Abstract
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a lncRNA playing oncogenic role in several cancers, including cervical cancer. However, its role in radiosensitivity of cervical cancer is not yet well understood. This study explored the role of MALAT1 in radiosensitivity of high-risk human papillomavirus (HR-HPV)-positive cervical cancer and whether there is a ceRNA mechanism which participated in its regulation over radiosensitivity. Based on tissue samples from 50 cervical cancer cases and 25 healthy controls, we found MALAT1 expression was significantly higher in radioresistant than in radiosensitive cancer cases. In addition, MALAT1 and miR-145 expression inversely changed in response to irradiation in HR-HPV+ cervical cancer cells. By using clonogenic assay and flow cytometry analysis of cell cycle distribution and apoptosis, we found CaSki and Hela cells with knockdown of MALAT1 had significantly lower colony formation, higher ratio of G2/M phase block and higher ratio of cell apoptosis. By performing RNA-binding protein immunoprecipitation (RIP) assay and RNA pull-down assay, we confirmed that miR-145 and MALAT1 were in the same Ago2 complex and there was a reciprocal repression between them. Then, we explored the function of MALAT1-miR-145 in radiosensitivity of cervical cancers cells and demonstrated that si-MALAT1 and miR-145 had some level of synergic effect in reducing cancer cell colony formation, cell cycle regulation, and inducing apoptosis. These findings provide an important clue about microRNA-lncRNA interaction in the mechanism of radioresistance of cervical cancer.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the third most common female malignancy [1]. The most common treatments are surgery or a combination of chemotherapy and radiotherapy [2]. Radiotherapy is usually prescribed for patients who are not tolerant for a major operation or the cancer has spread into the tissues or lymph nodes surrounding the cervix [2]. However, the underlying mechanism of radioresistance is not clear, making identification and treatment of radioresistant cervical cancer an unsolved problem [3].
Infection of high-risk human papillomavirus (HR-HPV), such as HPV-16 and HPV-18, is known as the major risk factor of cervical cancer [4]. These viral infection causes aberrant expression and constitutive activation of oncogenic molecule, leading to cervical carcinogenesis [5]. Long non-coding RNAs (lncRNAs) are non-protein coding transcripts longer than 200 nucleotides [6]. It is increasingly understood that lncRNAs are important molecules involved not only in normal development but also in tumorigenesis [7]. In fact, there are emerging evidence showing that lncRNAs are involved in pathological development of cervical cancer [8]. For example, MEG3 (maternally expressed 3) is a lncRNA playing tumor suppressor role in several cancers by inducing cell cycle arrest and apoptosis [9–12]. Reduced MEG3 expression is associated with increased cervical cancer cell proliferation [12]. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a lncRNA dramatically upregulated due to HPV infection in cervical cancer [13]. MALAT1 is capable of promoting cervical cancer cell proliferation partially through decreasing cell cycle regulation molecules cyclinD1, cyclinE, and CDK6 in CaSki cells, thereby suppressing cell cycle transition [13]. In addition, one recent study found the MALAT1-miR-124-RBG2 axis is involved in growth and invasion of HR-HPV-positive cervical cancer cells [14]. However, its role in regulating radiosensitivity of cervical cancer has not yet been studied.
MiR-145 is a tumor suppressive miRNA usually downregulated in cervical cancer. Its tumor suppressive role is partially achieved through regulating cell cycle-related proteins, such as CDK6 [15], CDK2 [16], and Cyclin D1 [17]. But how it is downregulated in cervical cancer is not clear. In the current study, we firstly reported that MALAT1 can modulate radioresistance of cervical cancer and its upregulation directly decreases miR-145 expression.
