Abstract
Basal cell carcinoma (BCC) is the most common tumor in humans. Reduced expression of sirtuins interferes with DNA repair, which may cause mutations and genomic instability, and eventually leads to tumor development. In the present study, we investigate the expression levels of SIRT genes in non-tumoral and tumor tissues of patients with BCC. A total of 27 patients (16 males, 11 females) with BCC were included in the study; the mean age was 65.40 ± 10.74 years and mean follow-up was 2.5 ± 0.5 years. There were multiple synchronous lesions in six patients, and the remaining 21 patients had a single lesion. Tumor and non-tumoral tissue samples were collected from all patients, and mRNA expression levels of SIRT1–7 (Sirt1.1, Sirt1.2, Sirt2, Sirt3, Sirt4, Sirt5, Sirt6, and Sirt7) were examined by real-time PCR. The results showed that expressions of SIRT1.1, SIRT1.2, SIRT4, SIRT5, SIRT6, and SIRT7 mRNAs were unchanged in tumor tissues of BCC patients compared with non-tumoral tissue samples. Importantly, the expressions of SIRT2 and SIRT3 mRNAs were significantly reduced in tumor tissue samples from BCC patients compared with non-tumoral tissues (P = 0.02 and P = 0.03, respectively). In light of the previous reports that have demonstrated a link between SIRT proteins and cancer, our findings suggest that SIRT2 and SIRT3 may plan important roles in BCC pathogenesis and could be candidate prognostic biomarkers for BCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Basal cell carcinoma (BCC) is the most commonly seen tumor type in humans and shows local invasion and slow progression [1, 2]. BCC is thought to originate from the basal layer of the epidermis or hair follicles [3]. Although the mortality rate of BCC is low, its incidence rate is high. If treatment is delayed or inadequately performed, BCC may become an important public health issue because of local recurrence. BCC treatment strategies include surgical excision, chemotherapy, and radiotherapy [4]. Curative treatment is possible with early diagnosis [5].
Sirtuins are NADH-dependent deacetylases in mammals that are encoded by seven different gene loci [6, 7]. In this entire sirtuins, 275-amino-acid catalytic core domain is located in the well-preserved regions [8]. SIRT1, 6, and 7 are localized primarily in the nucleus, SIRT2 is in the cytoplasm, and SIRT 3, 4, and 5 are located in mitochondria [9]. SIRT proteins function in a variety of metabolic processes including cell differentiation, viability, senescence, and inflammation. SIRT proteins also play a prominent role in the modification of many biochemical components, such as cytoskeletal proteins, transcription factors, and cell signaling components [10]. Previous studies have shown that some of the members of this protein family function as tumor suppressors [11, 12], and SIRT proteins are involved in a variety of important physiological processes including oxidative stress, genomic stability, cell survival, development, metabolism, aging, and longevity [13–15]. Because of these biological functions, increased expression of sirtuins is considered beneficial for longevity and provides a protective function against cancer [16–18].
Previous studies showed that sirtuins exert protective effects against DNA damage and promote longevity via reducing oxidative stress and genomic instability [19]. In contrast, loss of their expression interferes with proper DNA repair, thus leading to mutations, genomic instability, and, consequently, tumor growth [13]. We hypothesized that SIRT proteins may influence the pathogenesis of BCC. Therefore, we evaluated expression levels of SIRT genes in tumor and non-tumoral tissue samples of BCC patients.
