Abstract
Cell motility and chemotaxis play pivotal roles in the process of tumor development and metastasis. Protein kinase C ζ (PKC ζ) mediates epidermal growth factor (EGF)-stimulated chemotactic signaling pathway through regulating cytoskeleton rearrangement and cell adhesion. The purpose of this study was to develop anti-PKC ζ therapeutics for breast cancer metastasis. In this study, a novel and high-efficient PKC ζ inhibitor named PKCZI195.17 was screened out through a substrate-specific strategy. MTT assay was used to determine the cell viability of human breast cancer MDA-MB-231, MDA-MB-435, and MCF-7 cells while under PKCZI195.17 treatment. Wound-healing, chemotaxis, and Matrigel invasion assays were performed to detect the effects of PKCZI195.17 on breast cancer cells migration and invasion. Adhesion, actin polymerization, and Western blotting were performed to detect the effects of PKCZI195.17 on cells adhesion and actin polymerization, and explore the downsteam signaling mechanisms involved in PKC ζ inhibition. MDA-MB-231 xenograft was used to measure the in vivo anti-metastasis efficacy of PKCZI195.17. The compound PKCZI195.17 selectively inhibited PKC ζ kinase activity since it failed to inhibit PKC α, PKC β, PKC δ, PKC η, AKT2, as well as FGFR2 activity. PKCZI195.17 significantly impaired spontaneous migration, chemotaxis, and invasion of human breast cancer MDA-MB-231, MDA-MB-435, and MCF-7 cells, while PKCZI195.17 did not obviously inhibited cells viability. PKCZI195.17 also inhibited cells adhesion and actin polymerization through attenuating the phosphorylations of integrin β1, LIMK, and cofilin, which might be the downstream effectors of PKC ζ-mediated chemotaxis in MDA-MB-231 cells. Furthermore, PKCZI195.17 suppressed the breast cancer metastasis and increased the survival time of breast tumor-bearing mice. In summary, PKCZI195.17 was a PKC ζ-specific inhibitor which dampened cancer cell migration and metastasis and may serve as a novel therapeutic drug for breast cancer metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer metastasis constitutes the major cause of deaths in many cancer patients [1, 2]. Metastasis results from a sequential step process, in which tumor cells first detach from the original lesions and then intravasate into circulation, survive and travel along the blood circulation, extravasate to the target tissue, and grow a new focus [2]. Increasing evidence suggests that cell motility and chemotaxis play pivotal roles in the process of tumor metastasis [3, 4]. Chemotaxis is a cellular capacity to detect an extracellular gradient of chemical stimuli and to move to the higher concentration site [5, 6]. Both the processes of extravasation and intravasation are induced by chemotaxis [7]. Chemotaxis of cancer cells is mediated by both G-protein-coupled receptors (GPCR) and receptor tyrosine kinases (RTK), such as CXCR4 and epidermal growth factor (EGF) receptor, respectively [8–10], and EGF was considered to be a more potent chemoattractant than CXCL12 for human breast cancer cells [11] and markedly enhanced cell motility, indicative of chemotaxis.
Although the molecular mechanism of cells chemotaxis and metastasis has not been fully elucidated, protein kinase C (PKC) family of protein has been proved to be crucial for signal transduction in chemotactic cell migration. PKC family comprises about 12 serine/threonine kinases which can be sorted into three subfamilies based on their activation mechanism [12–14]: conventional PKCs (α, β1, β2, γ) are activated by both diacylglycerol (DAG) and calcium, novel PKCs (ε, δ, θ, η) are activated by DAG but insensitive to calcium, and atypical PKCs (ζ and λ) are not activated by either DAG or calcium [12]. PKC family plays pleiotropic roles in many cellular processes including division, proliferation, survival, anoikis, polarity, adhesion, and migration [14–16]. Moreover, PKC is abundant in many human cancers, and aberrant PKC signaling has been demonstrated in many cancer models [17, 18]. For example, PKC-mediated phosphorylation of Cdc42 effector protein-4 (CEP4) at Ser (18) and Ser (80) was involved in Rac activation, filopodium formation, and cell motility of MCF-10A human breast cells, via causing its dissociation from Cdc42 and thereby increasing its affinity for tumor endothelial marker-4 (TEM4) [19]. PKC/GSK-3β signaling pathway controls both the stability and transcription of Snail, which is crucial for epithelial-mesenchymal transition (EMT) induced by EGF and thereby to promote metastasis in PC-3 human prostate and A549 human lung cancer cells [20]. Paeonol (2'-hydroxy-4'-methoxyacetophenone), the main active compound of traditional Chinese remedy Paeonia lactiflora Pallas, suppresses migration and invasion of chondrosarcoma cells through up-regulation of miR-141 by modulating PKC δ and c-Src signaling pathways [21].
PKC ζ belongs to atypical PKC and has been reported to be concerned with cell growth, cell survival, ligand-induced cell adhesion, determination of cell polarity and actin polymerization, and so on [22–24]. Early studies have revealed that PKC ζ is essential for migration and invasion of multiple cancer cell types [22, 23], and isozyme-specific inhibitors of PKC ζ suppressed Gi protein-mediated cell adhesion and actin polymerization in polymorphonuclear cells [25, 26]. PKC ζ is involved in the control of glioblastoma cell migration and invasion by regulating the cytoskeleton rearrangement, cell adhesion, and matrix etalloprotease-9 expression through small-interference RNA technology [27]. It has also confirmed that PKC ζ mediated CSF-1-induced migration of human acutemonytic leukemia cell line THP1 as well as mouse peritoneal macrophages [28], emphasizing a potential and essential mechanism of PKC ζ in tumor metastasis which involved crosstalk between tumor and tumor-associated macrophage. Particularly, our previous work have indicated that PKC ζ is uniquely required for EGF-induced chemotaxis of breast and lung cancer cell lines [29, 30], and the data also confirmed that receptor tyrosine kinase (RTK)- and G-protein-coupled receptor-mediated chemotactic signaling pathways converge at PKC ζ [29]. Therefore, blocking activation of PKC ζ will be a novel strategy to inhibit cancer metastasis by blocking migration of cancer cells [28]. Taken together, we investigated the inhibition of a small molecular compound named PKCZI195.17 targeting of PKC ζ as a potential anti-metastasis drug.