Methods
Human specimens
This study was approved by the ethics committee of the First Hospital of Qinhuangdao. Fifty HR-HPV+ cervical patients histologically diagnosed as IIB and IIIA stage were recruited from the hospital from 2013 to 2014. The patients had never received previous chemo- or radiotherapy. All of them were given standard, pelvic radiation therapy, concurrent cisplatin-containing chemotherapy, and brachytherapy as recommended by the 2013 NCCN Clinical Practice Guideline in Oncology for Cervical Cancer [18]. Radiosensitivity was assessed 6 months after radiotherapy through histological examination of colposcopically directed biopsy of residual tumor tissues. Informed consent was obtained from all of the participants.
Cell culture
HPV-18-positive human cervical cancer cell lines HeLa and HPV-16-positive CaSki cell lines were grown in RPMI-1640 medium (Gibco-BRL, USA) supplemented with 10 % fetal bovine serum (HyClone, USA). All cells were cultured in a humidified atmosphere containing 5 % CO2 at 37 °C.
Transfection reagent and cell transfection
Two candidates of si-MALAT1 were chemically synthesized by RiboBio (Shanghai, China). Preliminary experiments were performed in both CaSki and Hela cells to identify the most efficient siRNA sequences. Briefly, CaSki and Hela cells were transfected with 100-nM MALAT1 siRNA and the negative control using Lipofectamine 2000 (Invitrogen). The most efficient siRNA sequences: sense 5′-GAGGUGUAAAGGGAUUUAUTT-3′, anti-sense 5′-AUAAAUCCCUUUACACCUCTT-3′ were used for the following studies. To overexpress miR-145 in CaSki and Hela cells, the cells were transfected with 100-nM miR-145 mimics (Ribobio) and the negative control using Lipofectamine 2000 (Invitrogen). MALAT1 knockdown or miR-145 overexpression was verified using qRT-PCR 24 h after transfection.
QRT-PCR analysis of MALAT1 and miR-145 expression
Total RNA in tumor and normal tissues was extracted using the RNeasy kit (Qiagen) according to the manufacturer’s instructions. cDNA was reversely transcribed using the PrimeScript® RT reagent kit (TaKaRa). MALAT1 expression level was quantified using the following primers: forwards: 5′-AGGCGTTGTGCGTAGAGGA-3′; reverse: 5′-GGATTTTTACCAACCACTCGC-3′ and SYBR® Premix DimerEraser kit (TaKaRa) in an ABI Prism 7500 (Applied Biosystems). GAPDH was used as the endogenous control gene. QRT-PCR analysis of miR-145 was performed using TaqMan MicroRNA Assay Kit (Applied Biosystems) with U6 snRNA served as the endogenous control.
To quantify pri-miR-145 and pre-miR-145 expression, qRT-PCR was performed using the following primers: pri-miR-145, forward: 5′-TGGATTTGCCTCCTTCCCA-3′ and reverse: 5′-TTGAACCCTCATCCTGTGAGCC-3′; pre-miR-145, forward: 5′-GTCCAGTTTTCCCAGGAATC-3′ and reverse: 5′-AGAACAGTATTTCCAGGAAT-3′, and SYBR® Premix DimerEraser kit (TaKaRa) in an ABI Prism 7500 (Applied Biosystems). β-actin served as the endogenous gene. Each test was carried out in triple replication, and the expression change was quantified using 2−ΔΔCT method.
Irradiation
Cells of different conditions (MALAT1 knockdown, miR-145 overexpression, or combined MALAT1 knockdown and miR-145 overexpression) were irradiated in 25-T flasks. Cells were exposed to 0-, 2-, 4-, 6-, and 8-Gy treatment using a linear accelerator. For time-course experiments, cells were sham irradiated or received 2 Gy and were collected every 2 h within 24 h post-irradiation. MALAT1 and miR-145 expression was quantified using qRT-PCR. For clonogenic assay, 1 × 102 to 1 × 105 cells (according to the radiation dose) were plated in six-well plates immediately after irradiation and were further cultured for another 12 days. The colonies were fixed with glutaraldehyde (6.0 % v/v) and stained with crystal violet (0.5 % w/v). Colonies (>50 cells) were counted using a stereomicroscope. The surviving fraction was defined as the number of colonies/number of plated cells. All the procedures were repeated in triplicate.