Materials and methods
Patients and tissue samples
All patients were admitted to the Plastic, Reconstructive, and Aesthetic Surgery Clinic and diagnosed with BCC. Patients were informed about the study and provided informed consent before inclusion in the study. This study was approved by the local ethics committee. Exclusion criteria included the following: patients under the age of 18, pregnant women, patients unauthorized to sign, patients who refused to participate in the study, tumor diameter of less than 1 cm, presence of external tumors in other areas of the body than BCC, patients receiving chemotherapy and/or radiotherapy for cancer in other parts of the body, patients with immunodeficiency, and patients with predisposition to skin cancer formation (Gorlin syndrome, basal cell nevus syndrome, Bazexim syndrome, albinism, xeroderma pigmentosum). Patients were subjected to local and general anesthesia prior to surgical operation, and tumoral and macroscopically non-tumoral tissue samples of patients were excised during surgical operation. Some specimens taken from patients were preserved in 10 % formalin solution after necessary markings and pathologically correlated with the presence of skin tumors. The non-tumoral tissues of the patients were used as control groups of the tumor tissues. The cancer location, histological subtypes, demographic characteristics of patients, lesion location, presence of recurrence or primary disease, and closure technique of the defect after excision are presented in Table 1.
Total RNA isolation from tissues
Tumoral and non-tumoral tissue samples were cut in pieces, and 25 mg was homogenized on ice by a homogenizer (Kinematica, Gmdh, Switzerland). The High Pure RNA Tissue Kit (Roche, Cat. No. 12 033 674 001, Mannheim, Germany) was used for isolation of total RNA from tissue samples according to the manufacturer’s protocol.
Quantitative real-time polymerase chain reaction
cDNA was obtained from mRNAs of all samples using the miScript II RT Kit (Qiagen, Hilden, Germany, Cat. No. 218161) and the 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA). Primer sets for specific reverse transcription for SIRT1–7 and endogenous control ActB genes were obtained from Qiagen. qRT-PCR was carried out using a LightCycler 480 II (Roche, Mannheim, Germany). The PCR master mix (Qiagen, Cat. No. 218073) containing 2× QuantiTect SYBR Green PCR Master Mix, 10× miScript Universal Primer, 10× miScript Primer Assay (related mRNA primer), RNase-free water, and template cDNA in 25 μL volume was analyzed using the following conditions: 95 °C for 15 min and 40 cycles of 94 °C for 15 s, 55 °C for 30 s, and 70 °C for 30 s. The signal was collected at the endpoint of every cycle.
Statistical analysis
The collected data were analyzed using SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Tumor and non-tumor tissues of BCC patients were compared with the Wilcoxon signed rank test. P values <0.05 were interpreted as statistically significant.
Results
This study included a total of 27 patients (16 males, 11 females) diagnosed with BCC. The mean patient age was 65.40 ± 10.74 years, and mean follow-up was 2.5 ± 0.5 years. No recurrence or wound healing problem was detected in any patient. BCC lesions were observed in the nose (n = 11), eyelid (n = 4), malar region (n = 6), forehead (n = 4), ear (n = 1), and leg (n = 1). To close defects, primary closure was employed in four patients, while grafting was performed in 12 patients and defects were closed by appropriate local flaps in the remaining 11 patients. There were multiple synchronous lesions in six patients, while the remaining 21 patients had a single lesion (Table 1).
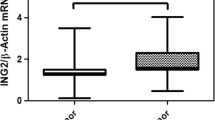
Expression levels of SIRT mRNA were assessed in tumoral and non-tumoral tissues of BCC patients. We examined the expression levels of two different transcription variants of SIRT1, SIRT1 transcript variant 1 (NCBI accession number NM 012238) and SIRT1 transcript variant 2 (NCBI accession number NM 001142498.1). Levels of both transcription variants of SIRT1, SIRT1.1, and SIRT1.2 as well as SIRT4, SIRT5, SIRT6, and SIRT7 mRNAs were not altered in tumoral tissues compared with non-tumoral tissue samples. However, expression levels of SIRT2 and SIRT3 mRNAs were significantly reduced in tumor tissues of BCC patients compared with non-tumoral tissues, as presented in Fig. 1 and Table 2 (P = 0.02 and P = 0.03, respectively).
