Abstract
Polymeric immunoglobulin receptor (pIgR) is a key component of the mucosal immune system that mediates epithelial transcytosis of immunoglobulins. The expression of pIgR was reported to be up-regulated and related to the prognosis of several human cancers. However, the clinical significance of pIgR in nasopharyngeal carcinoma (NPC) remains unclear. The purpose of this study was to detect the pIgR expression and its prognostic value in NPC. The expression of serum pIgR was measured in NPC patients and healthy controls by real-time quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) and western blotting analyses. The relationship between its expression and clinical factors was analyzed by chi-square test. Then, the overall survival of patients was assessed by Kaplan-Meier analysis while the prognostic value of serum pIgR was estimated using univariate and multivariate analyses with cox regression analysis. Serum pIgR was down-regulated in NPC patients compared to that in healthy controls both at messenger RNA (mRNA) and protein levels. Especially, its expression was also significantly lower in patients at advantage stages (III–IV) than those at early stages (I–II). And, the low pIgR expression was strongly associated with advanced clinical stages, T stage, N stage, and distant metastasis. Kaplan-Meier analysis demonstrated that patients with low pIgR expression had a significantly shorter overall survival than those with high expression at any stages. Cox regression analysis suggested that pIgR was closely related to the prognosis of NPC. Serum pIgR expression was reduced in NPC, and it could be an independent prognostic predictor for patients with this cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) is a rare malignant tumor in the word, and its average incidence is less than 1/105 population worldwide [1]. However, it is considered to be an endemic carcinoma in southern China [2, 3]. The pathogenesis of NPC may be related to Epstein-Barr virus (EBV) infection, genetic or environmental factors [4–6]. As it is difficult to detect because of lack of clinical manifestations and the hidden location at the early stages, the majority of NPCs are diagnosed with lymph node metastasis which is the major cause of death in patients with NPC [7, 8]. Although the treatments of NPC have great achievements, approximately 20 % of patients will suffer from recurrence which is the leading cause of treatment failures, and lead to the 5-year overall survival rate remains 50–60 % [9, 10]. Therefore, it is necessary to detect a new bio-marker that could predict the prognosis of NPC patients.
Polymeric immunoglobulin receptor (pIgR), a member of the immunoglobulin superfamily, is expressed in mucosal surfaces and can mediate polymeric IgA (pIgA) transcytosis from the basolateral pole to the apical surface of epithelial cells [11, 12]. Due to proinflammatory cytokines, responding to viral or bacterial infection, thus linking innate and adaptive immunity as well as leading to pIgR, it is often overexpressed [13–16]. In previous studies, pIgR had been confirmed to be up-regulated in various cancers such as colon cancer, epithelial ovarian cancer, esophageal and gastric adenocarcinoma, and hepatocellular carcinoma while it was down-regulated in lung cancer, pancreatic and periampullary adenocarcinoma, and NPC [11, 17–23]. However, its clinical significance in NPC was never clarified so far.
In this study, we detected the expression of pIgR and analyzed its relationship with clinical factors. We also estimated the prognostic value of pIgR via analyzing its influence on overall survival of patients with Kaplan-Meier analysis as well as cox regression analysis. To our knowledge, this was the first time for the evaluation of the prognostic performance of pIgR in human NPC.
Materials and methods
Patient samples
Blood samples were collected from 126 patients (including 61 at early stages (I–II) and 65 at advanced stages (III–IV) according to the tumor-node-metastasis (TNM) classification of the sixth American Joint Committee on Cancer [24]) who were newly histologically diagnosed with NPC at the The Affiliated Hospital of Weifang Medical College. Forty healthy blood donors in the hospital were selected as healthy controls. Blood samples of healthy controls with empty stomach were also obtained. Then, all the blood samples were centrifuged for 10 min at 3000 rpm within 1 h and stored at −80 °C till use. The subjects with cancer history were excluded. No NPC patients had received any form of specific therapy before the study. All the patients received radiotherapy treatment in this study. Clinical information is summarized in Table 1, and the complete follow-up information was obtained by a telephone to the patients or their relatives. Patients who were died from unexpected events or other diseases were excluded from our study.