Flow cytometry analysis of cell cycle distribution and apoptosis
Twenty-four hours after 0- or 10-Gy irradiation treatment, cells were harvested and fixed with 70 % ethanol. To assess the distribution of cells in different phases, cells were stained with 20 μg/mL PI (Sigma) and 100 μg/mL RNase A in PBS for 15 min at room temperature. To identify cells with active caspase-3, cells were stained using Fluorescein Active Caspase-3 Staining Kit (Abcam, ab65613) according to the manufacturers’ instructions. DNA content and cells with active caspase-3 were analyzed on FACSCalibur (BD Bioscience). Data acquisition was performed using CellQuest 3.2 software (BD Bioscience). Each test was carried out in triplicate.
RNA-binding protein immunoprecipitation assay
RIP assay was performed using the Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) following the manufacturer’s protocol. Briefly, Hela cells were lysed by RIP lysis buffer. Then, 100-μL cell extract was incubated with RIP buffer containing magnetic beads conjugated with human anti-Ago2 antibody (Millipore) or negative control (normal mouse IgG, Millipore). After the antibody was recovered by protein A/G beads, qRT-PCR was performed to detect MALAT1 and miR-145 level in the precipitates.
RNA pull-down assay
To explore whether MALAT1 is associated with the RISC complex, we performed RNA pull-down assay using synthesized MALAT1 as a probe and to detect Ago2 from the pellet using western blot and miR-145 using qRT-PCR. Briefly, the DNA fragment with the whole MALAT1 sequence was PCR amplified using a T7-containing primer and then cloned into pCR8 (Invitrogen). Besides, lncRNA loc285194 [19] was also cloned and used as the positive control in RNA pull-down assay. The resultant plasmid DNA was linearized using restriction enzyme NotI. Biotin-labeled RNAs were reversely transcribed using Biotin RNA Labeling Mix (Roche Diagnostics, USA) and T7 RNA polymerase (Roche, Switzerland). The products were treated with RNase-free DNase I (Roche, USA) and purified with the RNeasy Mini Kit (Qiagen, USA) and were further used for the extraction of RNA for qRT-PCR or for western blot analysis according to method described in the previous study [20].
Statistical analysis
Data analysis was performed using SPSS 17.0. Data were represented as mean ± standard deviation (SD) based on at least three repeats. Group difference was assessed using Student’s t test. P < 0.05 was considered as statistically significant. *, **, and *** denote significance at 0.05, 0.01, and 0.001 level, respectively.
Results
MALAT1 and miR-145 expression inversely changes in response to irradiation in HR-HPV+ cervical cancer
The basic characteristics of the 21 radiosensitive and 29 radioresistant cases were summarized in Table 1. MALAT1 expression in radioresistant and radiosensitive cancer cases was quantified. QRT-PCR analysis showed that MALAT1 expression was substantially higher in the radioresistant than that in the radiosensitive cases (Fig. 1a). Then, we measured miR-145 expression in the cancer cases and analyzed its correlation with MALAT1. The results showed that MALAT1 expression was negatively correlated to that of miR-145 (P < 0.0001) (Fig. 1b). Due to inverse expression trend of MALAT1 and miR-145, we decided to explore their expression changes in response to ionizing radiation. In both CaSki and Hela cells, miR-145 was significantly downregulated since the 4th hour after irradiation exposure (Fig. 1c, d). In contrast, MALAT1 showed an inverse tendency. Its expression was significantly upregulated since the 2nd hour after irradiation exposure (Fig. 1e, f). These results suggest that MALAT1 and miR-145 expression inversely changes in response to irradiation in HR-HPV+ cervical cancer.
