Abstract
To investigate the immunogenicity of Homo sapiens putative translation initiation factor (Sui1) in hepatocellular carcinoma (HCC), enzyme-linked immunosorbent assay (ELISA) and Western blot were utilized to assess autoantibody responses to Sui1 in sera from HCC patients and healthy individuals. Indirect immunofluorescence (IIF) assay with cancer cells and immunohistochemistry (IHC) study with tissue array slides were performed to examine Sui1 expression profile in cancer cells and tissues. The data confirmed that the frequency of autoantibody to Sui1 in sera of HCC patients was 15.5 % (16/103), which was remarkably higher than that in sera of liver cirrhosis (LC) patients (3.3 %, 1/30), chronic hepatitis (CH) patients (0 %, 0/29), and normal human serum (NHS) (0 %, 0/82) (p < 0.01). IHC study showed that the Sui1 expression in HCC tissues was 26.7 % (16/60). The expression of Sui1 had the trend of increasing along with the cancer grades but no statistical significance (p > 0.05). In immunodiagnosis of HCC, the sensitivity and specificity of the anti-Sui1 antibody were 15.5 and 99.3 %, respectively. If both anti-Sui1 and alpha fetal protein (AFP) were simultaneously utilized as detective markers, 66.7 % (30/45) of HCC patients could be correctly distinguished. The results suggested that anti-Sui1 could be utilized as a supplementary serological marker for the detection of HCC and Sui1 might be associated to HCC carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Our previous study has demonstrated that serum autoantibodies to tumor-associated antigens (TAAs) can be used as supplemental biomarkers of alpha fetal protein (AFP) in immunodiagnosis of hepatocellular carcinoma (HCC) [1]. But, the sensitivity of any of these single TAAs was very low (10–20 %) for HCC immunodiagnosis [2]. Thus, new TAAs that could be added to current TAAs as biomarkers to improve the early diagnosis of HCC are urgently needed. To identify tumor antigens, serological analysis of recombinant complementary DNA (cDNA) expression libraries (SEREX) were widely performed in different types of cancer. Autologous serum was used to screen the cDNA expression libraries coming from messenger RNA (mRNA) collected from cell lines or tumor tissues [3]. The advantage of this method is that intracellular proteins related to carcinogenesis can activate autoantibody responses, and thus, immunoscreen cDNA expression libraries with autoantibodies can isolate potential proteins related to carcinogenesis. Till now, more than 2000 antigens were defined by SEREX in different types of cancer, which accounted for one third of identified new antigens during the same period [1]. Unfortunately, most of these antigens defined by SEREX eventually have been proved not directly related to cancer [4]. To further confirm the immunogenicity of the defined antigen, our strategy is using serological screening in patients with cancer and non-neoplastic diseases and normal people in a large scale utilizing defined protein as a target antigen by ELISA and Western blot to assess whether it could be utilized as immunodiagnostic markers for cancer detection or potential cancer immunotherapeutic target [5]. Using this method, several new TAAs including Ra1A, p90/CIP2A, and p62/IMP2 were successfully identified in our previous studies [1, 4].
Homo sapiens putative translation initiation factor (Sui1), also called eukaryotic translation initiation factor 1 (eIF1), was known as a translation initiation factor, which was found in some eukaryotes including human, fungus, Saccharomyces cerevisiae (Baker’s yeast), and prokaryotes as well as archaea [6]. With the help of eIF2 and the initiator tRNA-Met, Sui1 could direct the ribosome to the translation beginning site [6]. Sui1 guarantees that there is beginning codon, AUG, at the outset of the protein which aides to stabilize the preinitiation complex. Sui1 is indispensable for discovering the right initiation site and eventually supports the development of the preinitiation complex [7]. In our previous study, we determined Sui1 cDNA with HepG2 cDNA expression library screening [8]. In this study, we subcloned Sui1 cDNA into an expression vector to obtain recombinant Sui1 protein. ELISA and Western blot were performed in sera from healthy individuals and patients with chronic hepatitis (CH), liver cirrhosis (LC), and HCC to evaluate autoantibody responses to Sui1. The Sui1 expression patterns in HCC tissues were further assessed by indirect immunofluorescence (IIF) examination with HepG2 cancer cells and IHC examination with commercially obtained HCC tissue array slides.