Materials and methods
Cells and materials
Human breast cancer cell line MCF-7, MDA-MB-231, and MDA-MB-435 were obtained from ATCC, Shanghai, China. The cells were grown in Dulbecco’s modified Eagle medium (DMEM) with 10 % fetal bovine serum (FBS) under 5 % CO2. The DMEM was bought from Invitrogen (Grand Island, New York, USA); FBS was from Hyclone (South Logan, UT, USA). Chemotaxis chambers and membranes were obtained from Neuroprobe (Gaithersburg, MD, USA); human EGF was from Peprotech (Rocky Hill, NJ, USA); fibronectin (0.1 %) from Sigma (St. Louis, MO); Oregon Green 568 phalloidin from Molecular Probes Inc. (Eugene, OR); Ki67 Rabbit mAb (IHC Specific) from Cell Signaling Technology, Inc. (Beverly, USA); antibodies against PKC ζ, Akt, cofilin and p-cofilin from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA); antibodies against p-PKC ζ, p-Akt from Cell Signaling Technology, Inc. (Beverly, USA); antibodies against integrin β1 from BD Transduction (San Jose, CA); antibodies against p-integrin β1 and p-LIMK from Calbiochem (La Jolla, CA); the micromolecular compound PKCZI195.17 (C9H9NO4) was bought from Sigma-Aldrich; and GӦ6850 was from Alexis Biochemicals (San Diego, CA). MTT assay kit was from Sigma, and Z′-LYTETM KINASE ASSAY KIT-SER/THR 7 PEPTIDE Kit (Cat. No. PV3180), Z'-LYTETM KINASE ASSAY KIT-SER/THR 6 PEPTIDE Kit (Cat. No. PV3179), PKC ζ (Part No. P2273), PKC α (Part no. P2227), PKC β (Part No. P2291), PKC δ (Part No. P2293), PKC η (Part No. P2633), AKT2 (Part No. PV3184), and FGFR2 (Part No. PV3368) were purchased from Invitrogen Corporation.
Z′-LYTE™ assay
The Z′-LYTETM assay was carried out according to the manufacturer’s instruction [24]. Briefly, 10 μl per well reactions were set up in 384-well plates containing kinase buffer, 10 μm ATP, 2 μm Z′-LYTE Ser/Thr peptide substrate, 125 ng/ml PKC ζ (or 9 ng/ml PKC α, PKC β, PKC δ, PKC η, AKT2, FGFR2), and inhibitors. After 1-h incubation, development solution was added to each well, and 1 h further incubation was allowed followed by reaction stopping. The fluorescent signal ratio of 445 nm (Coumarin)/520 nm (Fluorescin) was analyzed, which reflects the peptide substrate cleavage status and/or kinase inhibitory activity in the reaction.
MTT cytotoxicity assay
Human breast cancer cells were planted into 96-well plates, attached overnight, and subsequently exposed to different concentrations of PKCZI195.17 for different times (24, 48, and 72 h). Cytotoxicity of PKCZI195.17 was assessed by MTT assay as previously described [24], and the data was measured at the 490-nm wavelength.
Cell proliferation assay
Human breast cancer cells were planted in 35-mm dishes. At the same time, 30-nM concentration of PKCZI195.17 or DMSO was added to every dish. Cells were harvested and counted every 24 h. At least three wells were used for each data point.
Scratch assay (would healing)
Cells were plated in 35-mm dishes for 2 days to grow a mono layer, pre-treated with inhibitors or DMSO in medium for 12 h; after which, a linear scratch was lined out in the middle using a pipette tip. The cells were then incubated at 37 °C in 5 % CO2, and the widths of the wounds were measured at different time points (0, 6, and 24 h) under a light microscope.
Chemotaxis assay
Chemotaxis assay was done as described by Sun R.H. et al. [29]. Briefly, chemoattractants (EGF) were loaded into the lower chamber and cells (5 × 105 cells/ml) suspended in binding medium (DMEM, 0.1 % bovine serum albumin, and 25 mM HEPES) and were then loaded into the upper chambers. For the inhibition assay, cells were pre-treated with inhibitors at 37 °C for 3 h. Thereafter, the filter membrane was washed, fixed, and then stained. The number of migrating cells was counted under a light microscope. Chemotaxis index = the number of migrating cells under a chemoattractant gradient/the number of migrating cells in the control group.
Matrigel invasion assay
The upper surface of a Transwell (6.5-mm diameter and 8-μm pore size; Corning) was coated with 20 μl of diluted Matrigel (BD Biosciences). Cells were seeded at 3.5 × 104 cells per well in the upper chamber of the Transwell in serum-free medium with or without EGF (10 ng/ml) in the presence or absence of 30 nM PKCZI195.17. The lower chamber was filled with the same medium as in the upper chamber but with 10 % FBS. After incubation for 20 h, the cells remaining on the upper surface of the membrane were removed with a cotton swab, and the average invaded cell number per field of view was obtained from five random fields.
