Abstract
FBXW7 (F-box and WD repeat domain-containing 7) is the F-box protein component of a Skp1–Cul1–F-box protein–type (SCF-type) ubiquitin ligase. Previous studies have shown that FBXW7 serves as a tumor suppressor and is frequently downregulated in many types of human neoplasms. However, the molecular mechanisms for its downregulation remain poorly understood. Hyperactivation of Wnt/β-catenin signaling pathway is viewed as crucial for tumorigenesis, including hepatocellular carcinoma (HCC). In the present study, we show that protein levels, but not message RNA, of FBXW7 were suppressed by Wnt3a treatment or transfection of a constitutively activated β-catenin in HCC cells. Besides, microRNA-770 was identified as an important downstream target of Wnt/β-catenin signaling, to inhibit FBXW7 expression through targeting its 3′-untranslated region. Thus, our results suggest a previously unknown Wnt/β catenin–miR-770–FBXW7 molecular network in the HCC development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ubiquitin–proteasome system (UPS) plays an important role in many biological events, such as regulation of cell proliferation, differentiation, apoptosis, and many more [1, 2]. Recent studies have shown that defects in the UPS components could contribute to human diseases, including tumorigenesis [3, 4]. For instance, overexpression of Skp2 (S-phase kinase-associated protein 2) is required for cell–cycle progression by ubiquitination and degradation of several cell–cycle regulators, including p27, Foxo1, and p130 [5, 6]. Therefore, targeting the UPS might provide efficient anti-cancer drugs for patients.
FBXW7 (F-box and WD repeat domain-containing 7), a component of Skp1-Cul1-F box protein ubiquitin ligase, is considered as a tumor suppressor protein in human cancers, including hepatocellular carcinoma (HCC) [7, 8]. This is supported by several lines of evidence. Firstly, FBXW7 targets multiple oncoproteins including mTOR, c-myc, Cyclin E, c-Jun, and SRC-3 for ubiquitination-mediated degradation [9–13]. Secondly, hepatic deletion of FBXW7 led to hepatomegaly and steatohepatitis, accompanied with increased cell proliferation [14]. Thirdly, mutation or reduced expression of FBXW7 has been observed in many types of human malignancy, such as gastric, prostate, pancreatic, and colorectal cancer [15–17]. However, the upstream signaling pathway that inhibits its expression remains largely unknown.
Persistent activation of oncogenic pathways, such as Wnt/β-catenin, NF-κB, and Stat3 signalings, is a hallmark of human cancer [18, 19]. In this study, we aim to investigate whether FBXW7 could be regulated by these signalings, and our results indicate that Wnt/β-catenin signaling is a major regulator of FBXW7 expression in HCC.
Materials and methods
Cell culture and reagents
HCC cell lines (HepG2 and Hep3B cells) were obtained from The Cell Bank of Type Culture Collection of Chinese Academy of Sciences (CAS, Shanghai). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Shanghai) supplemented with 10 % fetal bovine serum (Gibco) and maintained at 37 °C in a humidified atmosphere with 5 % CO2.
Adenovirus and small interfering RNA
Recombinant adenovirus expressing constitutively activated β-catenin (CA-β-catenin) or empty vector was generated using the AdEasy™ Adenoviral Vector System (Qbiogene, Irvine, CA, USA) according to the manufacturer’s instructions. Small interfering RNA targeting β-catenin (5′-GCGCAGCTCGTGTATACTA-3′) or negative control (5′-ATCTCTCAACGACATGCGTAA-3′) was designed and synthesized by Genepharma Company (Shanghai, China).
mRNA isolation and quantitative real-time PCR
Total RNA was extracted using the RNeasy Plus Mini Kit (74134, QIAGEN) according to the manufacturer’s instructions. Quantitative real-time PCR was performed by using an Applied Biosystems 7300 Real-time PCR System and a TaqMan Universal PCR Master Mix. Expression of the target genes was normalized to that of β-actin. The primer sequences were listed as follows: β-catenin (Forward: 5′-CCAACGACTACCACCAACTTT-3′, Reverse: 5′-GCCAGGTCTGGTTCATTGCT-3′); β-actin (Forward: 5′-CTCGACACCAGGGCGTTATG-3′, Reverse: 5′-CCACTCCATGCTCGATAGGAT-3′).