Materials and methods
Serum specimens and antibodies
All the serum specimens used in present study were selected from the serum bank of Cancer Autoimmunity Research Laboratory (CARL) in The University of Texas at El Paso (UTEP), which including 103 serum specimens from HCC patients, 29 from CH patients, 30 from LC patients, and 82 from normal human individuals. The criteria used to diagnose HCC were described in our previous study [9]. General information of 103 HCC patients is shown in Table 1. Among them, 25 (24.3 %) were female, and 78 (75.7 %) were male. Age range was 24–79 years (56.9 ± 13.9 years). Hepatitis B virus (HBV)-positive patients were 74 (71.8 %), HCV-positive patients were 11 (10.7 %), and both HBV- and HCV-positive patients were 5 (4.9 %). Patients with CH history were 66 (64.1 %), with LC history were 22 (21.4 %), and with neither CH nor LC history were 37 (35.9 %). According to the Chinese clinical guideline of liver cancer, clinical stage I patients were 31 (30.1 %), stage II patients were 17 (16.5 %), stage III patients were 32 (31.1 %), stage IV patients were 11 (10.7 %), respectively, and 12 (11.7 %) patients had no accessible information on clinical stages. Ninety-five (92.2 %) patients were confirmed by the histological testing. Of 103 HCC patients, AFP test results could be obtained in 45 patients. The AFP test kit used in this study was purchased from GenWay Biotech (San Diego, CA). The results showed that 57.8 % (26/45) serum specimens had abnormal AFP levels (>100 ng/ml) whereas 19 (42.2 %) had normal levels (<100 ng/ml).
All the 82 normal human serum (NHS) specimens in this study were collected from people with routine annual physical examinations, who had no clear evidence of cancer. To exclude patients with asymptomatic HCC and primary biliary cirrhosis, all the LC and CH patients were followed up at least 1 year and a half after the blood gathered. All the 103 HCC serum specimens were collected from patients just diagnosed with HCC, and all the patients had not received radiotherapy or chemotherapy. The name and ID number of all the patients and normal human were blinded to researchers in order to comply with the rules of human subjects’ studies. The polyclonal anti-Sui1 antibody used in this study was obtained from immunization of rabbit with Sui1 protein, which was commercially obtained from ProSci Incorporated, Poway, CA.
Expression and purification of Sui1 recombinant protein
Sui1 was identified by SEREX in our previous study, in which autoantibodies in serum from a HCC patient were utilized to immunoscreen an expression library of HepG2 cDNA for the distinguishing of TAAs related to HCC carcinogenesis [8]. In the following study, Sui1 cDNA was amplified by PCR from a human-expressed sequence tag (EST) clone (GenBank Accession number: #CX163967). To get purified recombinant Sui1 protein, the Sui1 full-length cDNA was subcloned into the pET28 expression vector which was designed to produce a fusion protein with N-terminal 6× histidine and T7 epitope tags. Utilizing nickel column chromatography, the recombinant Sui1 protein was further expressed in E. coli BL21 (DE3) cells and purified. The purified Sui1 recombinant proteins were further assessed by electrophoresis on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and distinguished by Western blot utilizing polyclonal anti-Sui1 antibody.
Enzyme-linked immunosorbent assay
The detailed ELISA protocol utilized in this study was described in our previous study [8]. In short, the final concentration of purified recombinant Sui1 protein in phosphate-buffered saline (PBS) was 0.5 μg/ml and was coated onto each well of a 96-well microtiter plate. Human serum specimens at 1:200 dilution, goat anti-human IgG-horseradish peroxidase (HRP) (Invitrogen, NY, USA) at 1:4000 dilution, and the substrate ABTS (Invitrogen, NY, USA) were utilized as the first antibody, the second antibody, and detecting reagents, respectively. The cutoff value to distinguish positive response was defined as the mean optical density (OD) of 82 NHS +3 SD (standard deviations).