MALAT1 and miR-145 expression inversely changes in response to irradiation in HR-HPV+ cervical cancer. a qRT-PCR analysis of MALAT1 expression in radiosensitive (n = 21) and radioresistant (n = 29) tumor tissues. b Linear regression analysis of MALAT1 and miR-145 expression in cervical cancer tissues (n = 50). c–f qRT-PCR analysis of miR-145 (c, d) and MALAT1 (e, f) expression in CaSki (c, e) and Hela (d, f) cells at indicated time points after 2 Gy exposure. *P < 0.05; **P < 0.01, ***P < 0.001
MALAT1 increases radioresistance of HR-HPV+ cancer cells
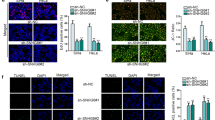
To explore the role of MALAT1 in radiosensitivity of the cancer cells, we firstly knocked down endogenous MALAT1 in HR-HPV-16+ CaSki and HR-HPV-18+ Hela cells using MALAT1 siRNA (Fig. 2a). Knockdown of MALAT1 significantly reduced colony formation rate of both CaSki (Fig. 2b) and Hela (Fig. 2c) cells under irradiation. By assessing cell cycle distribution of the cells, we found si-MALAT1 significantly decreased the ratio of cells in G1 phase but increased the cells in G2/M phase in both CaSki (Fig. 2d1–3) and Hela (Fig. 2e1–3) cells, no matter with or without irradiation exposure. Cells are most sensitive to radiation in G2 phase before mitosis and are least sensitive in the mid- to late S and early G1 phases. Therefore, the function of MALAT1 in radiosensitivity might be related its role in cell cycle regulation. Besides, we also observed that si-MALAT1 increased apoptosis of both CaSki (Fig. 2f1 and 3) and Hela (Fig. 2g1 and 3) and enhanced apoptosis under irradiation (Fig. 2f2 and 3, g2 and 3). These results suggest that MALAT1 can modulate radiosensitivity of HR-HPV+ cancer cells through regulating cell cycle progression.
MALAT1 increases radioresistance of HR-HPV+ cancer cells (a) qRT-PCR analysis of MALAT1 expression in CaSki and Hela cells transfected with si-MALAT1. (b and c) The clonogenic survival curves were compared in CaSki (b) and Hela (c) cells with or without MALAT1 knockdown. Forty-eight hours after transfection, cells were irradiated by the indicated dose and a clonogenic assay was performed 12 days after irradiation. (d1, d2, e1, and e2) Representative images of flow cytometry analysis of cell cycle phases of CaSki (d1 and d2) and Hela (e1 and e2) cells transfected with si-MALAT1 without irradiation (d1 and e1) or 24 h exposed to 10 Gy (d2 and e2). Cells were stained with propidium iodide for flow cytometry analysis. (d3 and e3) Quantitative analysis of CaSki (d3) and Hela (e3) cells in different cell phases shown in d1, d2 and e1, e2. (f1, f2, g1, and g2) Representative images of flow cytometry analysis of CaSki (f1 and f2) and Hela (g1 and g2) cells with active caspase-3 transfected with si-MALAT1without irradiation (f1 and g1) or 24 h exposed to 10 Gy (f2 and g2). (f3 and g3) Quantitative analysis of CaSki (f3) and Hela (g3) cells with active caspase-3 showed in f1, f2, and g1, g2. *P < 0.05; **P < 0.01, ***P < 0.001
MALAT1 directly interacts with miR-145 and decreases its expression
We then explored how miR-145 expression is regulated in HR-HPV+ cervical cancer. Through searching in online bioinformatics database (starBase, v2.0, http://starbase.sysu.edu.cn/), we observed that miR-145 has a putative binding site with MALAT1 (Fig. 3a). To further verify the mutual influence between miR-145 and MALAT1, CaSki and Hela cells were firstly transfected with miR-145 mimics (Fig. 3b). MiR-145 overexpression significantly decreased MALAT1 level (Fig. 3c). On the contrary, knockdown of MALAT1 significantly increased miR-145 level (Fig. 3d), suggesting a reciprocal interaction between MALAT1 and miR-145. To further explore the underlying mechanism of negative regulation of MALAT1 over miR-145, we examined the expression of pri-miR-145 and pre-miR-145 in response to MALAT1 knockdown. Knockdown of MALAT1 had almost no effect on the expression of pri-miR-145 and pre-miR-145 in both CaSki and Hela cells (Fig. 3e, f). Therefore, MALAT1-mediated negative regulation of miR-145 is probably through a posttranscriptional mechanism.