Adhesion assay
Adhesion assay was carried out as described by Sun R.H. et al. [29]. Shortly, MDA-MB-231 cells were pre-treated with 30 nM PKCZI195.17 or DMSO for 1 h, trypsinized, and re-suspended in serum-free medium (3 × 105 cells/ml) with or without 10 ng/ml EGF. The suspensions were incubated at 37 °C for 30 min; after which, 1.5-ml cell suspensions were added in 35-mm dishes containing a glass coverslip which had been coated with 10 μg/ml fibronectin in serum-free medium at 4 °C. After 5-, 10-, and 15-min incubations, cells were washed with cold PBS and then fixed with 4 % paraformaldehyde (PFA). The cells attached to the coverslips were counted under a light microscope.
F-actin polymerization assay
Filamentous actin was quantified through methanol extraction of Oregon Green 568/phalloidin-stained cells as described previously [29]. Briefly, MDA-MB-231 cells were planted in 35-mm dish and cultured overnight in complete medium followed by further culturing in serum-free medium for 3 h. Meanwhile, the cells were treated with 30 nM of PKCZI195.17 or DMSO for 1 h and stimulated by 50 ng/ml EGF at 37 °C for 0, 15 s, 30 s, 1 min, 2 min, and 5 min. Thereafter, cells were fixed, permeabilized, and stained in the dark with Oregon Green 568 phalloidin diluted in F-buffer (10 mM HEPES, 20 mM KH2PO4, 5 mM EGTA, 2 mM MgCl2, Dulbecco’s PBS, pH 6.8) at room temperature for 1 h. After five washes, the bound phalloidin was extracted with methanol at 4 °C for 1 h, and the F-actin content was measured with a fluorescence reader at 578-nm excitation wavelength and 600-nm emission wavelength. The results were expressed as relative F-actin content, where F-actin△ t / F-actin0 = (fluorescence△ t / mg per ml) / (fluorescence 0/mg per ml).
Western blotting assay
Western blotting assay was performed as described by Sun R.H. et al. [29]. The MDA-MB-231 cells were starved with serum-free medium for 3 h, pre-treated with 30 nM PKCZI195.17 or DMSO for another 3 h, and stimulated by 50 ng ml−1 EGF for various times. The reactions were stopped and washed twice with cold PBS, and then lysed using 1× SDS lysis buffer (Tris–HCl, pH 6.8, 62.5 mM, 2 % SDS, 10 % glycerol.). Thereafter, equal amounts of cell lysates (20 μg per lane) were loaded onto 10 % SDS-PAGE systems and transferred onto a polyvinylidene fluoride membrane. The membranes were blocked with 5 % nonfat milk, probed with corresponding primary antibodies followed by HRP-conjugated secondary antibody. Western blots were visualized by using enhanced chemiluminescence reagents ECL (Pierce, Rockford, IL).
In vivo metastasis
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animal of Tianjin Third Central Hospital. Briefly, 4–6-week-old female severe combined immunodeficient mice (SCID) were from Wei Tong Li Hua Experimental Animal Co.Ltd (Beijing, China), and 5 × 106 MDA-MB-231 cells were injected intravenously into the tail vein of SCID mice. And then, mice were divided into two experimental groups, specifically (i) physiological saline (control, n = 8) and (ii) PKCZI195.17 10 mg/kg (n = 8). PKCZI195.17 was i.v. administrated through the tail vein once a day for 6 weeks thereafter. The body weight of mice was measured individually every three days, and the survival times were monitored. The tumors were excised for size determination and IHC staining for Ki67 protein. The lungs tissues of mice were finally removed and embedded for the dissection on a cryostat (14 μm). The immunohistochemical staining will be operated on the slide-mounted sections, and metastatic tumor nodules were counted under a Zeiss inverted microscope.
Statistical analysis
SPSS 17.0 software was used for data analysis. The results of Western blotting were quantified using software ImageJ. Data were presented as mean ± SD, and t test and ANOVA were used.
Results
Screening of a PKC ζ inhibitor
PKCZI195.17 (C9H9NO4) was screened as a PKC ζ inhibitor from almost three hundred compounds by the Z′-LYTE™ KINASE ASSAY KIT-SER/THR 7 PEPTIDE Kit, which has been proved as a mature method basing on the differential sensitivity of phosphorylated and nonphosphorylated peptides for proteolytic cleavage [31]. According to manufacturer’s instruction, a pilot assay was carried out which determined that 125 ng/ml of PKC ζ kinase was an optimal concentration for the following screening procedures, and 30-nM concentration of PKCZI195.17 exhibited a 42.8 % inhibition of PKC ζ activity (Fig. 1a). The molecular structure of PKCZI195.17 was shown in Fig. 1b. The dose-response curve identified that the half maximal (50 %) inhibitory concentration (IC50) of PKCZI195.17 was 32 nM (Fig. 1c). In order for the specificity analysis of PKCZI195.17, the same assay was performed only that PKC ζ was substituted PKC α, PKC β, PKC δ, PKC η, AKT2, or FGFR2, and the results showed that PKCZI195.17 has no significant inhibition on PKC α (Fig. 1d), PKC β, PKC δ, PKC η (Fig. 1g), AKT2 (Fig. 1e), or FGFR2 (Fig. 1f).