Western blotting
Sixteen serum specimens from HCC patients and 1 serum specimen from LC patient that were positive in ELISA examination were further confirmed by Western blot analysis. A polyclonal anti-Sui1 antibody was used as a positive control. Western blot was operated essentially as the same protocol used in our previous study [9]. In short, denatured recombinant Sui1 protein was electrophoresed on 10 % SDS-PAGE gel and then transferred onto a nitrocellulose membrane. Human serum specimens at 1:200 dilution and the polyclonal anti-Sui1 antibody at 1:500 dilution, goat anti-human IgG-HRP, and goat anti-rabbit IgG-HRP (Invitrogen, NY, USA) at a 1:10,000 dilutions were applied as the first antibody and the secondary antibody, respectively. Immunoreactive bands were examined by the ECL kit (Thermo Scientific, Waltham, MA).
Absorption of serum antibodies by recombinant protein Sui1
All the positive serum specimens in ELISA examination at 1:200 dilutions were incubated with Sui1 recombinant protein at room temperature for 2 h (the concentration of Sui1 protein was 0.03 μg/μL). After being centrifuged at 10,000×g for 10 min, the supernatants of the mixtures were utilized for ELISA and IIF examination. Each serum sample was tested no less than three times.
Indirect immunofluorescence assay
IIF was performed on methanol- and acetone-fixed HepG2 cell slides to identify autoantibody response to Sui1 in HCC serum specimens as same protocol used in our previous study [9]. In short, HepG2 cells were grown on coverslips and pretreated with methanol and acetone. Polyclonal anti-Sui1 antibody 25 μL at 1:2000 dilutions, normal human serum 1:200 dilution, and PBS were utilized as positive, negative, and blank controls, respectively. HCC serum specimens at 1:200 dilution which showed positive reactivity to Sui1 in ELISA and the same serum specimens which had been preabsorbed with recombinant Sui1 protein were added to HepG2 cell-coated wells on the slide. The secondary antibodies used in the present study were the FITC-conjugated goat anti-human IgG (Caltag Laboratories, San Francisco, CA, USA) and anti-rabbit IgG Fab2 (Alexa Fluor 488) diluted 1:80. Confocal laser scanning microscope (LSMS PASCAL, Carl Zeiss GmbH, Jena, Germany) was used to evaluate the slides at ×400 magnification.
Immunohistochemistry with tissue array slides
The superfrost plus tissue slides including 60 paraffin-embedded HCC specimens with pathological diagnosis and pathological grades were commercially obtained (Cybrdi, Bethesda, MD, USA). IHC was operated essentially as the same protocol used in our previous study [4]. In short, the concentration of polyclonal anti-Sui1 antibody (1:2000 dilution) utilized for immunostaining the tissue specimens was according to the company’s suggestion and dictated by the pretest. The remaining experiments were according to the company’s suggestion (Vector Laboratories, Burlingame, CA).
Statistical analysis
Utilizing the χ 2 test with Yate’s correction to compare the frequencies of antibodies positive to Sui1 of different groups was done, and two significant levels were utilized (0.01 and 0.05). The method for calculation of sensitivity and specificity was described in our previous study [2].
Results
Prevalence of autoantibody to Sui1 in hepatocellular carcinoma
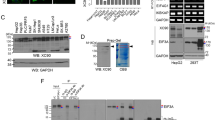
As shown in Table 2, the frequency of autoantibody to Sui1 in sera of HCC patients was 15.5 % (16/103), which was remarkably higher than that in sera of LC patients (3.3 %, 1/30), CH patients (0 %, 0/29), and NHS (0 %, 0/82) (p < 0.01). As a marker to immunodiagnosis HCC, the sensitivity and specificity of anti-Sui1 antibody were 15.5 and 99.3 %, respectively. Moreover, 12 out of 16 of the positive HCC sera were observed OD values more than twofold over the cutoff (mean + 3SD of NHS), which indicated that in some HCC patients, the antibody responses to the Sui1 antigen were very strong and not simply somewhat hoisted. Further research of Western blotting analysis confirmed the ELISA result. As shown in Fig. 1, four representative HCC sera also showed strong positive in Western blotting detection, which have positive response to Sui1 in ELISA. Moreover, antibody absorption study also confirmed the specificity of Sui1 in ELISA (data were not demonstrated).