MALAT1 interacts with miR-145 and reduces its expression. a Predicted binding sites between MALAT1 and miR-145. b qRT-PCR analysis of miR-145 levels in CaSki and Hela cells transfected with 100 nM miR-145 mimics. c qRT-PCR analysis of MALAT1 levels in CaSki and Hela cells 48 h after transfection with miR-145 mimics. d qRT-PCR analysis of miR-145 expression in CaSki and Hela cells 48 h after transfection with si-MALAT1. e, f qRT-PCR analysis of pri-miR-145 and pre-miR-145 expression in CaSki (e) and Hela (f) cells 48 h after transfection with 100nM si-MALAT1. g Association of MALAT1 and miR-145 with Ago2. Cellular lysates from Hela cells were used for RIP assay with Ago2 antibody. Ago2 was detected using IP-western (up panel). MALAT1 and miR-145 level were detected using qRT-PCR (down panel). h Identification of MALAT1 and miR-145 in the same RISC complex by RNA pull-down assay. Pull-down of Ago2 by biotin-labeled MALAT1 or loc285194 RNA probe was detected by western blotting (up panel). MiR-145 in the RNA-precipitated samples was detected using qRT-PCR (down panel). *P < 0.05; **P < 0.01, ***P < 0.001
Since miRNAs exert gene silencing through RISC [21], we hypothesized that MALAT1 and miR-145 might be in the same RISC complex. Therefore, we performed a RIP assay using antibody against Ago2, a core component of RISC complex necessary for siRNA or miRNA-mediated gene silencing [22]. The results showed that Ago2 antibody precipitated the Ago2 protein from the cell lysates (Fig. 3g, up panel). QRT-PCR analysis detected higher miR-145 and MALAT1 in the Ago2 pellet than that in the input control (Fig. 3g, down panel). To further confirm that miR-145 and MALAT1 are in the same Ago2 complex, we synthesized biotin-labeled MALAT1 RNA probe and mixed with the cellular extract. After pull-down experiment with streptavidin beads, we detected Ago2 by western blot analysis, suggesting MALAT1 directly interacted with Ago2 (Fig. 3h, up panel). In addition, we also detected a significant amount of miRNA-145 in the MALAT1 pulled down pellet, while the amount of miR-145 in the loc285194 pulled down pellet was only slightly increased compared with control (Fig. 3h, down panel). These results suggest that MALAT1 is a bona fide miR-145-targeting lncRNA.
MALAT-miR-145 axis can regulate radiosensitivity of HR-HPV+ cancer cells
Considering the verified regulation of MALAT1 over miR-145 expression, we further explored whether this axis is involved in radiosensitivity of HR-HPV+ cancer cells. MiR-145 overexpression can significantly decrease colony formation rate under irradiation in both CaSki (Fig. 4a) and Hela (Fig. 4b) cells. Simultaneous MALAT1 knockdown and miR-145 overexpression had a more evident effect than miR-145 alone. We then examined the role of MALAT1-miR-145 axis in cell cycle change and apoptosis under irradiation treatment. Enforced miR-145 expression enhanced G2/M phase block (Fig. 4c, d) and cell apoptosis (Fig. 4e, f). In addition, simultaneous MALAT1 knockdown and miR-145 overexpression had a stronger effect than miR-145 alone. These results suggest that MALAT1-miR-145 axis can regulate radiosensitivity of HR-HPV+ cancer cells.
