Abstract
High expression of cell division cycle 20 homolog (CDC20), a key component of the spindle assembly checkpoint (SAC), has been reported in various malignancies and plays a vital role in tumorigenesis and progression. The goal of this study was to evaluate the utility of CDC20 immunostaining in a wide range of malignant tumors. CDC20 immunohistochemistry was evaluated in normal tissues and compared to the most frequently occurring malignant tumors in these tissues (bladder, breast, cervical, colonic, endometrial, gastric, head and neck, liver, lung, ovarian, pancreatic, prostatic, renal, thyroid carcinomas, and testicular seminoma). Normal/non-neoplastic tissues showed positive CDC20 expression in 19.44 % of all examined cases. CDC20 staining was negative in normal and non-neoplastic tissues from the bladder, cervix, liver, stomach, and thyroid. From the all malignant tumors examined 55.7 % showed high CDC20 expression while low expression was found in 44.3 %. High expression of CDC20 was associated with high tumor grade in the bladder (p = 0.027), cervical (p = 0.032), colonic (p = 0.026), endometrial (p = 0.016), gastric (p = 0.033), liver (p = 0.028), ovarian (p = 0.044), prostatic (p = 0.040), and renal (p = 0.048) carcinomas. There was a significant correlation between high CDC20 expression and advanced tumor stage in carcinoma of the breast, colon, endometrium, and prostate (p = 0.021, p = 0.040, p = 0.047, p = 0.031, respectively). CDC20 expression may be useful as a biomarker of tumor prognosis and as a therapeutic target of human cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carcinogenesis is a multistep process with several steps of which genetic instability, especially chromosomal instability (CIN), plays an essential role [1, 2].
It has been evidenced that CIN often leads to a cellular phenotype with aberrant chromosome number (aneuploidy), which is a hallmark of cancer [3, 4].
An increasing aneuploidy is the mean event of disturbance in the spindle assembly checkpoint (SAC) which is a surveillance mechanism that plays a crucial role in chromosomal segregation during mitosis [5, 6].
The mitotic metaphase-to-anaphase checkpoint is activated when a mis-segregation of the sister chromatid has occurred. This activation temporarily arrests the cell cycle until all of the kinetochores are attached to the bipolar spindle and equal allocation of chromosomes is ascertained thus accurate participation of chromosomes from each mother cell to its two daughter cells is achieved during mitosis [7, 8].
The function of mitotic spindle assembly checkpoint is controlled by several genes including BUB1, BUBR1, BUB3, MAD1, MAD2, MAD3, and cell division cycle 20 homolog (CDC20) [7, 9]. Although the exact mechanism of how these genes work is not fully recognized, it is generally accepted that these genes must be present for spindle checkpoint activation to occur [5, 6]. These genes are responsible for precise chromosome segregation during mitosis [10]. A growing body of evidence that BUB1, BUBR1, and BUB3 gene mutations were involved in many malignant tumors, albeit at a very low frequency [11–13]. Decreased expression of MAD1, MAD2, BUB1, and BUBR1 has been identified in several human cancers [14–17].
Despite an established data on the relation between the BUB-MAD gene expression and the defects in the SAC, the role of another important SAC protein, CDC20, in tumor aneuploidy and carcinogenesis is not completely understood. Upon attachment of all of the kinetochores to their respective spindle poles, CDC20 activates the anaphase-promoting complex (APC/C), an E3 ubiquitin ligase, that ubiquitinates certain substrates for sequential degradation through the ubiquitin proteasome pathway to initiate anaphase [6, 18].
APC/C targets degradation of securin and cyclin B1 when activated by CDC20. The cycle of activation of the APC–CDC20 complex and degradation of securin and cyclin B1 marks the anaphase onset in normally regulated cell division [19, 20], Diagram 1. CDC20 is usually kept sequestered during prophase and metaphase by MAD2 and BUBR1/BUB3 in the form of a mitotic checkpoint complex (MCC) [21, 22]. This diffusible MCC serves as a wait-anaphase signal from the kinetochores and prevents premature anaphase progression. Thus, an abnormality in the cellular CDC20 level or in its function may deregulate APC activation and promote premature anaphase, which often results in aneuploidy in both daughter cells [5, 16].
