Abstract
Tumor recurrence and metastasis remain the major obstacles for the successful treatment of patients diagnosed with prostate cancer (PCa). In recent years, long non-coding RNAs (lncRNAs) have been considered as key regulators of tumor behavior. In this study, we investigated the biological role and clinical relevance of the lncRNA LOC400891 in prostate cancer. Using of lncRNAs expression chips screening and the biological analysis, we found the target lncRNA (LOC400891). Moreover, the expression levels of lncRNA LOC400891 in PCa tissues and cell lines were evaluated by quantitative real-time PCR (qRT-PCR), and its association with biochemical recurrence-free survival of patients was analyzed by statistical analysis. Furthermore, the effect of LOC400891 on proliferation, migration, and invasion was studied in PCa cells. We found that the expression level of LOC400891 was higher in PCa tissues and cells compared to adjacent non-tumor tissues and normal prostate stromal immortalized cells WPMY-1. The patients with higher LOC400891 expression had an advanced clinical features and a shorter biochemical recurrence-free survival time than those with lower LOC400891 expression. Furthermore, multivariate analysis showed that the status of LOC400891 expression was an independent predictor of biochemical recurrence-free survival in PCa. We also found that knockdown of LOC400891 could inhibit cell proliferation, migration, and invasion in vitro study. Our data suggested that lncRNA LOC400891 was a novel molecule involved in PCa progression, which provided a potential prognostic biomarker and therapeutic target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) is the second most common cause of malignancy in humans worldwide [1]. In America, the incidence and mortality rates of PCa are greater than lung carcinoma, and PCa has become the most common cancer among male patients [2]. What is more, in recent years, the occurrence of PCa in Asia has increased more rapidly than in Europe, the USA, and other developed countries [3]. According to the China National Cancer Prevention and Control Center annual report, the latest data for malignant tumors in males show that PCa is sixth in incidence and ninth in mortality. Because the mechanisms underlying PCa development have not been elucidated, the study of PCa remains extremely important.
The maturation and spread of high-throughput sequencing technologies has enabled increasingly complex analyses of the cellular transcriptome, with the nomination of numerous novel RNA species [4, 5], which can provide lncRNA profiles and other important information. Among these, long non-coding RNAs (lncRNAs) over 200 bp in length have been implicated as fundamental players in numerous molecular processes, including cell differentiation, lineage specificity, neurologic disorders, and cancer [6–8].
LncRNAs have been shown to be involved in the development and progression of PCa. One LncRNA, PCAT-18, which could be detected in plasma, incrementally increased its expression as prostate cancer progresses from localized to metastatic stages [9]. Another LncRNA, SChLAP1, increased its expression with prostate cancer progression. Moreover, higher expression level of SChLAP1 was associated with poor outcome among patients with clinically localized prostate cancer after radical prostatectomy [10]. However, research concerning lncRNA involvement in prostate cancer is in its preliminary stage. In hundreds of studies, PCA3 has been used in many different applications, including the diagnosis, treatment, and prediction of PCa. Additionally, TMPRSS2-ERG were reported in urinary exosomes, showing that microvesicles may contain disease-specific molecules which then can serve as promising novel biomarkers [11, 12]. However, the expression of lncRNAs and their biological functions still remain unknown.
In this study, we analyzed the novel lncRNA LOC400891 expression in 81 pairs of PCa samples compared with normal prostate tissue samples and investigated its potential biomedical functions. Our results proved that LOC400891 may provide new molecular biomarkers or a new basis for the treatment of PCa.
Materials and methods
Patient samples and microarray analysis
Written informed consent was obtained from all patients, and the study was approved by the Institutional Review Board of First Affiliated Hospital of Nanjing Medical University. Eighty-one patients with PCa who underwent open radical prostatectomy or laparoscopic radical prostatectomy were included in the study. Fresh tissue adjacent to carcinoma tissues and the carcinoma specimen of PCa were collected and confirmed by histopathology. Of these patients, 3 were used for microarray analysis of lncRNAs and 78 were used for additional evaluations. Microarray analysis was used to investigate the candidate molecular targets. PCa and matched histologically normal prostate tissue from each subject were snap-frozen in liquid nitrogen immediately after resection.