The study protocol was approved and supervised by the Ethics Committee of The Affiliated Hospital of Weifang Medical College, and written informed consent was obtained from each participant.
RNA extraction and qRT-PCR analysis
Total RNA from serum samples was extracted using the TRIzol LS Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol, respectively. Then, the first chain of cDNA was synthesized by reverse transcription with a kit (TaKaRa, Japan). RT-PCR was conducted in the 7300 Real-Time PCR System (Applied Biosystems, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as endogenous control. The primer sequences were as follows: pIgR: forward-5′-CTCTCTGGAGGACCACCGT-3′, reverse-5′-CAGCCGTGACATTCCC- TG-3′; GAPDH: forward-5′-CTCCTCCTGTTCGACAGTCAGC-3′, reverse-5′-CC- CAATACGACCAAATCCGTT-3′. The 2−ΔΔCt method was used to calculate the relative messenger RNA (mRNA) expression of pIgR. Each sample was in triplicate.
Western blotting
The total protein was extracted from the serum of patients with NPC and healthy controls using TPER Protein Extraction Reagent (Pierce, Rockford), respectively. Then, the protein was separated by SDS-PAGE gel and the brands were transferred onto polyvinylidene fluoride membranes (PVDF). After blocking with 5 % non-fat milk (Bio-Rad), the membrane was incubated with anti-pIgR antibody (1:200) at 4 °C overnight. Secondary antibody HRP-conjugated anti-rabbit IgG antibody was then added and incubated with membranes. The immunoreactive signal of antibody-antigen pairs was visualized using the Chemiluminescence Plus Western Blot analysis kit (Santa Cruz Biotechnology). β-Actin was used as internal control. Experiments were performed at least three times.
Statistical analysis
All statistical analyses were performed using SPSS 13.0 software (Chicago, USA), and the figures were designed by GraphPad prism 5. The continuous data were presented as the mean ± SD. Student’s t test was used to identify the differences between NPC patients and healthy controls or early stages and advanced stages in pIgR mRNA and protein expression. The relationship between pIgR expression and clinical factors was analyzed via chi-square test. Kaplan-Meier analysis with the log-rank test was used for estimating the overall survival of patients with NPC. Univariate and multivariate analyses were performed to evaluate the prognostic value of pIgR with cox regression analysis. The difference was considered to be statistically significant when P < 0.05.
Results
The expression of pIgR was down-regulated in patients with NPC
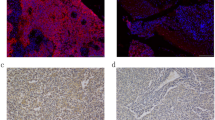
The mRNA expression of serum pIgR was detected by qRT-PCR assay in 126 NPC patients and 40 healthy controls. As shown in Fig. 1a, the pIgR mRNA level in NPC serum was significantly lower than that in healthy controls (2.04 ± 0.79 vs 4.28 ± 1.08, P < 0.001). Additionally, in contrast with the pIgR expression at early stages, pIgR expression at advanced stages of NPC was significantly decreased (1.77 ± 0.80 vs 2.46 ± 0.56, P < 0.001). Meanwhile, the protein expression of pIgR was measured with western blotting analysis. And, the result showed that pIgR protein was also decreased in patients with NPC compared to that in healthy controls (Fig. 1b, P < 0.001).
Relationship between pIgR expression and clinical features of patients with NPC
To further investigate whether pIgR was involved in the development of NPC, the relationship between its expression and clinicopathological features was assessed and is summarized in Table 1. One hundred twenty-six patients were divided into two groups according to the median expression of pIgR. Sixty patients were attributed into high pIgR expression group while the others were low pIgR expression group. The outcome demonstrated that the down-regulation of pIgR was closely associated with clinical stages (P = 0.001), T stage (P = 0.007), N stage (P = 0.001), and distant metastasis (P = 0.036). However, there was no significant association between pIgR expression and age, gender, and smoking (P > 0.05).