Screening results of protein kinase C ζ (PKC ζ) inhibitor. a Some of the screen results for PKC ζ kinase inhibitor, PKCZI195.17, showed significantly inhibition of 42.8 %. b Molecular structures of PKCZI195.17. c Dose-response curve of PKCZI195.17 inhibiting PKC ζ phosphorylation. d Dose-response curve of PKCZI195.17 inhibiting PKC α phosphorylation. e Dose-response curve of PKCZI195.17 inhibiting AKT2 phosphorylation. f Dose-response curve of PKCZI195.17 inhibiting FGFR2 phosphorylation. g %Inhibition of 30 nmol/l PKCZI195.17 on PKC ζ, PKC β, PKC δ, as well as PKC η kinase activity
The effect of PKCZI195.17 on cell viability and proliferation
MTT assays were used to test the cytotoxic effect of PKCZI195.17 against the three kinds of breast cancer cells. As shown in Fig. 2a, gradient concentrations of PKCZI195.17 did not obviously inhibit the cell viability of MDA-MB-231, MDA-MB-435, and MCF-7 cells after 24-, 48-, and 72-h treatments. Cell proliferation analyses in Fig. 2b revealed that 30 nM PKCZI195.17 moderately increased the overdoubling time of MDA-MB-231 and MDA-MB-435 cells, which suggesting an inhibition effect against cell growth and mitosis.
Effects of PKCZI195.17 on cells viability and proliferation. a Cytotoxicity assay: MDA-MB-231, MDA-MB-435, and MCF-7 cells were treated with indicated amounts of PKCZI195.17 or DMSO for up to 3 days followed by MTT assay. b Proliferation assay: cells were planted into 35-mm dishes, and 30 nM PKCZI195.17 or DMSO was added into the dishes. The cells were trypsinized and counted on the days indicated (*P < 0.05, **P < 0.01, paired Student’s t test)
PKCZI195.17 abrogates EGF-induced cell migration, chemotaxis, and invasion
Previous studies [29, 30] have demonstrated that inhibition of PKC ζ through a peptide pseudosubstrate blocks the chemotaxis of a series of human lung and breast cancer cell lines including A549, H1299, and T47D. In order to determine whether PKCZI195.17 might have the same effect, we investigated the chemotaxis of breast cancer cells, and the results showed that EGF-induced chemotaxis of MDA-MB-231, MDA-MB-435, and MCF-7 cells was greatly inhibited by 3-h pre-treatment with PKCZI195.17 compared to the control group, especially at 10 ng/ml EGF, and the effect was equivalent to the treatment with GӦ6850, which is a verified and positive pan-PKC inhibitor (Fig. 3a).
Effects of PKCZI195.17 on cells chemotaxis, wound-healing, and invasion. a Chemotaxis assay: MDA-MB-231, MDA-MB-435, and MCF-7 cells were pre-treated with inhibitors or DMSO for 3 h in culture incubator, followed by chemotaxis assay with indicated amount of EGF as chemoattractant for 3 h. Comparison of EGF-induced chemotaxis of the control cells and pre-treated cells with inhibitors (*P < 0.05, two-way analysis of variance). b Scratch assay: cells were pre-treated with inhibitors or DMSO for 12 h before scratching. Wound widths were recorded at indicated time points. Asterisks indicate the t test difference at 24 h (**P < 0.01, paired Student’s t test). c Scratch assay: the image of MDA-MB-231 cells migration at 6 and 24 h. d Matrigel invasion assay: cells were incubated in serum-free medium with or without EGF (10 ng/ml) in the presence or absence of 30 nM PKCZI195.17 for 20 h. Data were mean ± SD of the number of invaded cells per field of view (n = 5) (*P < 0.05, **P < 0.01, paired Student’s t test)
To study the effect of PKCZI195.17 on the cellular migration and invasion of cells, we performed wound-healing and Matrigel invasion assays. In the wound-healing assay, the cells pre-treated with either PKCZI195.17 or GӦ6850 showed a much slower directional migration compared with those in the control groups, which suggesting that PKCZI195.17 inhibited the directional motility or spreading of MDA-MB-231, MDA-MB-435, and MCF-7 cells (Fig. 3b, c). In the Matrigel invasion assay, PKCZI195.17 evidently attenuated the abilities of the three kinds of cells to invade through the Matrigel membrane (Fig. 3d). Thus, PKCZI195.17 strongly suppressed the migration and invasion of breast cancer cells.
PKCZI195.17 inhibits EGF-induced cell adhesion and actin polymerizations
Ligand-induced adhesion and cytoskeleton rearrangement are two key responses closely correlated with chemotactic activity of cells [32, 33], which were frequently regulated by PKC ζ [29]. Thus, we investigated the effect of PKCZI195.17 on EGF-induced MDA-MB-231 cells adhesion. As shown in Fig. 4a, 10 ng/ml EGF stimulated a marked increase in the numbers of cell adhesion after 5, 10, and 15 min. However, cell attachment in general was significantly decreased under the condition of PKCZI195.17 pre-treatment when compared with the corresponding cells in the control group. Ligand-induced actin polymerization, de-polymerization, and regulating research are of great significance for microfilament assembly and the molecular mechanism of cell motility. EGF at 50 ng/ml elicited a transient actin assembly in MDA-MB-231 cells, whose first phase peaked at 15 s and second phase peaked at 1 min (Fig. 4b). In the presence of PKCZI195.17, actin polymerization stimulated by EGF was significantly reduced, and its peak was delayed until at 1 min. Our observations further confirmed that PKCZI195.17 inhibited cell adhesion and actin polymerization induced by EGF.