Cell lines and culture conditions
Human PCa cell lines (PC-3, DU-145, 22RV1) and a normal myofibroblast stromal cell line (WPMY-1) were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in F-12K or RPMI 1640 or DMEM (GIBCO-BRL, Carlsbad, CA, USA) medium supplemented with 10 % fetal bovine serum (10 % FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin in humidified air at 37 °C with 5 % CO2.
RNA extraction and qRT-PCR analyses
Total RNA was extracted from tissues or cultured cells with TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocols. For qPCR, RNA reverse transcribed to complementary DNA (cDNA) from 1 μg of total RNA was reverse transcribed in a final volume of 10 μL using random primers and a Reverse Transcription Kit (Takara, Dalian, China). According to the manufacturer’s instructions, the reverse transcription was performed at 37 °C for 15 min, then 85 °C for 5 s. Real-time PCR (RT-PCR) analyses were performed using a standard protocol from Power SYBR Green (Takara) on an ABI StepOne Plus instrument (Applied Biosystems, Carlsbad, CA, USA) and in a total reaction volume of 10 μL, including 5 μL of SYBR Premix (2x), 0.4 μL of PCR forward primer (10 μM), 0.4 μL of PCR reverse primer (10 μM), 0.2 μL ROX Reference Dye II (50x), 1 μL of cDNA, and 3 μL of double-distilled water. The quantitative real-time PCR reaction included an initial denaturation step of 10 min at 95 °C, and 95 °C (30 s), 95 °C (5 s), 61 °C (30 s) in a total 50 cycles with a final extension step at 72 °C for 5 min. △C t values were normalized to β-actin. The relative quantitative value was expressed by the 2−ΔΔCt method. Each experiment was performed in triplicates and repeated three times.
The PCR primers were as follows:
- LOC400891::
-
Forward: 5′-GAGGGAGACACTTGGACCAG-3′
Reverse: 5′-CACGTTGTTGGCCTTCAGAG-3′
- β-actin::
-
Forward: 5′-ACTGGAACGGTGAAGGTGAC-3′
Reverse: 5′-AGAGAAGTGGGGTGGCTTTT-3′
Small interfering RNA transfection
Small interfering RNA that targeted LOC400891 RNA and scrambled negative control was purchased from GenePharma (GenePharma, Shanghai, China). The target sequences for si-Loc400891 included si-Loc400891-1 (CCCAUAGUGUUGUGCCCAUTT), si-Loc400891-2 (GCUGGACCUUCGAGACAAUTT), and si-Loc400891-3 (CCAGACGCUGAGGAUUCUUTT), and the experiments showed that the later had the highest inhibition efficiency. Synthetic sequence-scrambled small interfering RNA (siRNA) was used as a negative control siRNA. Cells were plated and cultured in growth media until cell density reached 70 % prior to siRNA transfection using Lipofectamine 2000 (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. Cells were harvested after 48 h for qRT-PCR and Western blot analyses.
Plasmid constructs
The sequence of LOC400891 was synthesized and subcloned into pCDNA3.1 (BioEasy, Shanghai, China) vector. Ectopic expression of LOC400891 was achieved by using the pCDNA LOC400891 transfection and empty pCDNA vector (empty) was used as a control. The expression level of LOC400891 was detected by qPCR.
Cell proliferation assays
Forty-eight hours after siRNA or pcDNA LOC400891 transfection, 2 × 103 cells per well were allowed to grow in 96-well plates with five replicate wells. After 6–8 h of culture, as well as at hours 24, 48, 72, and 96, cells were treated with 10 μL Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) by adding it to the medium. The cells were incubated at 37 °C for another 4 h. Finally, the absorbance was measured at 490 nm. All experiments were performed in triplicates.
Colony formation and clonogenic assays
Cells were trypsinized into single-cell suspension 48 h after transfection. For the colony formation assay, 8 × 102 cells were plated into each media plate and maintained in media containing 10 % FBS to allow colony formation, replacing the nutrient solution every 4 days. After 2 weeks, colonies were fixed with methanol and stained with 0.1 % crystal violet (Beyotime, Beijing, China). Visible colonies were manually counted. Triplicate plates were measured in each treatment group.