Western blot
Cells were lysed with ice-cold lysis buffer consisting of 50 mM Tris-HCl, 100 mM 2-mercaptoethanol, 2 % w/v SDS, and 10 % glycerol, supplemented with protease inhibitor cocktail (Beyotime). After incubation on ice for 30 min, the mixture was centrifuged at 10,000g for 10 min at 4 °C. Proteins in the supernatant were collected, quantified, separated by 10 % SDS PAGE, and transferred onto a PVDF membrane (Millipore, Bedford, MA, USA). Primary antibodies [anti-β-catenin (ab32572), anti-β actin (ab6276)], all from Abcam Company (Abcam, Cambridge, MA, USA)], were incubated overnight at 4 °C, and specific proteins were visualized by ECL Plus (Amersham Biosciences Inc, Buckinghamshire, UK).
Promoter construction, transfection, and luciferase reporter assays
Mutations were introduced in TCF binding sites using the QuikChange site-directed mutagenesis Kit (Stratagene, USA). Transfection was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s recommendations. The pRL-TK vector (Promega, USA) carrying the Renilla luciferase gene was used to normalize the transfection efficiency. Luciferase values were measured using the Dual-Luciferase Reporter Assay System (Promega).
BrdU incorporation
A cell proliferation enzyme-linked immunosorbent assay (BrdU kit; Beyotime) was used to analyze the incorporation of BrdU during DNA synthesis following the manufacturer’s protocols. Absorbance was measured at 450 nm in the Spectra Max 190 ELISA reader (Molecular Devices, Sunnyvale, CA).
Statistical analysis
All data are expressed as mean ± standard error (SE) values of four independent experiments. Significance was analyzed using two-tailed Student’s t test. P < 0.05 was considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
Activation of Wnt/β-catenin signaling suppresses protein level of FBXW7
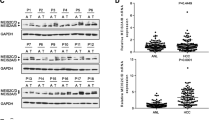
In order to determine the upstream regulators of FBXW7 in HCC tumorigenesis, HepG2 cells were treated with agonists of Wnt/β-catenin, NF-κB, and Stat3 signaling pathways (TNFα, IL-6, and Wnt3a). As a result, message RNA levels of FBXW7 were not affected by these cytokines (Fig. 1a). However, its protein contents were dramatically reduced upon Wnt3a treatment (Fig. 1b). Similar results were also detected in Hep3B cells (Fig. 1c, d). To rule out possible non-specific effects of Wnt3a, adenovirus-mediated overexpression of constitutively activated β-catenin (CA-β-catenin) was introduced into HepG2 and Hep3B cells. In agreement, CA-β-catenin significantly suppressed the protein levels of FBXW7 (Fig. 2a–d). Therefore, our results clearly demonstrate that activation of Wnt/β-catenin inhibits FBXW7 expression in HCC cells.
Wnt3a inhibits FBXW7 protein expression in HCC cells. a, b Relative mRNA (a) and representative protein (b) levels of FBXW7 in HepG2 cells treated with TNFα, IL-6, Wnt3a, or vehicle ctrl. c, d Relative mRNA (c) and representative protein (d) levels of FBXW7 in Hep3B cells treated with Wnt3a or vehicle ctrl. Ctrl control
Knockdown of β-catenin increases FBXW7 expression
To further show the regulation of FBXW7 by β-catenin, endogenous β-catenin was depleted by small interfering RNA (siRNA) in HCC cells. As expected, ablation of β-catenin increased FBXW7 protein levels in HepG2 and Hep3B cells (Fig. 3a–d), further showing a negative correlation between β-catenin and FBXW7.
Upregulation of miR‑770 by Wnt/β-catenin activation
Given that Wnt/β-catenin signaling regulates FBXW7 expression at the post-transcription/translation level, we speculate that microRNAs, a class of short and non-coding RNAs, might be involved in this process. Using the miRWalk algorithm based on seed recognition [20], several miRNAs were identified that potentially interact with the 3′-untranslated region (3′-UTR) of FBXW7 (data not shown). Notably, only miR-770 was increased in HepG2 cells treated with Wnt3a or infected with CA-β-catenin (Fig. 4a, b). Consistently, knockdown of endogenous β-catenin reduced miR-770 expression (Fig. 4c). Besides, a β-catenin/TCF binding site was also detected by luciferase reporter assays, in which the wild-type and mutant promoter region of miR-770 was cloned and inserted into the luciferase vectors (Fig. 4d). Together, these data indicate that miR-770 represents a downstream target of Wnt/β-catenin signaling.