The prognostic value of pIgR in patients with NPC
To assess the prognostic value of pIgR in NPC patients, a 5-year follow-up was performed. During the follow-up, the 5-year overall survival (OS) rate of 126 NPC patients was 74.2 % (Fig. 2a). Kaplan-Meier analysis with log-rank test manifested that patients with low expression of pIgR had a significantly shorter OS than those with high expression of pIgR expression group (log-rank test, P = 0.006, Fig. 2b). In view of the difference of pIgR expression in different clinical stages, we further assessed the OS patients at early and advanced stages. The results showed that patients with low pIgR expression had worse OS not only at early stages (I, II; log-rank test: P = 0.036; Fig. 2c) but also at advanced stages (III, IV; log-rank test: P = 0.003; Fig. 2d) compared to those with high expression of pIgR. These indicated that pIgR was related to the prognosis of NPC.
Kaplan-Meier analysis for the overall survival of patients with NPC according to the expression of pIgR. The 5-year overall survival rate was 74.2 % in 126 NPC patients (a). Patients with low pIgR expression had a worse overall survival than those with high expression (log-rank test, P = 0.006, b). Patients with high pIgR expression had better overall survival compared with those with low pIgR expression at both early stages (I–II, c) and advanced stages (III–IV, d)
Univariate and multivariate analyses adjusted for clinical factors were further carried out to estimate the prognostic value of pIgR with cox regression analysis (Table 2). In univariate analysis, clinical stages, T stage, and pIgR expression turned out to be prognostic indicators of NPC (HR = 2.533, 95 % CI 1.114–5.760, P = 0.027; HR = 2.494, 95 % CI 1.060–5.868, P = 0.036; HR = 3.145, 95 % CI 1.331–7.434, P = 0.009). The multivariate analysis exhibited that pIgR expression (HR = 4.886, 95 % CI 1.876–12.724, P = 0.001), clinical stages (HR = 2.450, 95 % CI 1.008–5.954, P = 0.048), and T stage (HR = 3.014, 95 % CI 1.202–7.558, P = 0.019) were correlated with the prognosis of patients with NPC and they might be independent prognostic bio-markers for NPC patients.
Discussion
To date, the NPC is mostly prevalent with a high incidence and still remains unsatisfactory prognosis. Therefore, it is necessary to identify potential prognostic bio-markers and expected to provide a new therapy target for NPC patients.
During the past several decades, a lot of molecule bio-markers for the prognosis of NPC had been confirmed. For instance, acylglycerol kinase was increased in patients with NPC and could act as a potential prognostic marker [25]. Ooft et al. found that the overexpression of EGFR could predict a poor prognosis of NPC [26]. You et al. considered that the up-regulated ADAM10 was not only a promoter for the progression and migration but also a potential therapeutic target for the treatment of NPC [27]. The overexpression of long non-coding RNA AFAP1-AS1 was also proved to be related to the prognosis of NPC [28]. To detect the expression of interleukin-35 and relative survival analyses, Zhang et al. had confirmed the prognostic value of it in NPC [29]. Nevertheless, the prognostic role of pIgR in NPC was never reported.
PIgR is a transporter of dimeric IgA (dIgA) and pentameric IgM which are the first-line antibodies produced in response to infection. It has been reported to be associated with many cancers. Jonna et al. found that pIgR was overexpressed and related to the prognosis of epithelial ovarian cancer [18]. Wang et al. detected the expression of pIgR in osteosarcoma and confirmed that it was an important prognostic marker in this cancer [30]. pIgR was positively expressed and could predict the prognosis of patients with glioma [31]. On the contrast, pIgR was considered to be down-regulated in some other cancers [11, 21]. In this study, we detected the expression of pIgR both at mRNA and protein levels. The outcome showed that serum pIgR was decreased in NPC patients compared to that in healthy controls which revealed that pIgR might be a tumor suppressor in NPC. This was consistent with previous studies.
Subsequently, we explored the association between serum pIgR expression and clinical factors of patients with NPC. As a result, we found that its expression was significantly influenced by some clinical factors which suggested that pIgR was involved in the development of NPC. Based on the result above, we further investigated the prognostic value of serum pIgR in NPC. According to the Kaplan-Meier analysis which demonstrated that the expression of pIgR was related to the OS of patients, we concluded that pIgR was correlated with the prognosis of NPC. Then, cox regression analysis proved our view and confirmed that pIgR could be a potential and independent prognostic indicator for patients with NPC.