Cell migration/invasion assay
Cells (2.5 × 104) were suspended in 200 μL with serum-free medium and seeded in the top chamber of the transwell (Millicell, PIEP12R48) with a membrane pore with an 8-μm diameter at 48 h after siRNA transfection. Culture medium containing 10 % FBS was placed in the bottom chamber. Cells were incubated for 48 h at 37 °C, and the cells that did not migrate through the pores of the transwell inserts were manually removed with a cotton swab. Cells which passed through the filter were fixed and stained using 0.1 % crystal violet. Numbers of invaded cells were counted in five randomly selected fields under a microscope, and the average value was calculated. Each experiment was conducted in triplicate. Matrigel invasion assays were performed as described previously [13].
Western blot assay and antibodies
Cells were lysed using the mammalian protein extraction reagent RIPA (Beyotime, Beijing, China) supplemented with a protease inhibitor cocktail (Roche, Shanghai, China) and PMSF (Roche, Shanghai, China). Protein was separated by 10 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and then transferred to polyvinylidene difluoride membranes (Sigma, St. Louis, MO, USA). The membranes were incubated with the specific primary antihuman antibodies. The secondary antibody was horseradish peroxidase-conjugated goat anti-rabbit IgG. Enhanced chemiluminescence chromogenic substrate was used to visualize the bands, and the intensity of the bands was quantified by densitometry (Quantity One software; Bio-Rad, City, CA, USA). GAPDH antibody was used as control. Rabbit monoclonal antibody PI3k, p-PI3k, AKT, p-AKT, mTOR, p-mTOR, PTEN, p-PTEN, vimentin, and β-catenin (1:1000) were purchased from Cell Signaling Technology, Inc. (St. Louis, MO, USA). Mouse monoclonal antibody Twist and snail (1:1000) were purchased from Abcam (USA).
Statistically analysis
All data were expressed as mean ± SD (standard deviation, SD), and analyses were performed using SPSS version 17.0 software (IBM). Statistical significance was tested by a Student’s t test, or a chi-square test as appropriate. Patient survival was evaluated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate Cox regression analyses were performed to analyze the survival data. Values of P < 0.05 were considered statistically significant.
Results
Expression of LOC400891 in prostate cancer
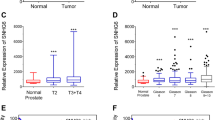
Based on the features of the differentially expressed lncRNAs from microarray data, we identified the lncRNA (LOC400891) listed in Table S1 for the qRT-PCR analysis in 81 pairs of PCa samples. The result supported a strong consistency between the qRT-PCR result and microarray data (Fig. S1) and also demonstrated that LOC400891 was significantly up-regulated in PCa samples compared to adjacent non-tumor samples (P < 0.05, Fig. 1a). Expression was further examined by qRT-PCR in three PCa cell lines and a normal human myofibroblast stromal cell line WPMY-1. This experiment revealed that LOC400891 was more over-expressed in DU-145 and 22RV1 than in WPMY-1 (P < 0.05, Fig. 1b).
Relative LOC400891 expression in prostate cancer and its clinical significance. Relative expression of LOC400891 in prostate cancer (PCa) tissues compared with corresponding non-tumor tissues (a). Relative expression of LOC400891 in three prostate cell lines (not expressed in PC3) compared with normal myofibroblast stromal cell line WPMY-1 (b). According to the cut-off value of the relative LOC400891 expression, the 81 PCa patients were divided into relative low-LOC400891 (n = 31) and a relative high-LOC400891 expression groups (n = 50) (c). Data represent the mean ± SD from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001
Relationship between LOC400891 expression and clinicopathological factors in patients with prostate cancer
Patients with PCa were classified into two groups based on a twofold cut-off of relative LOC400891 expression (Fig. 1c). The high-LOC400891 group had LOC400891 expression levels higher than the cut-off value (n = 50), and the low-LOC400891 group had LOC400891 expression levels lower than the cut-off value (n = 31). Clinicopathologic factors were compared between the two groups (Table 1). High-LOC400891 group was correlated with higher histological grade (P = 0.008), and deeper distant metastasis (P = 0.002) compared with the low-LOC400891 group. However, LOC400891 expression level was not associated with other parameters such as age (P = 0.642), tumor diameter (P = 0.166), and Gleason score (P = 0.864).