MALAT1-miR-145 axis can regulate radiosensitivity of HR-HPV+ cancer cells. Analysis of the effect of the MALAT1-miR-145 axis on clonogenic survival fraction, cell cycle phases, and cell apoptosis. CaSki and Hela cells were transfected with miR-145 mimics (100 nM) or combined si-MALAT1 (100 nM) and miR-145 mimics (100 nM) for overexpression. a, b The clonogenic survival fraction were compared in CaSki (a) and Hela (b) cells under 8-Gy treatment. c, d Quantitative analysis of flow cytometry analysis of cell cycle phases of CaSki (c) and Hela (d) cells 24 h after 10-Gy treatment. e, f Quantitative analysis of CaSki (c) and Hela (d) cells with active caspase-3 24 h after 10-Gy treatment. *P < 0.05; **P < 0.01, ***P < 0.001
Discussion
Increased MALAT1 is associated with tumorigenesis of several types of cancer, such as lung cancer [23], colorectal cancer [24], gastric cancer [25], and cervical cancer [13]. One recent study showed that MALAT1 expression in the cervical cancer tissues is remarkably higher than that in non-neoplastic tissues [13], suggesting MALAT1 is involved in tumorigenesis of cervical cancer. Another study reported that downregulation of MALAT1 can compromise the cytoplasmic translocation of hnRNP C in the G2/M phase and results in G2/M arrest [26]. In gastric cancer, MALAT1 can recruit SF2/ASF, promoting cell proliferation through facilitating cell cycle progression [25]. Silencing of MALAT1 in esophageal squamous cell carcinoma cells can significantly suppress the cell proliferation via G2/M cell cycle arrest [27]. In cervical cancer, suppressing endogenous MALAT1 can decrease expression of cell cycle regulation molecules cyclinD1, cyclinE, and CDK6 in CaSki cells, thereby suppressing cell cycle transition [13]. Therefore, MALAT1 might play a vital role in cell cycle regulation. In fact, dysregulated cell cycle control is closely related to radiosensitivity of cancer cells. In the current study, we observed significantly increased MALAT1 expression in radioresistant cervical cancer tissues than that in radiosensitive cases. In addition, we also found MALAT1 and miR-145 expression inversely changed in response to irradiation in HR-HPV+ cervical cancer cells.
Considering the crucial role of MALAT1 in cell cycle control and its upregulation in radioresistant cases, we decided to further explore its roles in radiosensitivity of cervical cancer. In an in vitro study, CaSki and Hela cells with knockdown of MALAT1 had significantly lower capability of colony formation, higher ratio of G2/M phase block, and higher ratio of cell apoptosis. Under irradiation treatment, si-MALAT1 further enhanced these effects. These results suggest that MALAT1 can modulate cell proliferation and apoptosis of cervical cancer cells and also modulate the effect of radiotherapy.
MiR-145 plays a tumor suppressor role in several types of cancer, such as gastric cancer [17], hepatic cancer [28], breast cancer [29], and non-small cell lung cancer [30]. In these cancers, the tumor suppressive role of miR-145 is partially achieved through regulating cell cycle-related proteins, such as CDK6 [15], CDK2 [31], and Cyclin D1 [17]. Therefore, miR-145 is an important miRNA involved in cell cycle regulation, which means it may participate in regulation of radiosensitivity. In fact, one recent study found that overexpression of miR-145 or knockdown of DNMT3b sensitizes prostate cancer cells to X-ray radiation [32]. In HPV-positive cervical cancer cells, induction of E6 proteins significantly reduces p53 and miR-145 expression, leading to reduced chemotherapy-induced apoptosis [33]. However, the mechanism involved in its downregulation in cervical cancer is not clearly demonstrated.