Function of APC/CCDC20 during the somatic cell cycle. In early mitosis, Cdk1 phosphorylates the APC/C core, promoting its binding to CDC20. APC/CCDC20 is blocked by the spindle checkpoint and inactivation of the spindle checkpoint allows activation of APC/CCDC20, which promotes the degradation of securin and cyclin B. This in turn leads to the anaphase onset. APC/CCDC20-dependent degradation of cyclin B binding to cyclin B reduces the Cdk1 activity in late anaphase resulting in facilitation of mitotic exit and cytokinesis
High CDC20 expression has been reported in several human cancers and its expression has been linked to poor prognosis in pancreatic [23], lung [24], bladder [25], colon [26], and oral squamous cell carcinomas [27].
Given the growing attention to this cell cycle regulatory protein, we investigated the role of CDC20, the activator of the APC/C complex, in common malignant tumors which have a high lifetime risk of causing cancer death among men and women in Egypt. We also evaluated the correlation between CDC20 expression and the grade and stage of these examined malignant tumors.
Materials and methods
Case selection
This retrospective study consisted of a total of 655 samples including 180 samples of normal/ non-neoplastic tissues and 475 samples from malignant tumors. Tissues included in the study were obtained from the bladder, breast, cervix, colon, endometrium, head and neck, kidney, liver, lung, ovary, pancreas, prostate, stomach, testis, and thyroid. The cases were collected from Department of Pathology, Minia University Hospital as well as Minia Oncology Center, Minia, Egypt during the period between January 2010 and October 2014. Data were extracted from the pathology reports and medical records. Only cases with available adequate tumor tissue were considered eligible. Patients did not receive neoadjuvant therapy. The specimens were fixed in 10 % buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin for histological assessment. All cases were reviewed by two pathologists (NMR Abd El-Maqsoud and MF Gayyed) for pathological parameters, including histologic grade and TNM stage. Tumor grade was based upon the criteria of the World Health Organization classification for each tumor. Tumor staging was performed according to the guidelines by the TNM staging system for each tumor. This work was covered by the approval of the ethics committee of both Minia University and Minia Oncology Center.
Immunohistochemistry
Immunohistochemical staining for CDC20 was performed on 4-μm unstained sections. Tissue sections were deparaffinized and dehydrated. This was followed by antigen retrieval with preheated 1 mM EDTA buffer (pH 8.0) in a microwave for 10–20 min. To block the endogenous peroxidase activity, slides were incubated with 0.3 % H2O2/methanol for 10–15 min. Slides were stained with monoclonal antibodies to CDC20 (1:50; clone E-7; Santa-Cruz Biotechnology, CA) at room temperature for 1 h. After incubation with secondary antibody at room temperature for 30 min, immunostaining was done using 3,30-diaminobenzidine (DAB) and counterstained with hematoxylin. Nuclear and/or cytoplasmic staining for CDC20 was considered positive. As a negative control, immunostaining was carried out without the primary antibody. We used normal tonsils as positive control for CDC20 (germinal center B cells).
Assessment of immunohistochemical staining
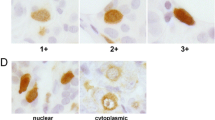
The immunostaining expression was evaluated by the two pathologist authors irrespective to the clinical data of the patients. The scoring system was adjusted from previously published studies [26]. The percent of positive cells was scored as 0 = ≤10 %, 1 = >10 to ≤25 %, 2 = >25 to ≤50 %, 3 = >50 to ≤75 %, and 4 = >75 %. The intensity of staining was scored as 0 = negative, 1 = weak, 2 = moderate, and 3 = strong. The two scores were then multiplied to calculate the final score. The cutoff points for CDC20 immunostaining were the median of CDC20 expression in each normal/non-neoplastic tissue. CDC20 expression was considered negative/low and high expression according to these cutoff points.