Activation of β-catenin promotes miR-770 expression. a–c Relative expression levels of miR-770 in HepG2 cells treated with Wnt3a (a), adenovirus expressing constitutively activated β-catenin (b), or small interfering RNA targeting β-catenin (c). d Relative luciferase activity of miR-770 promoter in HepG2 cells
miR-770 inhibits FBXW7 expression by targeting its 3′-UTR
To further examine the role of miR-770, we first performed luciferase reporter assays in HepG2 cells using 3′-UTR of FBXW7. As shown in Fig. 5a, overexpression of miR-770 mimics led to a reduction of luciferase activity when the reporter construct contained the wild-type 3′-UTR (Fig. 5a). However, mutation of the miR-770 binding site abolished the suppressive effect of miR-770 mimics (Fig. 5a). In addition, our Western blot analysis showed that endogenous protein levels of FBXW7 were substantially downregulated or upregulated by miR-367 mimics or antisense, respectively (Fig. 5b, c). Moreover, depletion of miR-770 largely attenuated the inhibitory effects of Wnt/β-catenin signaling on the expression of FBXW7 (Fig. 5d).
miR-770 represses FBXW7 protein expression through targeting its 3′-UTR. a Relative luciferase activity of wild-type or mutant FBXW7 3′-UTR in HepG2 cells transfected with NC, miR-770 mimics, or miR-770 antisense. b, c Representative protein levels of FBXW7 in HepG2 cells transfected with NC, miR-770 mimics (b), or miR-770 antisense (c). d Representative protein levels of FBXW7 in HepG2 cells transfected with NC or miR-770 antisense. Cells were treated with Wnt3a or vehicle control for another 24 h
miR-770 regulates HCC cell proliferation
Previous studies have shown that FBXW7 inhibits cell proliferation and tumorigenesis through destabilizing several oncoproteins. Therefore, HepG2 and Hep3B cells were transfected with miR-770 mimics and antisense, followed by cell viability, proliferation, and growth assays. As expected, cell viability, proliferation abilities, and growth rates were enhanced and suppressed by miR-770 mimics and antisense, respectively (Fig. 6a–f). Moreover, overexpression of FBXW7 in HepG2 cells phenocopies the effects of miR-770 depletion on the HCC cell growth (Fig. 7a–d), further suggesting the proliferative roles of miR-770 rely on, at least in part, its repression of FBXW7.
Overexpression of FBXW7 inhibits HCC proliferation. a Representative protein levels of FBXW7 in HepG2 cells transfected with adenovirus expressing FBXW7 or EV. b–d Cell viability (b), proliferation abilities (c), and growth curves (d) in HepG2 cells transfected with adenovirus expressing FBXW7 or EV. EV empty vector
Discussion
In the present study, Wnt/β-catenin signaling was shown to repress FBXW7 protein expression in HCC cells. At the molecular level, miR-770 was found to be a transcriptional target of Wnt/β-catenin signaling. Therefore, the present study proposes a novel mechanism for the downregulation of FBXW7 in human cancers.
Although recent studies have discovered the targets of FBXW7 ubiquitin ligase pathway, how FBXW7 itself is regulated is largely unclear in human cancers. Emerging evidence has demonstrated that several transcription factors could regulate the expression of FBXW7, including p53, C/EBP-δ (CCAAT/enhancer-binding protein-δ), and Hes5 (hairy and enhancer-of-split homologues 5) [21–24]. For instance, Kimura et al. found that FBXW7 was induced in a p53-dependent manner in response to genotoxic stresses caused by UV irradiation and adriamycin treatment [21]. Subsequently, Mao et al. identified FBXW7 gene as a p53-dependent tumor suppressor gene in tumorigenesis [22]. Therefore, targeting the p53 signaling pathway may potentially influence FBXW7 expression in human cancers.
Interestingly, accumulated evidence has also shown that several miRNAs including miR-223, miR-25, and miR-92a could regulate FBXW7 expression in tumorigenesis [25–27]. Therefore, together with previous studies, our results suggest that restoring the expression of FBXW7 by suppressing these miRNAs might be useful to design novel therapeutic drugs to treat cancer patients. Interestingly, Ma et al. showed that genistein, a natural compound, could upregulate FBXW7 by reducing the expression of miR-223 [28]. As a result, genistein could inhibit cell growth and induce apoptosis in pancreatic cancer cells [28]. Thus, further understanding the regulatory pathways that control FBXW7 expression will be beneficial to develop anti-cancer effects in the future.
References
Skaar JR, Pagan JK, Pagano M. SCF ubiquitin ligase-targeted therapies. Nat Rev Drug Discov. 2014;13(12):889–903.
Skaar JR, Pagan JK, Pagano M. Mechanisms and function of substrate recruitment by F-box proteins. Nat Rev Mol Cell Biol. 2013;14(6):369–81.
Wei R, Liu X, Yu W, Yang T, Cai W, Liu J, et al. Deubiquitinases in cancer. Oncotarget. 2015;6(15):12872–89.
Liu J, Shaik S, Dai X, Wu Q, Zhou X, Wang Z, et al. Targeting the ubiquitin pathway for cancer treatment. Biochim Biophys Acta. 2015;1855(1):50–60.