Association of LOC400891 expression and patient survival
We further examined whether the LOC400891 expression level correlated with the outcome of PCa patients after radical prostatectomy. Kaplan-Meier analysis showed that PCa patients with high LOC400891 expression had a significantly shorter biochemical recurrence-free survival time than those with low LOC400891 expression (log-rank test, P < 0.05) (Fig. 2). As shown in Table 2, LOC400891 expression level and tumor stage were significantly correlated with the biochemical recurrence-free survival rate in patients with PCa (P < 0.05). A multivariate analysis showed that relative expression of LOC400891 and tumor stage were independent prognostic markers for biochemical recurrence-free survival of PCa patients (Table 2). Taken together, these data indicate that a high LOC400891 expression level is an independent risk factor for PCa patients.
siRNA-mediated knockdown and overexpression of LOC400891 regulates cell proliferation
Since LOC554202 was highly expressed in PCa tissues compared with normal tissues, we hypothesized that the biological functions of LOC400891 might be involved in the control of cell proliferation. Experiments showed that the si-Loc400891-3 had the highest inhibition efficiency of approximately 95.3 and 89.7 %, respectively, in DU-145 and 22RV1 cell lines (P < 0.05; Fig. 3a, b). To examine this possibility, CCK8 assays were used to detect the impact of LOC400891 knockdown on proliferation of PCa cells DU-145 and 22RV1. The results revealed that si-LOC400891 transfection significantly decreased cell growth compared with the negative control and scrambled siRNA transfection at 96 h in DU-145 and 22RV1 cell lines (P < 0.05; Fig. 3c, d). To determine whether overexpression of LOC400891 contributed to cell growth, we performed pCDNA-LOC400891 transfected in DU145 and 22RV1 cells. The results of CCK8 assays revealed that cell growth was increased following up-regulation of LOC400891 in pCDNA-LOC40081 transfected DU-145 and 22RV1 cell lines compared with empty vector (P < 0.05; Fig. 3e–h)
siRNA-mediated knockdown and overexpression of LOC400891-regulated prostate cancer cells proliferation. The relative expression level of LOC400891 in DU-145 and 22RV1 cells was significantly decreased by si-LOC400891 and compared with the scramble and normal control (a, b). After 48 h of transfection, CCK8 assay (c, d) were performed to determine the proliferation of si-LOC400891 transfected DU-145 and 22RV1 cells. The relative expression level of LOC400891 in DU-145 and 22RV1 cells was significantly increased by pcDNA-LOC400891 and compared with the mock and empty vector (e, f). After 48 h transfection, CCK8 assay (g, h) were performed to determine the proliferation of pcDNA-LOC400891 transfected DU-145 and 22RV1 cells. GAPDH protein was used as an internal control. Data represent the mean ± SD from three independent experiments. *P < 0.05
To further investigate the effect of LOC400891 on the proliferation of DU-145 and 22RV1, a colony formation assay was performed. The results demonstrated that the colony numbers of PCa cells transfected with siRNA were lower than those of negative control and scrambled siRNA transfection (P < 0.05; Fig. 4a, b). All of the results were consistent with the results of cell proliferation assays.
Colony-forming growth assay and invasion/migration assay using transwell chambers for DU-145 and 22RV1. Colony-forming growth assay (a, b) were performed to determine the proliferation of si-LOC400891 transfected DU-145 and 22RV1 cells. Western blot analysis of PI3K/Akt/mTOR signaling pathway and PTEN relative protein expression level in si-LOC400891 transfected DU-145 and 22RV1 cells and respective control cells (c–e). PCa cells transfected with si-LOC400891 displayed significantly lower migration capacity (f, g) and lower invasion capacity (h, i) compared with those transfected with scramble and si-NC. Western bolt analysis of vimentin, β-catenin, Twist, and Snail protein expression level in si-LOC400891 transfected DU-145 and 22RV1 cells and respective control cells (j–l). GAPDH protein was used as an internal control. Data represent the mean ± SD from three independent experiments. *P < 0.05
Next, we investigated the effects of LOC400891 knockdown on the mTOR pathway and PTEN gene. The results of Western blot analysis showed that the expression of p-PI3k, p-AKT, PmTOR, and p-PTEN was significantly decreased in DU-145 and 22RV1 cells transfected with siRNA compared to scrambled siRNA (P < 0.05, Fig. 4c–e). These findings suggest that LOC400891 is involved in the promotion of PCa cell proliferation and seems to be mediated by modulation of the PI3K-AKT-mTOR pathway and PTEN gene.