In conclusion, our results prove that pIgR is down-regulated in NPC and it is linked with the development and progression of this cancer. Besides, it has a high prognostic value in predicting the clinical outcome of NPC.
References
Xu ZJ, Zheng RS, Zhang SW, Zou XN, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China in 2009. Chin J Cancer. 2013;32(8):453–60. doi:10.5732/cjc.013.10118.
Cao SM, Simons MJ, Qian CN. The prevalence and prevention of nasopharyngeal carcinoma in China. Chin J Cancer. 2011;30(2):114–9.
Trejaut J, Lee CL, Yen JC, Loo JH, Lin M. Ancient migration routes of Austronesian-speaking populations in oceanic Southeast Asia and Melanesia might mimic the spread of nasopharyngeal carcinoma. Chin J Cancer. 2011;30(2):96–105.
Jia WH, Qin HD. Non-viral environmental risk factors for nasopharyngeal carcinoma: a systematic review. Semin Cancer Biol. 2012;22(2):117–26. doi:10.1016/j.semcancer.2012.01.009.
Xiong W, Zeng ZY, Xia JH, Xia K, Shen SR, Li XL, et al. A susceptibility locus at chromosome 3p21 linked to familial nasopharyngeal carcinoma. Cancer Res. 2004;64(6):1972–4.
Zeng Z, Huang H, Zhang W, Xiang B, Zhou M, Zhou Y, et al. Nasopharyngeal carcinoma: advances in genomics and molecular genetics. Sci China Life Sci. 2011;54(10):966–75. doi:10.1007/s11427-011-4223-5.
Ho FC, Tham IW, Earnest A, Lee KM, Lu JJ. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. BMC Cancer. 2012;12:98. doi:10.1186/1471-2407-12-98.
Xiao WW, Huang SM, Han F, Wu SX, Lu LX, Lin CG, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117(9):1874–83. doi:10.1002/cncr.25754.
Suarez C, Rodrigo JP, Rinaldo A, Langendijk JA, Shaha AR, Ferlito A. Current treatment options for recurrent nasopharyngeal cancer. Eur Arch Otorhinolaryngol. 2010;267(12):1811–24. doi:10.1007/s00405-010-1385-x.
Ma BB, Hui EP, Chan AT. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: current and future directions. Cancer Sci. 2008;99(7):1311–8. doi:10.1111/j.1349-7006.2008.00836.x.
Ocak S, Pedchenko TV, Chen H, Harris FT, Qian J, Polosukhin V, et al. Loss of polymeric immunoglobulin receptor expression is associated with lung tumourigenesis. Eur Respir J. 2012;39(5):1171–80. doi:10.1183/09031936.00184410.
Norderhaug IN, Johansen FE, Schjerven H, Brandtzaeg P. Regulation of the formation and external transport of secretory immunoglobulins. Crit Rev Immunol. 1999;19(5–6):481–508.
Denning GM. IL-4 and IFN-gamma synergistically increase total polymeric IgA receptor levels in human intestinal epithelial cells. Role of protein tyrosine kinases. J Immunol. 1996;156(12):4807–14.
Kvale D, Lovhaug D, Sollid LM, Brandtzaeg P. Tumor necrosis factor-alpha up-regulates expression of secretory component, the epithelial receptor for polymeric Ig. J Immunol. 1988;140(9):3086–9.
Rojas R, Apodaca G. Immunoglobulin transport across polarized epithelial cells. Nat Rev Mol Cell Biol. 2002;3(12):944–55. doi:10.1038/nrm972.
Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi:10.1111/j.0105-2896.2005.00278.x.
Liu F, Ye P, Bi T, Teng L, Xiang C, Wang H, et al. Colorectal polymeric immunoglobulin receptor expression is correlated with hepatic metastasis and poor prognosis in colon carcinoma patients with hepatic metastasis. Hepato-Gastroenterology. 2014;61(131):652–9.
Berntsson J, Lundgren S, Nodin B, Uhlen M, Gaber A, Jirstrom K. Expression and prognostic significance of the polymeric immunoglobulin receptor in epithelial ovarian cancer. J Ovarian Res. 2014;7:26. doi:10.1186/1757-2215-7-26.