Effects of PKCZI195.17 on EGF-induced MDA-MB-231 cell adhesion and actin polymerization. a Cell adhesion assay: cells were pre-treated with 30 nM PKCZI195.17 or DMSO for 1 h, followed by measuring adhesion capacity on fibronectin over indicated time periods. Cell numbers in five fields were counted for each coverslip under microscopy with 200× magnitudes (**P < 0.01, independent t test). b Effects of PKCZI195.17 on EGF-induced actin polymerization. Cells were starved for 3 h, treated with 30 nM PKCZI195.17 or DMSO 1 h and followed by 50-ng/ml EGF stimulation for the indicated time periods. Cells were fixed, and F-actin contents were assayed as described in the “Materials and method” section (**P < 0.01, paired Student’s t test). c Western blotting analysis of phosphorylated PKC ζ and phosphorylated Akt in total cell lysates from DMSO and PKCZI195.17-treated cells upon stimulated with 50 ng/ml EGF for 0, 5, 15, and 30 min. PKC ζ and Akt were used as loading controls. d Western blotting analysis of phosphorylated integrin β1 in total cell lysates from DMSO and PKCZI195.17-treated cells upon stimulated with 50 ng/ml EGF for 0, 5, and 15 min. Integrin β1 was used as a loading control. e Western blotting analysis of phosphorylated LIMK and phosphorylated cofilin in total cell lysates from DMSO and PKCZI195.17-treated cells upon stimulated with 50 ng/ml EGF for 0, 30 s, 1 min, and 5 min. Cofilin was used as a loading control
Previous study has demonstrated that PI3K and Akt are the upstream regulators of PKC ζ in the chemotaxis signaling pathway of human non-small cell lung cancer cells [30], and inhibition of PKC ζ by myristolated pseudosubstrate did not interfere with EGF-induced phosphorylation of Akt, which further confirmed that PKC ζ functioned downstream of Akt and suggested that there were no feedback regulation of Akt by PKC ζ [34]. Our observation also indicated PKCZI195.17 which inhibited phosphorylation of PKC ζ did not attenuate the activations of Akt in human breast cancer cell line MDA-MB-231 (Fig. 4c). Our results had suggested that PKCZI195.17 impaired EGF-stimulated cell adhesion. Integrin β1 is a key component in cell adhesion, and phosphorylated-integrin β1 can promote cell attachment. To further confirm this observation, we investigated the effect of PKCZI195.17 on EGF-induced phosphorylation of integrin β1 in MDA-MB-231 cells. In the presence of PKCZI195.17, the phosphorylation of integrin β1 was obviously reduced (Fig. 4d). These results suggested that PKCZI195.17 impaired EGF-stimulated cell adhesion through reducing the phosphorylation of integrin β1, which might be a downstream effector of PKC ζ-mediated chemotaxis.
Our results suggested that PKCZI195.17 reduced EGF-induced actin polymerization. It has been demonstrated that LIM kinase (LIMK) modulates the actin assembly by inhibiting the activity of the actin depolymerizing factor (ADF)/cofilin. Next, in the presence of PKCZI195.17, we investigated the activation of LIMK by detecting the phosphorylation of threonine in MDA-MB-231 cells. As shown in Fig. 4e, EGF significantly induced the phosphorylation of LIMK, and PKCZI195.17 clearly impaired the activation of LIMK under EGF stimulation. Cofilin is one of the major regulators of actin polymerization and cell motility [35, 36]. Cofilin was activated by EGF stimulation through phospholipase C-mediated hydrolysis of PIP2, a membrane sequesterer and inhibitor of cofilin. Released cofilin induces actin polymerization in the presence of free globular actins [37]. Then, we investigated the effect of PKCZI195.17 on the phosphorylation of cofilin stimulated by EGF. As shown in Fig. 4e, 50 ng/ml EGF stimulated the phosphorylation of cofilin, and the phosphorylation level reached a plateau at 30 s. On the other hand, the phosphorylation of cofilin was severely reduced in the presence of PKCZI195.17, which was consistent with a reduction in actin polymerization. Thus, PKCZI195.17 reduced EGF-stimulated actin polymerization through attenuating the phosphorylations of LIMK and cofilin, which might be another two downstream effectors of PKC ζ-mediated chemotaxis.
PKCZI195.17 inhibits tumor metastasis and improves survival in vivo
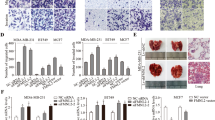
Cell migration plays a crucial role in the complex, multistep process of cancer metastasis. We finally intended to explore therapeutic effect of PKCZI195.17 in overcoming cancer metastasis. The anti-metastasis effect of PKCZI195.17 was examined using a spontaneous metastasis assay through tail vein injection of human breast cancer MDA-MB-231 cells into SCID mice. As shown in Fig. 5a, b, daily i.v. injection of PKCZI195.17 for 6 weeks (10 mg/kg) was effective in retarding tumor growth compared to the control group treated with physiological saline, and we found significant reduction of nuclear immunolabeling of the surrogate marker of proliferation Ki67 in PKCZI195.17-treated MDA-MB-231 xenograft as compared with that of the control tumors (Fig. 5c). Obviously, PKCZI195.17 treatment (10 mg/kg) also caused a dramatic decrease in the number of pulmonary metastatic nodules, yielding inhibition rates of 55.81 % (Fig. 5d, e). There was no difference in the body weight of mice between the PKC ZI195.17-treated group and control group. PKCZI195.17-treated mice showed no signs of toxicity throughout the experiments and showed enhanced survival (Fig. 5f) compared to the control mice. These results suggest the potential of PKCZI195.17 in cancer therapy via hindering cancer metastasis.