Statistical analysis
All statistical analyses were carried out using the SPSS 16.0 statistical software package (SPSS Inc., Chicago, IL). The relationship between CDC20 expression and tumor grade and stage was analyzed by chi-squared test. The p value of equal to or less than 0.05 was considered statistically significant.
Results
Results of immunohistochemical staining patterns for CDC20 were summarized in Tables 1 and 2 and Graph 1.
CDC20 immunohistochemical expression in normal and non-neoplastic tissues
Normal/non-neoplastic tissues showed either negative or weak nuclear and/or cytoplasmic CDC20 expression and their expressions were shown in Fig. 1. Positive CDC20 expression was found in 35/180 (19.44 %) of all examined normal/non-neoplastic tissues. We found positive CDC20 expression in normal/non-neoplastic breast 4/15 (26.7 %), colonic 5/15 (33.3 %), endometrial 7/15 (46.7 %), head and neck 2/10(20 %), renal 2/10(20 %), lung 3/10 (30 %), ovarian 4/10 (40 %), pancreatic 1/5 (20 %), prostatic 4/15 (26.7 %), and testicular 3/10 (30 %) tissues. CDC20 staining was not observed in normal/non-neoplastic tissues from the bladder, cervix, liver, stomach, and thyroid.
Representative immunohistochemistry for CDC20 expression in normal / non-neoplastic tissue samples (a-o). Representative examples of negative CDC20 expression in normal / non-neoplastic tissues of the a bladder, c cervix, h liver, m stomach, and o thyroid. High expression of CDC20 was shown in normal / non-neoplastic tissues of the b breast, d colon, e endometrium, f head and neck, g kidney, i lung, j ovary, k pancreas, l prostate, and n testis. (DAB chromogen, hematoxylin counterstain. X400)
CDC20 immunohistochemical expression in malignant tumors
CDC20 expression was detected in nuclei and/or cytoplasm of tumor cells as shown in Fig. 2. High CDC20 expression was detected in 265 cases (55.7 %) and low expression in 210 cases (44.3 %). As regarding CDC20 expression in bladder carcinoma, we found positive CDC20 expression in 24/40 cases (60 %). CDC20 expression in transitional cell carcinoma (TCC) was 14/25 cases (56 %) while the expression was 10/15 cases (66.7 %) in squamous cell carcinoma (SCC) which was slightly higher than in TCC but this difference did not reach a significant level (p = 0.505). In breast carcinoma, positive CDC20 expression was found in 24/40 (60 %) of all examined cases. Positive CDC20 expression in IDC and in ILC was 63 % and 50 %, respectively. The expression was higher in invasive duct carcinoma (IDC) than in invasive lobular carcinoma (ILC) but without a statistically significant difference (p = 0.351). In cervical SCC, 10/25 of cases (40 %) showed high CDC20 immunostaining. In colonic adenocarcinoma, high CDC20 expression was found in 22/40 of cases (55 %) compared to 18/40 of cases (45 %) showing low expression. In endometrial adenocarcinoma, we found low CDC20 immunostaining in 9/30 of cases (30 %), while the high CDC20 immunostaining was found in 21/30 of cases (70 %). In head and neck SCC, CDC20 expression was low in 14/25 of cases (56 %) and high in 11/25 of cases (44 %). In renal cell carcinoma, low CDC20 expression was found in 16/30 of cases (53.3 %) and high expression was found in 14/30 of cases (46.7 %). In HCC, the high CDC20 expression was detected in 23/40 of cases (57.5 %). Regarding lung carcinoma, positive CDC20 expression was 46.7 % of all cases. Positive CDC20 expression in ADC was 33.3 % which was lower than SCC (60 %) and the difference was also not statistically significant (p = 0.136). In ovarian carcinoma, CDC20 overexpression was found in 72.5 % of cases. CDC20 expression in mucinous carcinoma was higher than in serous carcinoma, 80 % and 68 %, respectively (p = 0.329). High CDC20 expression was found in 6/15 of cases (40 %) of pancreatic adenocarcinoma. In prostatic adenocarcinoma, CDC20 expression was high in 21/40 of cases (52.5 %). In gastric adenocarcinoma, 16/30 of cases (53.3 %) showed high CDC20 immunostaining. In seminoma, high CDC20 immunostaining was found in 13/20 of cases (65 %). In thyroid carcinoma, high CDC20 expression was noted in 43.3 % of all examined cases. Positive CDC20 expression in papillary and follicular thyroid carcinoma was 45 and 40 %, respectively, with no statistically difference level (p = 0.794).