Knockdown of LOC400891 inhibited cell migration and invasion
We also explored the effect of LOC400891 knockdown on PCa cell migration/invasion. Knockdown of LOC400891 inhibited DU-145 and 22RV1 cell migration capacity compared to the control group (P < 0.05, Fig. 4f, g). Transwell invasion assay was undertaken to assess the effect of LOC400891 on the invasiveness of prostate cancer cells. Our studies indicated that the invasion ability of si-LOC400891-transfected PCa cells was reduced compared to the control group (P < 0.05, Fig. 4h, i). Western blot analysis revealed that the expressions of vimentin, β-catenin, Twist, and Snail were significantly decreased in DU-145 and 22RV1 cells transfected with siRNA compared to the control group (P < 0.05, Fig. 4j–l). These data imply that LOC400891 involves mechanisms relevant to the promotion of PCa cell migration and invasion and that the underlying mechanisms may act by regulating the epithelial–mesenchymal transition (EMT) pathway.
Discussion
LncRNAs dysregulation may affect epigenetic information and provide a cellular growth advantage, resulting in progressive and uncontrolled tumor growth [14–18]. Like other cancers, PCa is a complicated biological process characterized by a myriad spectrum of molecular abnormalities. In a previous study, the first prominent lncRNA PCA3 was initially described as a novel biomarker of PCa [19] and subsequently defined as a promising urine test for this disease [20]. Similarly, the lncRNA PCGEM1 has been implicated in PCa as a regulator of apoptosis [21] and newer lncRNAs are being pursued through high-throughput technologies [22]. Therefore, identification of cancer-associated lncRNAs and investigation of their clinical significance and functions may provide a missing piece of the well-known oncogenic and tumor suppressor network puzzle.
Improved high-throughput technology may help us find possible therapeutic targets and identify new diagnostic and prognostic biomarkers. The LOC400891 gene, which is located at chromosome 22q11.21, is identified as Homo sapiens leucine-rich repeat containing 74B (LRRC74B), transcript variant 2. Studies have demonstrated that the LOC400891 contains multiple differences in the internal exons, compared to variant 1. This variant is represented as non-coding because the use of the 5′-most translational start codon, as used in variant 1, renders the transcript a candidate for nonsense-mediated mRNA decay [23]. However, the expression level and biomedical functions of LOC400891 in human cancers remain unclear.
According to the microarray data, which showed that LOC400891 is up-regulated in the PCa tissues and predicted some relative functions, the results were not further validated and confirmed. In our study, we focused on the identification and characteristics of LOC400891 in PCa and its role in proliferation, migration, and invasion. Consequently, we retrospectively analyzed the expression of LOC400891 in 81 patients with prostate carcinoma. We found that LOC400891 was over-expressed in PCa, which was consistent with the microarray data. Further studies also showed that LOC400891 was up-regulated in DU-145 and 22RV1 cell lines when compared to a normal prostate cell line (WPMY-1). Specifically, we investigated the clinical significance of LOC400891 in PCa patients for the first time. A higher expression of LOC400891 was detected in tumor of higher histological grade and deeper distant metastasis. In addition, LOC400891 elevated expression was correlated with a lower biochemical recurrence-free survival rate and could be an independent prognostic factor in patients with PCa. These results indicate that LOC400891 expression is an independent prognostic factor for patients with PCa, and plays an important role in the development and progression of PCa.