PKCZI195.17 inhibited breast cancer metastasis in vivo and improved survival. a The tumors of each group treated with physiological saline (control) and PKCZI195.17. b Tumor volumes of the two different treatment groups. Columns mean of a typical experiment; bars SD (**P < 0.01, paired Student’s t test). c Immunohistochemical analysis of the Ki67 protein expression in tissue specimens from MDA-MB-231 xenografts treated with physiological saline and PKCZI195.17 in vivo (magnification, 200×). d Effect of PKCZI195.17 on lung metastasis of MDA-MB-231 breast carcinoma i.v. xenograft in SCID mice. The representative photograph of metastatic nodules on lungs with H&E staining (magnification, 200×). e The histogram showed the inhibitory action of PKCZI195.17 on the number of pulmonary metastatic nodules. Columns mean of a typical experiment; bars SD (**P < 0.01, paired Student’s t test). f Kaplan-Meier survival curve showing increase in overall survival for the PKCZI195.17 treatment group of mice (n = 8) compared to the controls (n = 8, log-rank test **P < 0.01)
Discussion
PKC, a prototypical class of serine/threonine kinase, is certified to be associated with a number of diseases including breast cancer, which integrate in the cascades of multiple parallel and/or cross cell signaling pathways directing towards various cellular processes including cell proliferation, differentiation, survival, motility, and metastasis [18, 19]. Extensive work established PKC isoenzymes levels were always found elevated in many malignant cancers, which include breast, lung, liver, colon, leukemia, etc. [20]. Importantly, each PKC isoform has distinct biological function and may act differently in cancer progression. Accumulating reports have shown that PKC ζ plays pleiotropic roles in multiple signal transduction pathways including mitogen-activated kinase cascade, ribosomal S6-protein kinase signaling, NF-κB activation, and cell polarity pathway, which is also identified as a convergence point of RTK- and G-protein-coupled receptor-mediated chemotaxis signaling pathways in recent research [29]. Therefore, PKC ζ might be an effective target for more potent anti-metastasis therapeutic strategies.
Due to the apparent role of PKCs in cancer formation and progression, a variety of PKC inhibitors, for instance, enzastaurin, bryostatin, PKC412, LY317615 and G06976, etc., have been developed and tested in vitro and in vivo cancer models, and also in treatment of human cancers to some extent. These compounds have anti-neoplastic effects as estimated by decreased invasion or reduced growth of cancer cells both in vitro and in vivo but their clinical efficacy has unfortunately been low, and more importantly, rare effective inhibitors of PKC ζ have been identified. Here, we validated a novel and high-efficient PKC ζ inhibitor named PKCZI195.17 (IC50 = 32 nmol/l), which was screened out through a substrate-specific strategy, and its selectivity against PKC ζ has been further confirmed since it failed to inhibit PKC α, PKC β, PKC δ, PKC η, AKT2, as well as FGFR2 kinase activity. Besides, PKCZI195.17 displayed moderate inhibitory effects on breast cancer MDA-MB-231, MDA-MB-435, and MCF-7 cells proliferation at 30-nmol/l concentration.
Moreover, the EGF-induced chemotaxis assay, wound-healing assay, and Matrigel invasion assay were performed to evaluate the functional effects of PKCZI195.17 against MDA-MB-231, MDA-MB-435, and MCF-7 cells migration and invasion, and the results showed that PKCZI195.17 pre-incubation indeed impaired cells migration and invasion with high significance (Fig. 3, P < 0.05), which thus confirmed that PKC ζ is the key node connecting RTK- and GPCR-mediated cells migration including cancer cell metastasis, and that the effects of inhibition of PKC ζ alone were almost equivalent to the effects of GӦ6850 which disrupting all types of PKC activities. Cell adhesion and actin polymerization are two factors contributing to the cells motility and metastasis [38]. Our data further confirmed that cell adhesion ability and actin cytoskeleton remodeling of MDA-MB-231 cells were both severely impaired by PKCZI195.17 (Fig. 4a, b). PKCZI195.17 significantly inhibited phosphorylation of PKC ζ while showed no effects on activity of Akt, suggesting that Akt was an upstream regulator of PKC ζ. Furthermore, our data verified that EGF-induced phosphorylation of LIMK, cofilin, and integrin β1 was blocked by PKCZI195.17 (Fig. 4c, d, e), suggesting that LIMK, cofilin [38, 39], and integrin β1 might be downstream effectors of PKC ζ-mediated chemotaxis. In vivo, PKCZI195.17 was found to inhibit the tumor growth (Fig. 5a, b), the expression of proliferation marker ki67 (Fig. 5c), as well as the lung metastasis of breast cancer cell line MDA-MB-231 xenografts (Fig. 5d, e). This represents the first demonstration of in vivo anti-metastasis efficacy of a PKC ζ inhibitor using continued i.v. administration. Furthermore, PKCZI195.17 increased the survival time of mice bearing MDA-MB-231 breast tumors (Fig. 5f). This study demonstrated that the novel and selective small-molecule PKC ζ inhibitor, PKCZI195.17, inhibited EGF-induced breast cancer cell line chemotaxis, migration, invasion, presumably through preventing PKC ζ downstream effectors cofilin, LIMK, and integrin β1 from activations, and lung metastasis in mice bearing MDA-MB-231 breast tumors.
Taken together, PKCZI195.17 could be used as an anti-metastatic drug in oncology. Our data strongly indicate that our novel and selective small-molecule PKC ζ inhibitor, PKCZI195.17, showed therapeutic anti-metastatic effects in MDA-MB-231 breast tumor-bearing mice. However, further trials and clinical studies are necessary to validate the therapeutic potential of PKCZI195.17.