Fristedt R, Gaber A, Hedner C, Nodin B, Uhlen M, Eberhard J, et al. Expression and prognostic significance of the polymeric immunoglobulin receptor in esophageal and gastric adenocarcinoma. J Transl Med. 2014;12:83. doi:10.1186/1479-5876-12-83.
Ai J, Tang Q, Wu Y, Xu Y, Feng T, Zhou R, et al. The role of polymeric immunoglobulin receptor in inflammation-induced tumor metastasis of human hepatocellular carcinoma. J Natl Cancer Inst. 2011;103(22):1696–712. doi:10.1093/jnci/djr360.
Fristedt R, Elebro J, Gaber A, Jonsson L, Heby M, Yudina Y, et al. Reduced expression of the polymeric immunoglobulin receptor in pancreatic and periampullary adenocarcinoma signifies tumour progression and poor prognosis. PLoS One. 2014;9(11):e112728. doi:10.1371/journal.pone.0112728.
Chang Y, Lee TC, Li JC, Lai TL, Chua HH, Chen CL, et al. Differential expression of osteoblast-specific factor 2 and polymeric immunoglobulin receptor genes in nasopharyngeal carcinoma. Head Neck. 2005;27(10):873–82. doi:10.1002/hed.20253.
Su T, Chapin SJ, Bryant DM, Shewan AM, Young K, Mostov KE. Reduced immunoglobulin A transcytosis associated with immunoglobulin A nephropathy and nasopharyngeal carcinoma. J Biol Chem. 2011;286(52):44921–5. doi:10.1074/jbc.M111.296731.
Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73(5):1326–34. doi:10.1016/j.ijrobp.2008.07.062.
Zhu Q, Cao SM, Lin HX, Yang Q, Liu SL, Guo L. Overexpression of acylglycerol kinase is associated with poorer prognosis and lymph node metastasis in nasopharyngeal carcinoma. Tumour Biol J Int Soc Oncodev Biol Med. 2015. doi:10.1007/s13277-015-4148-x.
Ooft ML, Braunius WW, Heus P, Stegeman I, van Diest PJ, Grolman W, et al. Prognostic significance of the EGFR pathway in nasopharyngeal carcinoma: a systematic review and meta-analysis. Biomark Med. 2015. doi:10.2217/bmm.15.68.
You B, Shan Y, Shi S, Li X, You Y. Effects of ADAM10 upregulation on progression, migration, and prognosis of nasopharyngeal carcinoma. Cancer Sci. 2015. doi:10.1111/cas.12800.
Bo H, Gong Z, Zhang W, Li X, Zeng Y, Liao Q, et al. Upregulated long non-coding RNA AFAP1-AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget. 2015;6(24):20404–18.
Zhang Y, Sun H, Wu H, Tan Q, Xiang K. Interleukin 35 is an independent prognostic factor and a therapeutic target for nasopharyngeal carcinoma. Contemp Oncol (Pozn). 2015;19(2):120–4. doi:10.5114/wo.2014.44754.
Wang X, Du J, Gu P, Jin R, Lin X. Polymeric immunoglobulin receptor expression is correlated with poor prognosis in patients with osteosarcoma. Mol Med Rep. 2014;9(6):2105–10. doi:10.3892/mmr.2014.2110.
Niu H, Wang K, Wang Y. Polymeric immunoglobulin receptor expression is predictive of poor prognosis in glioma patients. Int J Clin Exp Med. 2014;7(8):2185–90.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Ethics, consent, and permissions
The study protocol was approved and supervised by the Ethics Committee of The Affiliated Hospital of Weifang Medical College, and written informed consent was obtained from each participant.
Consent to publish
All authors read and approved the final manuscript.
Funding
None.
Rights and permissions
About this article
Cite this article
Qi, X., Li, X. & Sun, X. Reduced expression of polymeric immunoglobulin receptor (pIgR) in nasopharyngeal carcinoma and its correlation with prognosis. Tumor Biol. 37, 11099–11104 (2016). https://doi.org/10.1007/s13277-016-4791-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4791-x