Western blot detection showing representative HCC sera which are reactive to Sui1 recombinant protein. Positive control utilized the polyclonal anti-Sui1 antibody. Lanes 1–4: four representative HCC sera had strong reactivity with Sui1 recombinant protein, which were also positive in ELISA. Lane 5: negative control utilized a normal human serum
In this study, 45 HCC sera were available to study the relationship of AFP and Sui1 response. Twenty-six of 45 (57.8 %) HCC sera had abnormally elevated levels of AFP (>100 ng/ml), and 8 of 45 (17.8 %) were autoantibody positive to Sui1. As a marker in HCC diagnosis, the sensitivity of AFP was similar to the previous study [10]. Of interest was that 4 of 19 (21.1 %) HCC sera with normal AFP level (<100 ng/ml) were autoantibody positive to Sui1. If anti-Sui1 and AFP were utilized as detective markers together, 30 of 45 (66.7 %) HCC patients could be distinguished correctly.
Immunofluorescence staining pattern of Sui1 in HepG2 cells
Utilizing HepG2 cells as substrate, HCC sera with positive anti-Sui1 in ELISA were analyzed by IIF to investigate the intracellular localization of Sui1 protein and further confirm the specificity of autoantibody positive to Sui1 in HCC sera. As demonstrated in Fig. 2, a representative anti-Sui1-positive HCC serum had a cytoplasmic staining pattern with more intense staining in the perinuclear areas, which was similar to that shown by the polyclonal Sui1 antibody in the fluorescent staining pattern and cellular localization. The fluorescent signal was significantly decreased when the same HCC serum was preabsorbed with recombinant Sui1 protein.
Representative immunofluorescence staining pattern of anti-Sui1 antibody-positive HCC serum. a Blank control utilized phosphate-buffered saline (PBS). b Negative control utilized normal human serum (NHS). c Positive control utilized polyclonal anti-Sui1 antibody which demonstrated a perinuclear immunofluorescence staining pattern. d A representative anti-Sui1 antibody-positive HCC serum showed similar perinuclear immunofluorescence staining pattern. e The same HCC serum utilized in d was preabsorbed with recombinant Sui1 protein and subsequently utilized for immunofluorescence assay. The fluorescent signal sign was basically
Expression of Sui1 in hepatocellular carcinoma tissues by immunohistochemistry with tissue array
Tissue array slides including 60 HCC tissue specimens were commercially obtained. Histopathology of all specimens was confirmed by the hematoxylin and eosin (H&E) staining analysis. The results indicated that the expression of Sui1 in HCC tissues was 26.7 % (16/60). In HCC grades I, II, and III, the expressions of Sui1 were 18.2 % (2/11), 23.5 % (8/34), and 40.0 % (6/15), respectively. With the increase of cancer grades, there is an increasing tendency of Sui1 expression but no statistical significance (p > 0.05). Figure 3 demonstrates the representative Sui1-negative and Sui1-positive immunostaining and their homologous H&E staining pattern in HCC tissues.
Immunohistochemistry (IHC) detection of Sui1 expression in HCC tissues. Hematoxylin and eosin (H&E) staining was performed to demonstrate the histopathology of the tissue specimens. Tissue array slide was stained with polyclonal anti-Sui1 antibody at a 1:2000 dilution. a A representative Sui1 negatively stained HCC tissue (×100). b A representative Sui1 positively stained HCC tissue (×100). A positively stained area (box) was enlarged (×400)
Discussion
The vast majority of liver cancer is HCC, whose mortality rate is very high. The incidence rate ranks sixth, but the mortality rate ranks second among cancers in the world [11]. Although early resection of subclinical smaller HCC resulted in significant improvement in long-term survival [12], for most patients with advanced or metastatic HCC, there is still no effective therapy in spite of new therapeutic strategies that have been continuously developed and applied to clinical treatment in recent decades [2]. Early diagnosis can significantly improve the efficacy and cure rate of HCC, but more than 80 % of the patients are advanced or metastatic HCC at the time of diagnosis because the symptoms of early HCC are not typical. Lack of diagnostic methods that enable the early detection attributed to the high fatality rate of HCC. AFP was demonstrated by Tatarinov in patients with HCC in 1968 and widely used for clinical screening HCC till now [12]. However, AFP can be positive in nonseminomatous germ cell tumor patients, germ cell tumor patients, metastatic liver cancer patients, ataxia telangiectasia patients, and pregnant women; on the other hand, AFP does not