Bochis OV, Irimie A, Pichler M, Berindan-Neagoe I. The role of Skp2 and its substrate CDKN1B (p27) in colorectal cancer. J Gastrointestin Liver Dis. 2015;24(2):225–34.
Kitagawa K, Kotake Y, Kitagawa M. Ubiquitin-mediated control of oncogene and tumor suppressor gene products. Cancer Sci. 2009;100(8):1374–81.
Wang L, Ye X, Liu Y, Wei W, Wang Z. Aberrant regulation of FBW7 in cancer. Oncotarget. 2014;5(8):2000–15.
Lau AW, Fukushima H, Wei W. The Fbw7 and betaTRCP E3 ubiquitin ligases and their roles in tumorigenesis. Front Biosci (Landmark Ed). 2012;17:2197–212.
Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321(5895):1499–502.
Yada M, Hatakeyama S, Kamura T, Nishiyama M, Tsunematsu R, Imaki H, et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23(10):2116–25.
Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW, et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science. 2001;294(5540):173–7.
Nateri AS, Riera-Sans L, Da Costa C, Behrens A. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science. 2004;303(5662):1374–8.
Wu RC, Feng Q, Lonard DM, O’Malley BW. SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell. 2007;129(6):1125–40.
Onoyama I, Suzuki A, Matsumoto A, Tomita K, Katagiri H, Oike Y, et al. Fbxw7 regulates lipid metabolism and cell fate decisions in the mouse liver. J Clin Invest. 2011;121(1):342–54.
Rajagopalan H, Lengauer C. hCDC4 and genetic instability in cancer. Cell Cycle. 2004;3(6):693–4.
Koh MS, Ittmann M, Kadmon D, Thompson TC, Leach FS. CDC4 gene expression as potential biomarker for targeted therapy in prostate cancer. Cancer Biol Ther. 2006;5(1):78–83.
Calcagno DQ, Freitas VM, Leal MF, de Souza CR, Demachki S, Montenegro R, et al. MYC, FBXW7 and TP53 copy number variation and expression in gastric cancer. BMC Gastroenterol. 2013;13:141.
Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26.
Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14(11):736–46.
Dweep H, Sticht C, Pandey P, Gretz N. miRWalk—database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform. 2011;44(5):839–47.
Kimura T, Gotoh M, Nakamura Y, Arakawa H. hCDC4b, a regulator of cyclin E, as a direct transcriptional target of p53. Cancer Sci. 2003;94(5):431–6.
Mao JH, Perez-Losada J, Wu D, Delrosario R, Tsunematsu R, Nakayama KI, et al. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature. 2004;432(7018):775–9.
Balamurugan K, Wang JM, Tsai HH, Sharan S, Anver M, Leighty R, et al. The tumour suppressor C/EBPδ inhibits FBXW7 expression and promotes mammary tumour metastasis. EMBO J. 2010;29(24):4106–17.
Sancho R, Blake SM, Tendeng C, Clurman BE, Lewis J, Behrens A. Fbw7 repression by hes5 creates a feedback loop that modulates Notch-mediated intestinal and neural stem cell fate decisions. PLoS Biol. 2013;11(6), e1001586.
Xu Y, Sengupta T, Kukreja L, Minella AC. MicroRNA-223 regulates cyclin E activity by modulating expression of F-box and WD-40 domain protein 7. J Biol Chem. 2010;285(45):34439–46.
Gong J, Cui Z, Li L, Ma Q, Wang Q, Gao Y, et al. MicroRNA-25 promotes gastric cancer proliferation, invasion, and migration by directly targeting F-box and WD-40 domain protein 7, FBXW7. Tumour Biol. 2015. [Epub ahead of print].
Zhou C, Shen L, Mao L, Wang B, Li Y, Yu H. miR-92a is upregulated in cervical cancer and promotes cell proliferation and invasion by targeting FBXW7. Biochem Biophys Res Commun. 2015;458(1):63–9.
Ma J, Cheng L, Liu H, Zhang J, Shi Y, Zeng F, et al. Genistein down-regulates miR-223 expression in pancreatic cancer cells. Curr Drug Targets. 2013;14(10):1150–6.
Acknowledgments
This work was supported by the National Natural Science Foundation, People’s Republic of China, Grant No. 31401185 (to Dr. Bin Liu) and No. 81402478 (to Dr. Zhi-Xiang Wu).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Additional information
Wen-Jie Wu and Jia Shi contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, WJ., Shi, J., Hu, G. et al. Wnt/β-catenin signaling inhibits FBXW7 expression by upregulation of microRNA-770 in hepatocellular carcinoma. Tumor Biol. 37, 6045–6051 (2016). https://doi.org/10.1007/s13277-015-4452-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4452-5