Recently, the concept of competing endogenous RNAs (ceRNAs) has been introduced to explain a novel regulatory mechanism of RNA. This concept indicates that RNAs can cross-talk with each other through competing shared for miRNAs and thus impose another level of posttranscriptional regulation [34]. HR-HPV infection significantly increases MALAT1 expression [13]. Through searching in online bioinformatics databases, we found MALAT1 has a putative binding site with miR-145. Since regression analysis of MALAT1 and miR-145 expression in the cancer cases showed a negative correlation, we explored whether ceRNA is involved in lowered miR-145 expression in cervical cancer. By using RIP and RNA pull-down assay, we confirmed that MALAT1 and miR-145 are in the RISC complex and there is a reciprocal interaction between them. We further explored the function of MALAT1-miR-145 axis in radiosensitivity of cervical cancer cells and demonstrated that si-MALAT1 and overexpressing miR-145 has some level of synergic effect in reducing cancer cell colony formation, cell cycle regulation, and inducing apoptosis.
Conclusion
In conclusion, this study showed that the MALAT1-miR-145 axis is involved in radioresistance of cervical cancer and there is a reciprocal repression between MALAT1 and miR-145. This provides an important clue about lncRNA-miRNA interaction in the mechanisms of radioresistance of cervical cancer.
References
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a Cancer Journal for Clinicians. 2014;64:9–29.
Rogers L, Siu SS, Luesley D, Bryant A, Dickinson HO. Radiotherapy and chemoradiation after surgery for early cervical cancer. The Cochrane Database of Systematic Reviews. 2012;5, CD007583.
Powell ME. Modern radiotherapy and cervical cancer. International Journal of Gynecological Cancer : Official Journal of the International Gynecological Cancer Society. 2010;20:S49–51.
Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53-dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697–705.
Lando M, Holden M, Bergersen LC, Svendsrud DH, Stokke T, Sundfor K, et al. Gene dosage, expression, and ontology analysis identifies driver genes in the carcinogenesis and chemoradioresistance of cervical cancer. PLoS Genetics. 2009;5, e1000719.
Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Molecular Cancer. 2011;10:38.
Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discovery. 2011;1:391–407.
Gibb EA, Becker-Santos DD, Enfield KS, Guillaud M, Niekerk D, Matisic JP, et al. Aberrant expression of long noncoding RNAs in cervical intraepithelial neoplasia. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society. 2012;22:1557–63.
Yin DD, Liu ZJ, Zhang E, Kong R, Zhang ZH, Guo RH. Decreased expression of long noncoding RNA MEG3 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Tumour Biology: the Journal of the International Society for Oncodevelopmental Biology and Medicine. 2015;36:4851–9.
Sun M, Xia R, Jin F, Xu T, Liu Z, De W, et al. Downregulated long noncoding RNA MEG3 is associated with poor prognosis and promotes cell proliferation in gastric cancer. Tumour Biology: the Journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:1065–73.
Ying L, Huang Y, Chen H, Wang Y, Xia L, Chen Y, et al. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Molecular BioSystems. 2013;9:407–11.
Qin R, Chen Z, Ding Y, Hao J, Hu J, Guo F. Long non-coding RNA MEG3 inhibits the proliferation of cervical carcinoma cells through the induction of cell cycle arrest and apoptosis. Neoplasma. 2013;60:486–92.
Jiang Y, Li Y, Fang S, Jiang B, Qin C, Xie P, et al. The role of MALAT1 correlates with HPV in cervical cancer. Oncology Letters. 2014;7:2135–41.
Liu S, Song L, Zeng S, Zhang L. MALAT1-miR-124-RBG2 axis is involved in growth and invasion of HR-HPV-positive cervical cancer cells. Tumour Biology: the Journal of the International Society for Oncodevelopmental Biology and Medicine. 2015.
Zhang J, Wang L, Li B, Huo M, Mu M, Liu J, et al. miR-145 downregulates the expression of cyclin-dependent kinase 6 in human cervical carcinoma cells. Experimental and Therapeutic Medicine. 2014;8:591–4.
Chang S, Gao L, Yang Y, Tong D, Guo B, Liu L, et al. miR-145 mediates the antiproliferative and gene regulatory effects of vitamin D3 by directly targeting E2F3 in gastric cancer cells. Oncotarget. 2015;6:7675–85.