To further highlight the function of LOC400891, in vitro experiments were conducted. From our clinicopathological data, we found that high LOC400891 expression was closely associated with histological grade and distant metastasis. Thus, we suspect that LOC400891 may also regulate the growth and metastasis of PCa cells. Therefore, it is necessary to identify the biological function of LOC400891 in PCa cells. siRNA-mediated knockdown of LOC400891 significantly decreased the proliferation and metastasis capability of DU-145 and 22RV1 cells compared with the control group, suggesting that knockdown LOC400891 expression could suppress the development of PCa. In addition, pCDNA-LOC400891 mediated overexpression of LOC400891 significantly promotes DU-145 and 22RV1 cells proliferation. Our results demonstrated that high expression of LOC400891 might increase the malignant phenotypes of PCa cells. Compared to other lncRNAs reported in PCa, LOC400891 have the following distinct characteristics: (1) LOC400891 expression was closely associated with the histological grade and distant metastasis of PCa; (2) the elevated LOC400891 expression was correlated with a lower biochemical recurrence-free survival rate of PCa; and (3) si-LOC400891 can significantly decreased the PCa proliferation and metastasis capability. All these indicated that LOC400891 would be a potential prognostic biomarker and therapeutic target in PCa.
To explore the underlying molecular mechanism by which LOC400891 contributes to cell proliferation of PCa, we investigated the potential PI3K/Akt/mTOR signaling pathway and PTEN gene responsible for accelerated cell growth [24, 25]. PTEN is reported to be dysfunctional in the pathogenesis of a number of human malignancies and aberrant methylation in tumor suppressor genes has been linked to cancer progression and outcome [26, 27]. The results showed that siRNA-mediated knockdown of LOC400891 significantly decreased the PI3K/Akt/mTOR signaling pathway relative inner protein expression level and PTEN compared with negative control and scrambled siRNA transfection. We speculate that LOC400891 might act via regulation of the PI3K/Akt/mTOR signaling pathway and suppresses PTEN expression via epigenetic modification, thus promoting tumorigenesis. Epithelial–mesenchymal transition (EMT) process is an important step in metastasis, during which non-motile, polarized epithelial cells dissolve their cell-cell junctions and convert into individual, motile mesenchymal cells [28–30]. Twist was frequently observed in the bone marrow of breast cancer patients and the expression of Twist correlated with the rapid occurrence of distant metastasis or local progression [31]. Snail-positive breast cancer tends to home into the bone in breast cancer patients [32]. In this study, we investigated the role of LOC400891 in the EMT process in PCa. Our results showed that up-regulation of LOC400891 promoted migration and invasion of PCa cells in vitro and further indicated that knockdown of LOC400891 expression significantly decreased vimentin, β-catenin, Twist, and snail protein expression compared with the control group. Mechanistically, we demonstrated that LOC400891 might function as an inducer of EMT to carry out its specific functions in PCa.
LncRNA perform its vital function by some potential targets involved in tumorigenesis [33]. In our study, INHBA and THBS2 may be the potential targets of LOC400891 from microarray data. However, it should be confirmed by further study.
Conclusion
In summary, we demonstrate for the first time that increased LOC400891 expression is a common event underlying PCa and that LOC400891 may act as an indicator of poor survival rate and a negative prognostic factor for PCa patients. The effects of this lncRNA on cell proliferation, cell migration, and invasion suggest that it promotes tumorigenesis in PCa. An increased level of LOC400891 promotes PCa growth and metastasis by inducing the PI3K/Akt/mTOR signaling pathway, PTEN gene, and the EMT process, and may be a promising prognosis factor and therapeutic target for the future treatment of PCa.
References
Center MM, Jemal A, Lortet-Tieulent J, Ferlay J, Brawley O, Bray F. International variation in prostate cancer incidence and mortality Rates. Eur Urol. 2012;61:1079–92.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Han S, Zhang S, Chen W, Li C. Analysis of the status and trends of prostate cancer incidence in China [J]. Chinese Clinical Oncology. 2013;18:330–4.
Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–8.
Derrien T, Johnson R, Bussotti G, Anzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–89.
Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–61.
Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38.
Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407.
Crea F, Watahiki A, Quagliata L, Xue H, Pikor L, Parolia A, et al. Identification of a long non-coding RNA as a novel biomarker and potential therapeutic target for metastatic prostate cancer. Oncotarget. 2014;5:764–74.