Comparison of SIRT gene expression in non-tumoral (Non-T) and tumor (T) tissue of BCC patients. Gene expression levels of SIRT1 (SIRT1-1 SIRT1 transcription variant-1, SIRT1-2 SIRT1 transcription variant-2), SIRT2–7, and β-actin (ACTB) were determined in mRNA samples of non-tumoral and tumor tissues of BCC patients. ACTB was used as an internal control for the normalization of SIRT gene expressions
Discussion
Previous studies have linked sirtuin family members with tumor suppressor functions. Here, we evaluated the expression levels of SIRT1–7 genes in tumor and non-tumoral tissue samples of BCC patients and found that the expression levels of SIRT2 and SIRT3 were significantly reduced in tumor tissues of BCC patients compared with non-tumoral tissues. SIRT2 and 3 act like tumor suppressors in patients with BCC as well as other tumors. As a result of the decrease in these sirtuins, the exposure to cancerous like ultraviolet radiation (UV) causes genomic instability and BCC due to the mutations. However, there is not a precise explanation about which path these molecules use during this process.
The SIRT1 NAD(+)-dependent histone deacetylase is involved in transcription, DNA replication, and DNA repair [20] and increases survival of cells by downregulating rRNA genes during undesirable conditions such as starvation [21]. SIRT1 is also involved in DNA repair together with p53, APE/Ref1, and PARP1 proteins [22–24]. To the best of our knowledge, our study is the first to examine the relationship between SIRT1 expression and BCC. The pathogenesis of BCC resembles squamous cell carcinoma (SCC) in many ways. Alterations of SIRT1 expression levels were reported in head and neck squamous cell carcinoma patients and shown to be associated with good prognosis [25]. SIRT1 was also overexpressed in malignant melanoma [26]. However, our results did not reveal any changes in SIRT1 expression in tumor tissues from BCC patients.
SIRT6 is a nuclear protein that is associated with heterochromatin and functions in base excision repair (BER). BER protects the genomic structure against alkylation, oxidation, and point mutations formed during deamination or by chemical mutagens [9, 27]. The ADP-ribosylation target of SIRT6 has not been determined; however, histone and DNA repair proteins have been considered possible targets. As SIRT2 and SIRT6 are localized together in the nucleus during DNA repair, the SIRT2–SIRT6 crosstalk can be thought to induce mitosis progression. The replicative lifespans of human fibroblast cells were postulated not to be long because of the increased expression levels of SIRT6 [27]. SIRT6 is considered a tumor suppressor because of its ability to block mutations and increase genomic stability [13]. UV-induced mutations are known to play significant roles in BCC pathogenesis, and we speculate that SIRT6 expression would increase during UV-induced DNA damage repair in BCC. However, in our study, we did not observe any changes in expression levels of SIRT6 in tumoral samples of BCC patients. We believe this may be due to two possible reasons. First, it is possible that re-evaluating SIRT6 protein levels from the same sample could generate different results. Second, we did not perform grouping according to cancer staging when determining the expression level of SIRT6 mRNA, and expression levels of genes are known to vary depending on the cancer stage.
SIRT7 is located in the nucleus and functions during chromatin condensation in mitosis. SIRT7 is highly expressed in tissues with increased proliferation ability, including the liver, testis, and spleen of the mouse [9, 28] and expressed at low levels in non-proliferative tissues of the heart, brain, and skeletal muscle. SIRT7 interacts with RNA polymerase I and increases the transcription of rRNA genes. SIRT7 expression levels were elevated in papillary thyroid carcinoma tumor tissues compared with normal tissues [29, 30]. SIRT7 was also highly expressed in breast cancer patients with lymph node metastasis compared with breast cancer patients without lymph node metastasis and normal breast tissues [31]. Some reports have indicated that human U2OS cells undergo apoptosis due to defective endogenous SIRT7 expression; thus, SIRT7 seems to play a crucial role in the survival of these cells [28]. SIRT is required both for the survival and proliferation of normal and transformed cells because it regulates the transcription of rRNAs. In our study, SIRT7 expression levels were found to be similar between tumor tissues and non-tumoral tissues.