References
Thakur S, Singla AK, Chen J, Tran U, Yang Y, Salazar C, et al. Reduced ING1 levels in breast cancer promotes metastasis. Oncotarget. 2014;5(12):4244–56.
Su Z, Yang Z, Xu Y, Chen Y, Yu Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol Cancer. 2015;14:48. doi:10.1186/s12943-015-0321-5.
DeCastro AJ, Cherukuri P, Balboni A, DiRenzo J. ΔNP63α transcriptionally activates chemokine receptor 4 (CXCR4) expression to regulate breast cancer stem cell activity and chemotaxis. Mol Cancer Ther. 2015;14(1):225–35. doi:10.1158/1535-7163.MCT-14-0194.
Dillenburg-Pilla P, Patel V, Mikelis CM, Zárate-Bladés CR, Doçi CL, Amornphimoltham P, et al. SDF-1/CXCL12 induces directional cell migration and spontaneous metastasis via a CXCR4/Gαi/ mTORC1 axis. FASEB J. 2015;29(3):1056–68. doi:10.1096/fj.14-260083.
Feng S, Zhu W. Bidirectional molecular transport shapes cell polarization in a two-dimensional model of eukaryotic chemotaxis. J Theor Biol. 2014;363:235–46. doi:10.1016/j.jtbi.2014.08.033.
Xie L, Lu C, Wu XL. Marine bacterial chemoresponse to a stepwise chemoattractant stimulus. Biophys J. 2015;108(3):766–74. doi:10.1016/j.bpj.2014.11.3479.
Riahi R, Yang YL, Kim H, Jiang L, Wong PK, Zohar Y. A microfluidic model for organ-specific extravasation of circulating tumor cells. Biomicrofluidics. 2014;8(2):024103. doi:10.1063/1.4868301.eCollection2014.
Wang F, Lin SL. Knockdown of kinesin KIF11 abrogates directed migration in response to epidermal growth factor-mediated chemotaxis. Biochem Biophys Res Commun. 2014;452(3):642–8. doi:10.1016/j.bbrc.2014.08.136.
Uddin M, Lau LC, Seumois G, Vijayanand P, Staples KJ, Bagmane D, et al. EGF-induced bronchial epithelial cells drive neutrophil chemotactic and anti-apoptotic activity in asthma. PLoS One. 2013;8(9):e72502. doi:10.1371/journal.pone.0072502.eCollection2013.
Ota I, Higashiyama S, Masui T, Yane K, Hosoi H, Matsuura N. Heparin-binding EGF-like growth factor enhances the activity of invasion and metastasis in thyroid cancer cells. Oncol Rep. 2013;30(4):1593–600. doi:10.3892/or.2013.2659.
Seshacharyulu P, Ponnusamy MP, Rachagani S, Lakshmanan I, Haridas D, Yan Y, et al. Targeting EGF-receptor(s)-STAT1 axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer. Oncotarget. 2015;6(7):5164–81.
Mori N, Ishikawa C, Senba M. Activation of PKC-δ in HTLV-1-infected T cells. Int J Oncol. 2015;46(4):1609–18. doi:10.3892/ijo.2015.2848.
Lorimer IA. Atypical PKCι as target for glioblastoma therapy. Curr Cancer Drug Targets. 2015;15(2):136–44.
Martin-Liberal J, Cameron AJ, Claus J, Judson IR, Parker PJ, Linch M. Targeting protein kinase C in sarcoma. Biochim Biophys Acta. 2014;1846(2):547–59. doi:10.1016/j.bbcan.2014.10.002.
Vorhagen S, Niessen CM. Mammalian aPKC/Par polarity complex mediated regulation of epithelial division orientation and cell fate. Exp Cell Res. 2014;328(2):296–302. doi:10.1016/j.yexcr.2014.08.008.
Chen J, Zhang M. The Par3/Par6/aPKC complex and epithelial cell polarity. Exp Cell Res. 2013;319(10):1357–64. doi:10.1016/j.yexcr.2013.03.021.
Parker PJ, Justilien V, Riou P, Linch M, Fields AP. Atypical protein kinase Cι as a human oncogene and therapeutic target. Biochem Pharmacol. 2014;88(1):1–11. doi:10.1016/j.bcp.2013.10.023.
Iitaka D, Moodley S, Shimizu H, Bai XH, Liu M. PKCδ-iPLA2-PGE2- PPARγ signaling cascade mediates TNF-α induced Claudin 1 expression in human lung carcinoma cells. Cell Signal. 2015;27(3):568–77. doi:10.1016/j.cellsig.2014.12.015.
Zhao X, Rotenberg SA. Phosphorylation of Cdc42 effector protein-4 (CEP4) by protein kinase C promotes motility of human breast cells. J Biol Chem. 2014;289(37):25844–54. doi:10.1074/jbc.M114.577783.
Liu ZC, Chen XH, Song HX, Wang HS, Zhang G, Wang H, et al. Snail regulated by PKC/GSK-3β pathway is crucial for EGF-induced epithelial-mesenchymal transition (EMT) of cancer cells. Cell Tissue Res. 2014;358(2):491–502. doi:10.1007/s00441-014-1953-2.
Horng CT, Shieh PC, Tan TW, Yang WH, Tang CH. Paeonol suppresses chondrosarcoma metastasis through up-regulation of miR-141 by modulating PKCδ and c-Src signaling pathway. Int J Mol Sci. 2014;15(7):11760–72. doi:10.3390/ijms150711760.