rise at all in about 40 % of HCC patients [1]. To find better biomarker of HCC, close attention was paid to TAAs by researchers in recent years. As we all know, the human body’s normal proteins do not have immunogenicity for the reason of self-tolerance. During the process of carcinogenesis, that mean from normal cells to tumor cells, aberrant proteins of oncogenes, tumor suppressor genes, and other mutated genes act as TAAs, which trigger immune responses in the host. The novel autoantibody response to TAAs can be detected in sera which can be used for cancer immunodiagnosis as biomarkers [3, 9, 13, 14]. In our previous study, more than ten proteins have been identified and confirmed as TAAs in HCC, whose autoantibodies have been studied in sera of HCC patients [8]. Unfortunately, the specificity and sensitivity of autoantibody to any single TAA are not high enough to become routinely useful in detection of HCC [1]. To overcome this problem, multiple carefully selected known TAAs were combined as a TAA array to obtain the required sensitivity and specificity of HCC diagnosis. One of our previous studies showed that using eight TAAs (full-length recombinant proteins of p62, Imp1, Koc, cyclin B1, p53, c-Myc, p16, and survivin) as a panel to distinguish HCC, the sensitivity and specificity of eight TAAs for detection of HCC were 59.8 and 87.8 %, respectively [2]. The following study showed that using 14 TAAs (full-length recombinant proteins of p62, p90, Koc, p53, CAPERα, p16, survivin, IMP1, RalA, NPM1, cyclin B1, 14-3-3 ζ, c-Myc, and MDM2) as a panel to distinguish HCC, the sensitivity and specificity of 14 TAAs for detection of HCC were 69.7 and 83.0 %, respectively [15]. With more TAAs were added into mini-array, a higher sensitivity and specificity of detection HCC can be acquired, but it is still not good enough till now and too many TAAs make it impossible to be applied in clinical diagnosis. Therefore, more studies should be focused on distinguishing and confirming new TAAs that could be added to current TAAs as biomarkers to further improve the early diagnosis of HCC.
Sui1 or eIF1 is involved in eukaryotic translation initiation with the help of at least 11 other initiation factors (eIFs) [6]. Sui1 protein is composed of 108 amino acids. The protein domain has a seven-bladed beta-propeller structure, and it additionally contains a C-terminal alpha helix [16]. Sui1 can promote recruitment of Met-tRNAi to assemble the 43S preinitiation complex (PIC) with the help of eIF2 and eIF5. Enhanced by eIF3, eIF4B, and eIF4F, the 43S PIC then binds the mRNA’s 5′end and scans its 5′ untranslated region (UTR) for an AUG codon for discovering the right initiation site [17]. Little studies were focused on the relationship between Sui1 and cancer, and the findings also have contradictions. Chin et al. reported that reduced expression of Sui1 blocks the translation of proteins that mediate growth suppression and/or enhances the translation of growth stimulatory molecules [18]. Van Agthoven et al. reported that in breast tumors, high Sui1 mRNA levels can cause the tamoxifen treatment resistance in the vitro cell model, while low Sui1 mRNA levels are related to poor clinical benefit of tamoxifen treatment and shorter progression-free survival [19]. Van Agthoven et al. reported in another article that eIF1 (Sui1) mRNA levels were significantly associated with clinical benefit and progression-free survival when the relation of eIF1 (Sui1) with tamoxifen treatment in patients with advanced breast cancer was evaluated [20]. Lian et al. have reported that Sui1 was a tumor suppressor in HCC carcinogenesis and was associated with apoptosis, but the aberrant Sui1 protein promotes unregulated cell growth and assists the HCC carcinogenesis [21]. According to our study, the frequency of autoantibody to Sui1 in sera of HCC patients was 15.5 % (16/103), which was remarkably higher than that in sera of LC patients (3.3 %, 1/30), CH patients (0 %, 0/29), and NHS (0 %, 0/82) (p < 0.01). The following research of western blotting analysis confirmed the ELISA results. As a marker in immunodiagnosis of HCC, the sensitivity and specificity of anti-Sui1 were 15.5 and 99.3 %, respectively. The results proposed that Sui1 may be related to HCC carcinogenesis, and anti-Sui1 could be utilized as a supplementary serological marker of HCC diagnosis. Data from our study also demonstrated that if both anti-Sui1 and AFP were simultaneously utilized as detective markers, 66.7 % (30/45) of HCC patients could be correctly distinguished, suggesting that anti-Sui1 might be a supplementary serological marker for the detection of HCC.