Previous studies showed that SIRT2 functions as a tumor suppressor [27, 32–37]. SIRT2 plays an essential role in the regulation of the mitosis and positively regulates anaphase by promoting the function of the anaphase complex/cyclosome function. SIRT2 dysfunction has been associated with genomic instability and tumorigenesis [36], and deregulation of SIRT2 was observed in gliomas [19]. Reduced levels of SIRT2 were shown to inhibit apoptosis through p53 in HeLa cells [38]. Here, we found reduced expression of SIRT2 in tumor tissues of BCC patients compared with non-tumoral tissues, suggesting that SIRT2 may play a significant role in BCC development. SIRT2 is involved in both mitotic control and apoptosis control through p53. Thus, decreased expression levels of the SIRT2 gene could lead to BCC development through deregulation of these cellular pathways.
SIRT3, SIRT4, and SIRT5 proteins are all localized in the mitochondria. Approximately 20 % of mitochondrial proteins are found in an acetylated form and are associated with lifespan and metabolism [39]. Mitochondria play key roles in lifespan and aging as these organelles contain reactive oxygen species (ROS) that cause oxidative stress. Mitochondrial sirtuins play a key role in the regulation of energy metabolism and in regulating response to oxidative stress in tumor cells. Although some literature is available on SIRT3 and SIRT4, little is known regarding SIRT5 [9, 40]. Several studies have shown an association between SIRT3 and aging in humans [41, 42].
SIRT3 changes the redox balance of the cell by affecting the function of a number of enzymes through deacetylation and regulates the response to oxidative stress. The increase of ROS in cells interferes with physiological functions by affecting DNA, proteins, and lipids and causes several pathological conditions such as diabetes, aging, neurodegenerative diseases, and cancer [43]. While defects in SIRT3 interfere with the deacetylation of mitochondrial proteins, defects in SIRT4 and SIRT5 had no impact on mitochondrial protein deacetylation [44]. Therefore, SIRT3 is considered the major enzyme responsible for mitochondrial deacetylation events. Previous studies have shown that SIRT3 acts as a mitochondrial tumor suppressor protein [45–47]. SIRT3 is synthesized as an inactive protein and activated by matrix peptidases [48, 49] and regulates oxidative stress [50–52]. Chen et al. evaluated SIRT3 levels in oral SCC cases and reported that while the SIRT3 enzyme level was relatively normal, SIRT3 enzyme activity was defective; the authors also found a loss of heterozygosity of the SIRT3 gene in five of 21 (23.8 %) patients [53]. Loss of function of SIRT3 increases the level of ROS and results in genomic instability, thus supporting its role as a tumor suppressor [45]. In our study, SIRT3 gene expression was significantly decreased in tumoral tissues of BCC patients compared with non-tumoral tissues. In a previous study, SIRT3 overexpression was shown to inhibit prostate cancer cell proliferation in both in vivo and in vitro experimental setups [54]. In the same study, silencing SIRT3 expression was found to promote the growth of prostate cancer cells. Wang et al. demonstrated that inhibition of SIRT3 affects NOTCH-1 mRNA and protein levels in gastric cancers [55]. Previous reports showed that the sonic hedgehog (SHH) pathway is involved in the development of BCC [56]. Besides, numerous studies that show NOTCH-1 as SHH transcription factor as effective on expression levels were reported [57, 58]. In the light of all these previously reported studies and the data we obtained, it makes us think that the lower levels of SIRT-3 expressions on tumors of patients with BCC affect NOTCH-1 levels through SHH and cause tumor development. Moreover, reduction of SIRT3 expression may lead to tumor development by increasing mitochondrial ROS levels. ROS level is an important factor in tumor development because increased ROS levels are responsible not only for DNA mutations but also for disrupting the balance of apoptosis in cells. The tumor suppressor function of SIRT4 was reported in many studies, and SIRT4 was suggested as a novel therapeutic target for some types of cancers, such as colorectal cancer [18]. However, our results showed no change in the expression levels of SIRT4 and SIRT5 mRNAs.