Mehra R, Shi Y, Udager AM, Prensner JR, Sahu A, Iyer MK, et al. A novel RNA in situ hybridization assay for the long noncoding RNA SChLAP1 predicts poor clinical outcome after radical prostatectomy in clinically localized prostate cancer. Neoplasia. 2014;16:1121–7.
Bussemakers MJ, van Bokhoven A, Verhaegh GW, Smit FP, Karthaus HF, Schalken JA, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. 1999;59:5975–9.
Nilsson J, Skog J, Nordstrandetal A, Mincheva-Nilsson L, Breakefield XO, Widmark A. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100(10):1603–7.
Ding ZB, Shi YH, Zhou J, Shi GM, Ke AW, Qiu SJ, et al. Liver–intestine cadherin predicts microvascular invasion and poor prognosis of hepatitis B virus-positive hepatocellular carcinoma. Cancer. 2009;115(20):4753–65.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–6.
Nie Y, Liu X, Qu S, Song E, Zou H, Gong C. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci. 2013;104(4):458–64.
Niinuma T, Suzuki H, Nojima M, Nosho K, Yamamoto H, Takamaru H, et al. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72(5):1126–36.
Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, Kitagawa M, et al. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15 (INK4B) tumor suppressor gene. Oncogene. 2011;30(16):1956–62.
Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48(3):R45–53.
de Kok JB, Verhaegh GW, Roelofs RW, Hessels D, Kiemeney LA, Aalders TW, et al. DD3(PCA3), a very sensitive andspecific marker to detect prostate tumors. Cancer Res. 2002;62:2695–8.
Tomlins SA, Aubin SM, Siddiqui J, Lonigro RJ, Sefton-Miller L, Miick S, et al. Urine TMPRSS2:ERG fusion transcript stratifies prostate cancer risk in men with elevated serum PSA. Sci Transl Med. 2011;3:94ra72.
Srikantan V, Zou Z, Petrovics G, Xu L, Augustus M, Davis L, et al. PCGEM1, a prostate-specific gene, is overexpressed in prostate cancer. Proc Natl Acad Sci USA. 2000;97:12216–21.
Du Z, Fei T, Verhaak RG, Su Z, Zhang Y, Brown M, et al. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat Struct Mol Biol. 2013;20:908–13.
Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A. 2002;99(26):16899–903.
Li L, Ittmann M, Ayala G, Tsai M, Amat OR, Wheeler T, et al. The emerging role of the PI3-K-Akt pathway in prostate cancer progression. Prostate Cancer Prostatic Dis. 2005;8:108–18.
Pommery N, Henichart J. Involvement of PI3K/Akt pathway in prostate cancer—potential strategies for developing targeted therapies. Mini Rev Med Chem. 2005;5:1125–32.
Rizvi MM, Alam MS, Ali A, Mehdi SJ, Batra S, Mandal AK. Aberrant promoter methylation and inactivation of PTEN gene in cervical carcinoma from Indian population. J Cancer Res Clin Oncol. 2011;137:1255–62.
Bai W, Wang L, Ji W, Gao H. Expression profiling of supraglottic carcinoma: PTEN and thrombospondin 2 are associated with inhibition of lymphatic metastasis. Acta Otolaryngol. 2009;129:569–74.
Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y, et al. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett. 2010;584:4575–80.
Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–23.
Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75–90.
Watson MA, Ylagan LR, Trinkaus KM, Gillanders WE, Naughton MJ, Weilbaecher KN, et al. Isolation and molecular profiling of bone marrow micrometastases identifies TWIST1 as a marker of early tumor relapse in breast cancer patients. Clin Cancer Res. 2007;13:5001–9.
Sihto H, Lundin J, Lundin M, Lehtimäki T, Ristimäki A, Holli K, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. Breast Cancer Res. 2011;13:R87.
Prensner JR, Chen W, Iyer MK, Cao Q, Ma T, Han S, et al. PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res. 2014;74:1651–60.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Jun Wang, Gong Cheng and Xiao Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, J., Cheng, G., Li, X. et al. Overexpression of long non-coding RNA LOC400891 promotes tumor progression and poor prognosis in prostate cancer. Tumor Biol. 37, 9603–9613 (2016). https://doi.org/10.1007/s13277-016-4847-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4847-y