While little was known about Sui1, whether or not Sui1 contributing to cell proliferation and transformation is still uncertain, but Bilanges et al. reported that in the signal transduction pathways, translation initiation was an important downstream target. Mutated oncogenes and tumor suppressors could deregulate it in cancer [22]. Other reports indicated that lymphomagenesis was promoted by aberrant control of protein translation [23, 24], which made it possible to target the translational machinery [25]. Over the past few decades, due to applications of new therapeutic methods in HCC, for example, surgery, transarterial chemoembolization, radiofrequency therapy, Argon-Helium cryoablation, ethanol injection therapy, liver transplantation, and targeted therapy, the treatment of HCC improved rapidly. But, because of the precursor liver diseases that limit the use of therapeutic methods, rapid intra-liver spread, and out-liver metastasis, the higher fatality rate of HCC is still a big problem. Therefore, a new approach, such as immunotherapy, is urgently needed. The perfect candidates for immunotherapeutic targets must have restricted tumor expression of tumor antigens that could be distinguished by the human immune system and resulted in tumor rejection [4]. Our study indicates that there was a significantly increased autoimmune response to Sui1 in HCC sera compared to NHS. Whether or not Sui1 expression is specifically in HCC and can contribute ideal targets for HCC immunotherapy still needs to be evaluated. Studies demonstrated that an IHC approach, staining tissue specimens with monoclonal or polyclonal antibody, could provide such a method to analyze the expression profile of the targeted tumor antigen [26]. In the current study, the IHC results indicated that the expression of Sui1 in HCC tissues was 26.7 % (16/60). In HCC grades I, II, and III, the expressions of Sui1 were 18.2 % (2/11), 23.5 % (8/34), and 40.0 % (6/15), respectively. With the increase of cancer grades, there is an increasing tendency of Sui1 expression but no statistical significance (p > 0.05). Due to the higher immunogenecity of Sui1 in HCC sera combined with higher expression of Sui1 in HCC tissues, we propose that Sui1 is related to HCC progression and maybe a potential target for HCC immunotherapy.
In conclusion, anti-Sui1 can be utilized as a supplementary serological marker for the detection of HCC and may be especially useful in patients with normal AFP levels. Based on our previous studies, the sensitivity in using a single TAA (for example, Sui1 in this study) is usually not very high (10–20 %) [2]. Further study should be focused on whether Sui1 could be added to current TAAs as biomarkers to improve the early diagnosis of HCC, and extensive investigation on the Sui1 expression profile in HCC may give more knowledge about the underlying mechanism of how this antigen is related to the HCC carcinogenesis.
References
Zhang JY, Tan EM. Autoantibodies to tumor-associated antigens as diagnostic biomarkers in hepatocellular carcinoma and other solid tumors. Expert Rev Mol Diagn. 2010;10(3):321–8.
Zhang JY, Megliorino R, Peng XX, Tan EM, Chen Y, Chan EK. Antibody detection using tumor-associated antigen mini-array in immunodiagnosing human hepatocellular carcinoma. J Hepatol. 2007;46(1):107–14.
Soo Hoo L, Zhang JY, Chan EK. Cloning and characterization of a novel 90 kDa ‘companion’ auto-antigen of p62 overexpressed in cancer. Oncogene. 2002;21(32):5006–15.
Wang K, Chen Y, Liu S, Qiu S, Gao S, Huang X, et al. Immunogenicity of Ra1A and its tissue-specific expression in hepatocellular carcinoma. Int J Immunopathol Pharmacol. 2009;22(3):459–67.
Fernandez Madrid F, Tang N, Alansari H, Karvonen RL, Tomkiel JE. Improved approach to identify cancer-associated autoantigens. Autoimmun Rev. 2005;4(4):230–5.
Hinnebusch AG. The scanning mechanism of eukaryotic translation initiation. Annu Rev Biochem. 2014;83:779–812.
Martin-Marcos P, Cheung YN, Hinnebusch AG. Functional elements in initiation factors 1, 1A, and 2beta discriminate against poor AUG context and non-AUG start codons. Mol Cell Biol. 2011;31(23):4814–31.