Several studies have demonstrated an association between the expression levels of mitochondrial sirtuins and cancer [18, 59–61]. These reports showed that SIRT3 and SIRT4 function in various types of cancers and could be novel therapeutic targets. However, our study demonstrated a significant finding that among all mitochondrial sirtuins, only SIRT3 expression was altered in BCC pathogenesis. The SHH pathway plays a critical role in BCC development, and SIRT3 is the only sirtuin that affects this pathway. This may explain the increased expression of this gene in BCC.
Conclusion
Investigation of the relationship between sirtuins and cancer has generated promising results for the treatment of cancer, and these molecules have been suggested as therapeutic targets. In the current work, we provide the first report of reduced expression of mRNA levels of two SIRT family members (SIRT2 and SIRT3) in tumoral tissues from BCC patients compared with non-tumoral tissues of BCC patients. In light of previous studies that have established a relationship between SIRT and cancer, SIRT2 and SIRT3 seem to have key roles in BCC pathogenesis and could be candidate prognostic biomarkers in BCC.
References
Savoia P, Deboli T, Previgliano A, Broganelli P. Usefulness of photodynamic therapy as a possible therapeutic alternative in the treatment of basal cell carcinoma. Int J Mol Sci. 2015;16(10):23300–17. doi:10.3390/ijms161023300.
Deng M, Marsch AF, Petronic-Rosic V. Basal cell carcinoma. Part 1: basal cell carcinoma has come of age. Skinmed. 2015;13(3):206–13. quiz 14.
Chu SW, Biswas A. Basal cell carcinomas showing histological features generally associated with cutaneous adnexal neoplasms. J Cutan Pathol. 2015. doi:10.1111/cup.12577.
Gualdi G, Monari P, Apalla Z, Lallas A. Surgical treatment of basal cell carcinoma and squamous cell carcinoma. G Ital Dermatol Venereol. 2015;150(4):435–47.
Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53. doi:10.12703/P7-53.
Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi:10.1038/35001622.
Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–38. doi:10.1038/nrm3293.
Hallows WC, Albaugh BN, Denu JM. Where in the cell is SIRT3?—functional localization of an NAD+-dependent protein deacetylase. Biochem J. 2008;411(2):e11–3. doi:10.1042/BJ20080336.
Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16(10):4623–35. doi:10.1091/mbc.E05-01-0033.
Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126(2):257–68. doi:10.1016/j.cell.2006.07.002.
Voelter-Mahlknecht S, Mahlknecht U. Cloning, chromosomal characterization and mapping of the NAD-dependent histone deacetylases gene sirtuin 1. Int J Mol Med. 2006;17(1):59–67.
Hida Y, Kubo Y, Murao K, Arase S. Strong expression of a longevity-related protein, SIRT1, in Bowen’s disease. Arch Dermatol Res. 2007;299(2):103–6. doi:10.1007/s00403-006-0725-6.
Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26(37):5489–504. doi:10.1038/sj.onc.1210616.
Haigis MC, Guarente LP. Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–21. doi:10.1101/gad.1467506.
Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404(1):1–13. doi:10.1042/BJ20070140.
Poulose N, Raju R. Sirtuin regulation in aging and injury. Biochim Biophys Acta. 2015;1852(11):2442–55. doi:10.1016/j.bbadis.2015.08.017.
Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. American journal of physiology Heart and circulatory physiology. 2015:ajpheart 00053 2015. doi:10.1152/ajpheart.00053.2015.
Miyo M, Yamamoto H, Konno M, Colvin H, Nishida N, Koseki J, et al. Tumour-suppressive function of SIRT4 in human colorectal cancer. Br J Cancer. 2015;113(3):492–9. doi:10.1038/bjc.2015.226.