Lee H, Park M, Shin N, Kim G, Kim YG, Shin JS, et al. High mobility group box-1 is phosphorylated by protein kinase C zeta and secreted in colon cancer cells. Biochem Biophys Res Commun. 2012;424(2):321–6. doi:10.1016/j.bbrc.2012.06.116.
Rimessi A, Zecchini E, Siviero R, Giorgi C, Leo S, Rizzuto R, et al. The selective inhibition of nuclear PKCζ restores the effectiveness of chemotherapeutic agents in chemoresistant cells. Cell Cycle. 2012;11(5):1040–8. doi:10.4161/cc.11.5.19520.
Wu J, Zhang B, Wu M, Li H, Niu R, Ying G, et al. Screening of a PKC zeta-specific kinase inhibitor PKCzI257.3 which inhibits EGF-induced breast cancer cell chemotaxis. Invest New Drugs. 2010;28(3):268–75. doi:10.1007/s10637-009-9242-8.
Giagulli C, Scarpini E, Ottoboni L, Narumiya S, Butcher EC, Constantin G, et al. RhoA and zeta PKC control distinct modalities of LFA-1 activation by chemokines: critical role of LFA-1 affinity triggering in lymphocyte in vivo homing. Immunity. 2004;20:25–35. doi:10.1016/S1074-7613(03)00350-9.
Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence of zeta protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem. 1998;273:30306–15. doi:10.1074/jbc.273.46.30306.
Guo H, Gu F, Li W, Zhang B, Niu R, Fu L, et al. Reduction of protein kinase C zeta inhibits migration and invasion of human glioblastoma cells. J Neurochem. 2009;109(1):203–13. doi:10.1111/j.1471-4159.2009.05946.x.
Guo H, Ma Y, Zhang B, Sun B, Niu R, Ying G, et al. Pivotal advance: PKCzeta is required for migration of macrophages. J Leukoc Biol. 2009;85(6):911–8. doi:10.1189/jlb.0708429.
Sun R, Gao P, Chen L, Ma D, Wang J, Oppenheim JJ, et al. Protein kinase C zeta is required for epidermal growth factor-induced chemotaxis of human breast cancer cells. Cancer Res. 2005;65:1433–41. doi:10.1158/0008-5472.CAN-04-1163.
Liu Y, Wang B, Wang J, Wan W, Sun R, Zhao Y, et al. Down-regulation of PKC zeta expression inhibits chemotaxis signal transduction in human lung cancer cells. Lung Cancer. 2009;63:210–8. doi:10.1016/j.lungcan.2008.05.010.
Rodems SM, Hamman BD, Lin C, Zhao J, Shah S, Heidary D, et al. A FRET-based assay platform for ultra-high density drug screening of protein kinases and phosphatases. Assay Drug Dev Technol. 2002;1:9–19. doi:10.1089/154065802761001266.
Barati MT, Scherzer J, Wu R, Rane MJ, Klein JB. Cytoskeletal rearrangement and Src and PI-3K-dependent Akt activation control GABA(B)R-mediated chemotaxis. Cell Signal. 2015;27(6):1178–85. doi:10.1016/j.cellsig.2015.02.022.
Reinhardt B, Godfrey R, Fellbrich G, Frank H, Lüske A, Olieslagers S, et al. Human cytomegalovirus infection impairs endothelial cell chemotaxis by disturbing VEGF signalling and actin polymerization. Cardiovasc Res. 2014;104(2):315–25. doi:10.1093/cvr/cvu204.
Wang JN, Wan WZ, Sun RH, Liu Y, Sun XJ, Ma DL, et al. Reduction of Akt2 expression inhibits chemotaxis signal transduction in human breast cancer cells. Cell Signal. 2008;20(6):1025–34. doi:10.1016/j.cellsig.2007.12.023.
Wang JT, Song LZ, Li LL, Zhang W, Chai XJ, An L, et al. Src controls neuronal migration by regulating the activity of FAK and cofilin. Neuroscience. 2015;292:90–100. doi:10.1016/j.neuroscience.2015.02.025.
Wang Y, Kuramitsu Y, Kitagawa T, Baron B, Yoshino S, Maehara SI, et al. Cofilin-phosphatase slingshot-1L (SSH1L) is over-expressed in pancreatic cancer (PC) and contributes to tumor cell migration. Cancer Lett. 2015;360(2):171–6. doi:10.1016/j.canlet.2015.02.015.
Siton O, Bernheim-Groswasser A. Reconstitution of actin-based motility by vasodilator-stimulated phosphoprotein (VASP) depends on the recruitment of F-actin seeds from the solution produced by cofilin. J Biol Chem. 2014;289(45):31274–86. doi:10.1074/jbc.M114.586958.
Li HY, Wu J, Ying GG, Chen LW, Lai LH, Liu Z, et al. J-4: a novel and typical preclinical anticancer drug targeting protein kinase C ζ. Anti- Cancer Drugs. 2012;23(7):691–7.
Huang X, Sun D, Pan Q, Wen WW, Chen Y, Xin XL, et al. JG6, a novel marine-derived oligosaccharide, suppresses breast cancer metastasis via binding to cofilin. Oncotarget. 2014;5(11):3568–78.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81301985 and No. 81102292) and the Natural Science Foundation of Tianjin (No. 14JCQNJC14000 and No. 12JCQNJC06200; to Jing Wu and Rui Yang).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance with ethical standards
All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animal of Tianjin Third Central Hospital.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Wu, J., Liu, S., Fan, Z. et al. A novel and selective inhibitor of PKC ζ potently inhibits human breast cancer metastasis in vitro and in mice. Tumor Biol. 37, 8391–8401 (2016). https://doi.org/10.1007/s13277-015-4744-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4744-9