Chen Y, Zhou Y, Qiu S, Wang K, Liu S, Peng XX, et al. Autoantibodies to tumor-associated antigens combined with abnormal alpha-fetoprotein enhance immunodiagnosis of hepatocellular carcinoma. Cancer Lett. 2010;289(1):32–9.
Zhang JY, Chan EK, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189(7):1101–10.
Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S108–12.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Tang ZY. Hepatocellular carcinoma surgery—review of the past and prospects for the 21st century. J Surg Oncol. 2005;91(2):95–6.
Looi KS, Nakayasu ES, Diaz RA, Tan EM, Almeida IC, Zhang JY. Using proteomic approach to identify tumor-associated antigens as markers in hepatocellular carcinoma. J Proteome Res. 2008;7(9):4004–12.
Zhang J, Wang K, Zhang J, Liu SS, Dai L, Zhang JY. Using proteomic approach to identify tumor-associated proteins as biomarkers in human esophageal squamous cell carcinoma. J Proteome Res. 2011;10(6):2863–72.
Dai L, Ren P, Liu M, Imai H, Tan EM, Zhang JY. Using immunomic approach to enhance tumor-associated autoantibody detection in diagnosis of hepatocellular carcinoma. Clin Immunol. 2014;152(1–2):127–39.
Herrmannova A, Daujotyte D, Yang J-C, Cuchalova L, Gorrec F, Wagner S, et al. Structural analysis of an eIF3 subcomplex reveals conserved interactions required for a stable and proper translation pre-initiation complex assembly. Nucleic Acids Res. 2012;40(5):2294–311.
Hussain T, Llacer JL, Fernandez IS, Munoz A, Martin-Marcos P, Savva CG, et al. Structural changes enable start codon recognition by the eukaryotic translation initiation complex. Cell. 2014;159(3):597–607.
Chin LS, Singh SK, Wang Q, Murray SF. Identification of okadaic-acid-induced genes by mRNA differential display in glioma cells. J Biomed Sci. 2000;7(2):152–9.
van Agthoven T, Sieuwerts AM, Meijer-van Gelder ME, Look MP, Smid M, Veldscholte J, et al. Relevance of breast cancer antiestrogen resistance genes in human breast cancer progression and tamoxifen resistance. J Clin Oncol. 2009;27(4):542–9.
van Agthoven T, Sieuwerts AM, Meijer D, Meijer-van Gelder ME, van Agthoven TL, Sarwari R, et al. Selective recruitment of breast cancer anti-estrogen resistance genes and relevance for breast cancer progression and tamoxifen therapy response. Endocr Relat Cancer. 2010;17(1):215–30.
Lian Z, Pan J, Liu J, Zhang S, Zhu M, Arbuthnot P, et al. The translation initiation factor, hu-Sui1 may be a target of hepatitis B X antigen in hepatocarcinogenesis. Oncogene. 1999;18(9):1677–87.
Bilanges B, Stokoe D. Mechanisms of translational deregulation in human tumors and therapeutic intervention strategies. Oncogene. 2007;26(41):5973–90.
Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, et al. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10(5):484–6.
Ruggero D, Pandolfi PP. Does the ribosome translate cancer? Nat Rev Cancer. 2003;3(3):179–92.
Mazan-Mamczarz K, Hagner P, Dai B, Corl S, Liu Z, Gartenhaus RB. Targeted suppression of MCT-1 attenuates the malignant phenotype through a translational mechanism. Leuk Res. 2009;33(3):474–82.
Sullivan CAW, Chung GG. Biomarker validation: in situ analysis of protein expression using semiquantitative immunohistochemistry-based techniques. Clin Colorectal Cancer. 2008;7(3):172–7.
Acknowledgments
This work was supported by a grant (SC1CA166016) from the National Institutes of Health (NIH). We also thank the Border Biological Research Center (BBRC) Core Facilities at The University of Texas at El Paso (UTEP) for their support, which were funded by a NIH grant (5G12MD007592).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, JW., Li, Y., Yue, LX. et al. Autoantibody response to Sui1 and its tissue-specific expression in hepatocellular carcinoma. Tumor Biol. 37, 2547–2553 (2016). https://doi.org/10.1007/s13277-015-4074-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4074-y