Sayd S, Thirant C, El-Habr EA, Lipecka J, Dubois LG, Bogeas A, et al. Sirtuin-2 activity is required for glioma stem cell proliferation arrest but not necrosis induced by resveratrol. Stem Cell Rev. 2014;10(1):103–13. doi:10.1007/s12015-013-9465-0.
Abdelmohsen K, Pullmann Jr R, Lal A, Kim HH, Galban S, Yang X, et al. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25(4):543–57. doi:10.1016/j.molcel.2007.01.011.
Murayama A, Ohmori K, Fujimura A, Minami H, Yasuzawa-Tanaka K, Kuroda T, et al. Epigenetic control of rDNA loci in response to intracellular energy status. Cell. 2008;133(4):627–39. doi:10.1016/j.cell.2008.03.030.
Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107(2):137–48.
Rajamohan SB, Pillai VB, Gupta M, Sundaresan NR, Birukov KG, Samant S, et al. SIRT1 promotes cell survival under stress by deacetylation-dependent deactivation of poly(ADP-ribose) polymerase 1. Mol Cell Biol. 2009;29(15):4116–29. doi:10.1128/MCB.00121-09.
Yamamori T, DeRicco J, Naqvi A, Hoffman TA, Mattagajasingh I, Kasuno K, et al. SIRT1 deacetylates APE1 and regulates cellular base excision repair. Nucleic Acids Res. 2010;38(3):832–45. doi:10.1093/nar/gkp1039.
Noguchi A, Li X, Kubota A, Kikuchi K, Kameda Y, Zheng H, et al. SIRT1 expression is associated with good prognosis for head and neck squamous cell carcinoma patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(3):385–92. doi:10.1016/j.oooo.2012.12.013.
Wilking MJ, Singh C, Nihal M, Zhong W, Ahmad N. SIRT1 deacetylase is overexpressed in human melanoma and its small molecule inhibition imparts anti-proliferative response via p53 activation. Arch Biochem Biophys. 2014;563:94–100. doi:10.1016/j.abb.2014.04.001.
Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–29. doi:10.1016/j.cell.2005.11.044.
Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20(9):1075–80. doi:10.1101/gad.1399706.
De Nigris F, Cerutti J, Morelli C, Califano D, Chiariotti L, Viglietto G, et al. Isolation of a SIR-like gene, SIR-T8, that is overexpressed in thyroid carcinoma cell lines and tissues. Br J Cancer. 2002;87(12):1479. doi:10.1038/sj.bjc.6600636.
Frye R. “SIRT8” expressed in thyroid cancer is actually SIRT7. Br J Cancer. 2002;87(12):1479. doi:10.1038/sj.bjc.6600635.
Ashraf N, Zino S, Macintyre A, Kingsmore D, Payne AP, George WD, et al. Altered sirtuin expression is associated with node-positive breast cancer. Br J Cancer. 2006;95(8):1056–61. doi:10.1038/sj.bjc.6603384.
Movahedi Naini S, Sheridan AM, Force T, Shah JV, Bonventre JV. Group IVA cytosolic phospholipase A2 regulates the G2-to-M transition by modulating the activity of tumor suppressor SIRT2. Mol Cell Biol. 2015;35(21):3768–84. doi:10.1128/MCB.00184-15.
Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 2000;14(9):1021–6.
Kyrylenko S, Kyrylenko O, Suuronen T, Salminen A. Differential regulation of the Sir2 histone deacetylase gene family by inhibitors of class I and II histone deacetylases. Cell Mol Life Sci. 2003;60(9):1990–7. doi:10.1007/s00018-003-3090-z.
Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability, and aging. Cell. 2005;120(4):497–512. doi:10.1016/j.cell.2005.01.028.
Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu X, et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20(4):487–99. doi:10.1016/j.ccr.2011.09.004.
Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6.
