progression [18].
In addition to prognostic power, biomarkers have the potential to become therapeutic targets. One study demonstrated that the deletion of IL-33 receptor, ST2, could significantly attenuate mammary tumor growth and metastasis in the murine model [26], suggesting that targeting IL-33 signaling pathway with antagonist might be a novel and efficient anticancer therapy in the future. The potential roles of IL-33 and its antagonist in malignancies are worthy to be fully investigated.
We acknowledge that there were a few limitations in our study. First, due to the retrospective nature and no external validation, the prognostic significance of IL-33 in ccRCC patients remains to be investigated prospectively in other populations and larger cohorts. Second, the TMA technique only displays a small piece of the original tumor, and the information contained may not be typical. Third, blood samples in our study are not available, and we hope to evaluate the prognostic value of serum IL-33 in the future.
In conclusion, our study is the first to demonstrate the intratumoral IL-33 expression as an independent prognostic factor in ccRCC. High expression of IL-33 is associated with shortened survival, especially in patients with early stage of disease. When incorporated with TNM stage, UISS, and SSIGN, IL-33 could improve the prognostic accuracy. In addition, IL-33 signaling pathway might be a promising target for individualized ccRCC therapeutic strategy.””
References
Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–21.
Oosterwijk E, Rathmell WK, Junker K, Brannon AR, Pouliot F, Finley DS, et al. Basic research in kidney cancer. Eur Urol. 2011;60:622–33.
Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205.
Lang H, Lindner W, de Fromont M, Molinie V, Letourneux H, Meyer N, et al. Multicenter determination of optimal interobserver agreement using the Fuhrman grading system for renal cell carcinoma—assessment of 241 patients with >15-year follow-up. Cancer. 2005;103:625–9.
Zisman A. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–66.
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–400.
Sun M, Shariat SF, Cheng C, Ficarra V, Murai M, Oudard S, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol. 2011;60:644–61.
Jovanovic IP, Pejnovic NN, Radosavljevic GD, Arsenijevic NN, Lukic ML. IL-33/ST2 axis in innate and acquired immunity to tumors. Oncoimmunol. 2012;1:229–31.
Zhao Q, Chen G. Role of IL-33 and its receptor in T cell-mediated autoimmune diseases. BioMed Res Int. 2014;2014:587376.
Jiang HR, Milovanovic M, Allan D, Niedbala W, Besnard AG, Fukada SY, et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-gamma production and inducing alternatively activated macrophages. Eur J Immunol. 2012;42:1804–14.
Schmitz J, Owyang A, Oldham E, Song YL, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90.
Miller AM. Role of IL-33 in inflammation and disease. J Inflamm. 2011;8:22.
Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3, e3331.
Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114:3117–26.
Joshi AD, Oak SR, Hartigan AJ, Finn WG, Kunkel SL, Duffy KE, et al. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010;11:52.
Masamune A, Watanabe T, Kikuta K, Satoh K, Kanno A, Shimosegawa T. Nuclear expression of interleukin-33 in pancreatic stellate cells. Am J Physiol-Gastr L. 2010;299:G821–32.
Bergis D, Kassis V, Ranglack A, Koeberle V, Piiper A, Kronenberger B, et al. High serum levels of the interleukin 33 receptor soluble ST2 as a negative prognostic factor in hepatocellular carcinoma. J Hepatol. 2013;58:S257–7.
Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, et al. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer J Int Du Cancer. 2014;134:1669–82.
Hu L-A, Fu Y, Zhang D-N, Zhang J. Serum IL-33 as a diagnostic and prognostic marker in non-small cell lung cancer. Asian Pac J Cancer Prev. 2013;14:2563–6.
Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Pajares MJ, Agorreta J, Larrayoz M, Vesin A, Ezponda T, Zudaire I, et al. Expression of tumor-derived vascular endothelial growth factor and its receptors is associated with outcome in early squamous cell carcinoma of the lung. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30:1129–36.
Mager LF, Riether C, Schurch CM, Banz Y, Wasmer MH, Stuber R, et al. IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J Clin Invest. 2015;125:2579–91.
Yu XX, Hu Z, Shen X, Dong LY, Zhou WZ, Hu WH. IL-33 promotes gastric cancer cell invasion and migration via ST2-ERK1/2 pathway. Dig Dis Sci. 2015;60:1265–72.
Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–40.
Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6.
Jovanovic I, Radosavljevic G, Mitrovic M, Juranic VL, McKenzie AN, Arsenijevic N, et al. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur J Immunol. 2011;41:1902–12.
Acknowledgments
This study was funded by grants from the National Basic Research Program of China (2012CB822104), National Key Projects for Infectious Diseases of China (2012ZX10002012-007, 2016ZX10002018-008), National Natural Science Foundation of China (31100629, 31270863, 81372755, 31470794, 81401988, 81402082, 81402085, 81471621, 81472227, 81472376, 31570803, 81501999, and 81572352), and Program for New Century Excellent Talents in University (NCET-13-0146). All these study sponsors have no roles in the study design, in the collection, analysis, and interpretation of data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Zewei Wang, Le Xu and Yuan Chang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Kaplan-Meier analysis of OS in patients with TNM stage III (a) and stage IV (b) based on the expression of IL-33. Kaplan-Meier analysis of RFS in patients with T3-4N0M0 (c). P value was calculated by log-rank test. (GIF 16 kb)
Rights and permissions
About this article
Cite this article
Wang, Z., Xu, L., Chang, Y. et al. IL-33 is associated with unfavorable postoperative survival of patients with clear-cell renal cell carcinoma. Tumor Biol. 37, 11127–11134 (2016). https://doi.org/10.1007/s13277-016-4879-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4879-3