Qiu T, Zhou X, Wang J, Du Y, Xu J, Huang Z, et al. MiR-145, miR-133a and miR-133b inhibit proliferation, migration, invasion and cell cycle progression via targeting transcription factor Sp1 in gastric cancer. FEBS Letters. 2014;588:1168–77.
Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Chan J, et al. National Comprehensive Cancer Network: cervical cancer. Journal of the National Comprehensive Cancer Network: JNCCN. 2013;11:320–43.
Liu Q, Huang J, Zhou N, Zhang Z, Zhang A, Lu Z, et al. LncRNA loc285194 is a p53-regulated tumor suppressor. Nucleic Acids Research. 2013;41:4976–87.
Zhang A, Zhou N, Huang J, Liu Q, Fukuda K, Ma D, et al. The human long non-coding RNA-RoR is a p53 repressor in response to DNA damage. Cell Research. 2013;23:340–50.
Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–40.
Karginov FV, Conaco C, Xuan Z, Schmidt BH, Parker JS, Mandel G, et al. A biochemical approach to identifying microRNA targets. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19291–6.
Gutschner T, Hammerle M, Eissmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Research. 2013;73:1180–9.
Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han Z, et al. Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. British Journal of Cancer. 2014;111:736–48.
Wang J, Su L, Chen X, Li P, Cai Q, Yu B, et al. MALAT1 promotes cell proliferation in gastric cancer by recruiting SF2/ASF. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2014;68:557–64.
Yang F, Yi F, Han X, Du Q, Liang Z. MALAT-1 interacts with hnRNP C in cell cycle regulation. FEBS Letters. 2013;587:3175–81.
Wang X, Li M, Wang Z, Han S, Tang X, Ge Y, et al. Silencing of long noncoding RNA MALAT1 by miR-101 and miR-217 inhibits proliferation, migration, and invasion of esophageal squamous cell carcinoma cells. The Journal of Biological Chemistry. 2015;290:3925–35.
Bandopadhyay M, Banerjee A, Sarkar N, Panigrahi R, Datta S, Pal A, et al. Tumor suppressor micro RNA miR-145 and onco micro RNAs miR-21 and miR-222 expressions are differentially modulated by hepatitis B virus X protein in malignant hepatocytes. BMC Cancer. 2014;14:721.
Eades G, Wolfson B, Zhang Y, Li Q, Yao Y, Zhou Q. LincRNA-RoR and miR-145 regulate invasion in triple-negative breast cancer via targeting ARF6. Molecular cancer research: MCR. 2015;13:330–8.
Shen H, Shen J, Wang L, Shi Z, Wang M, Jiang BH, et al. Low miR-145 expression level is associated with poor pathological differentiation and poor prognosis in non-small cell lung cancer. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie. 2015;69:301–5.
Chang, S., Gao, L., Yang, Y., Tong, D., Guo, B., Liu, L., Li, Z., Song, T., and Huang C. 2015. miR-145 mediates the antiproliferative and gene regulatory effects of vitamin D3 by directly targeting E2F3 in gastric cancer cells. Oncotarget.
Xue G, Ren Z, Chen Y, Zhu J, Du Y, Pan D, et al. A feedback regulation between miR-145 and DNA methyltransferase 3b in prostate cancer cell and their responses to irradiation. Cancer Letters. 2015;361:121–7.
Shi M, Du L, Liu D, Qian L, Hu M, Yu M, et al. Glucocorticoid regulation of a novel HPV-E6-p53-miR-145 pathway modulates invasion and therapy resistance of cervical cancer cells. The Journal of Pathology. 2012;228:148–57.
Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, H., He, Y., Lin, L. et al. Long non-coding RNA MALAT1 modulates radiosensitivity of HR-HPV+ cervical cancer via sponging miR-145. Tumor Biol. 37, 1683–1691 (2016). https://doi.org/10.1007/s13277-015-3946-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3946-5