Aguissa-Toure AH, Wong RP, Li G. The ING family tumor suppressors: from structure to function. Cell Mol Life Sci. 2011;68(1):45–54. doi:10.1007/s00018-010-0509-1.
Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23(4):607–18. doi:10.1016/j.molcel.2006.06.026.
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11(2):437–44.
Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38(10):1065–70.
Glatt SJ, Chayavichitsilp P, Depp C, Schork NJ, Jeste DV. Successful aging: from phenotype to genotype. Biol Psychiatry. 2007;62(4):282–93. doi:10.1016/j.biopsych.2006.09.015.
Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–95. doi:10.1016/j.cell.2005.02.001.
Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27(24):8807–14. doi:10.1128/MCB.01636-07.
Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17(1):41–52. doi:10.1016/j.ccr.2009.11.023.
Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19(3):416–28. doi:10.1016/j.ccr.2011.02.014.
Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1alpha and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30(26):2986–96. doi:10.1038/onc.2011.37.
Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A. 2002;99(21):13653–8. doi:10.1073/pnas.222538099.
Schwer B, North BJ, Frye RA, Ott M, Verdin E. The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol. 2002;158(4):647–57. doi:10.1083/jcb.200205057.
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–5. doi:10.1038/nature08778.
Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–7. doi:10.1016/j.cmet.2010.11.015.
Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–12. doi:10.1016/j.cell.2010.10.002.
Chen IC, Chiang WF, Liu SY, Chen PF, Chiang HC. Role of SIRT3 in the regulation of redox balance during oral carcinogenesis. Mol Cancer. 2013;12:68. doi:10.1186/1476-4598-12-68.
Quan Y, Wang N, Chen Q, Xu J, Cheng W, Di M et al. SIRT3 inhibits prostate cancer by destabilizing oncoprotein c-MYC through regulation of the PI3K/Akt pathway. Oncotarget. 2015.
Wang L, Wang WY, Cao LP. SIRT3 inhibits cell proliferation in human gastric cancer through down-regulation of Notch-1. Int J Clin Exp Med. 2015;8(4):5263–71.
Lesiak A, Sobolewska-Sztychny D, Majak P, Sobjanek M, Wodz K, Sygut KP, et al. Relation between sonic hedgehog pathway gene polymorphisms and basal cell carcinoma development in the Polish population. Arch Dermatol Res. 2015. doi:10.1007/s00403-015-1612-9.
Morrow D, Cullen JP, Liu W, Guha S, Sweeney C, Birney YA, et al. Sonic Hedgehog induces Notch target gene expression in vascular smooth muscle cells via VEGF-A. Arterioscler Thromb Vasc Biol. 2009;29(7):1112–8. doi:10.1161/ATVBAHA.109.186890.
Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010;5(11):e13984. doi:10.1371/journal.pone.0013984.
He S, He C, Yuan H, Xiong S, Xiao Z, Chen L. The SIRT 3 expression profile is associated with pathological and clinical outcomes in human breast cancer patients. Cell Physiol Biochem. 2014;34(6):2061–9. doi:10.1159/000366401.
Liu R, Fan M, Candas D, Qin L, Zhang X, Eldridge A, et al. CDK1-mediated SIRT3 activation enhances mitochondrial function and tumor radioresistance. Mol Cancer Ther. 2015;14(9):2090–102. doi:10.1158/1535-7163.MCT-15-0017.
Gonzalez Herrera KN, Lee J, Haigis MC. Intersections between mitochondrial sirtuin signaling and tumor cell metabolism. Crit Rev Biochem Mol Biol. 2015;50(3):242–55. doi:10.3109/10409238.2015.1031879.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Funding
This research did not receive funding from any agency in the public, commercial, or not for profit sectors.
Rights and permissions
About this article
Cite this article
Temel, M., Koç, M.N., Ulutaş, S. et al. The expression levels of the sirtuins in patients with BCC. Tumor Biol. 37, 6429–6435 (2016). https://doi.org/10.1007/s13277-015-4522-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4522-8