CDC20 immunohistochemical expression in multiple malignant tumor tissues (a-o). Representative examples of high CDC20 expression in a TCC of the bladder, b IDC of the breast, c SCC of the cervix, d adenocarcinoma of the colon, e adenocarcinoma of the endometrium, f SCC of the head and neck, g RCC of the kidney, h non-small cell carcinoma of the lung, i HCC, j serous cystadenocarcinoma of the ovary, k duct carcinoma of the pancreas, l adenocarcinoma of the prostate, m adenocarcinoma of the stomach, n seminoma of the testis, and o papillary carcinoma of the thyroid. (DAB chromogen, hematoxylin counterstain. X400)
For beast carcinoma, we found that ER, PR, and HER2 expressions were 52.5, 50, and 42.5 %, respectively. An inverse significant association was found between CDC20 and ER (p = 0.020) and PR (p = 0.010) expressions while no significant association was seen between CDC20 and HER2 expressions (p = 0.601).
As shown in Table 3, in all tumors examined, we found that most of the high-grade tumors showed high CDC20 immunostaining, whereas most of the low-grade tumors showed low CDC20 expression. A statistical analysis revealed significant positive correlation between CDC20 expression and tumor grade in carcinomas of the bladder (p = 0.027), cervix (p = 0.032), colon (p = 0.026), endometrium (p = 0.016), kidney (p = 0.048), liver (p = 0.028), ovary (p = 0.044), prostate (p = 0.040), and stomach (p = 0.033) as shown in Graph 2.
Regarding tumor stage, we found higher positive CDC20 expression with advanced tumor stage. However, there was a significant correlation between high CDC20 expression and advanced tumor stage in carcinomas of the breast (p = 0.021), colon (p = 0.040), endometrium (p = 0.047), and prostate (p = 0.031) as shown in Graph 3.
Discussion
Aneuploidy is the hallmark of carcinogenesis. Normal cells are protected by the presence of SAC which maintains genomic stability through precise chromosomal segregation. SAC prevents the activation of APC/C ubiquitin ligase by sequestration of CDC20 into a complex of mitotic checkpoint complex (MCC). SAC performs its work through the generation of a diffusible wait-anaphase signal that inhibits the APC/C in the cytoplasm. APC/C is activated once all chromosomes attach in the metaphase. MCC can inhibit CDC20 that activated APC/C [28].
Human CDC20, a homolog of Saccharomyces cerevisiae cell division cycle 20 protein, is considered one of the major regularity components of the cell cycle. In mitosis, CDC20 activates APC/C which in role enables the onset of anaphase only after being certain that the centromeres of all sister chromatids are lined up in the metaphase plate, assembly attached to spindle poles and no one of the kinetochores left unattached [29]. In human malignancy, CDC20 overexpression has been associated with CIN and thus an aneuploidization [30].
Interestingly, it has been suggested that CDC20 possesses other functions independently of the APC/C. This was assumed by Clarke et al. who found that in budding yeasts, the accumulation of CDC20 was decreased in S phase via Mec1p and Rad53p, thus avoiding premature entry into mitosis during the S phase [31].
Resistance of the malignant cells to the antiproliferative therapy may be related to lack of apoptosis [32]. One of the apoptosis inhibitor mechanisms is the premature exit out of the mitotic arrest [33]. CDC20 knockout has been shown to block mitotic exit thus suggesting its role in decreasing apoptosis resistance in malignant cells [34]. CDC20 DNA damage caused an arrest in G2/M phase of cell cycle and subsequently decreased cell growth [35]. Regarding the previously mentioned roles of CDC20 in the cell cycle regulation, CDC20 is thought to be a potential therapeutic target in human cancers.
CDC20 immunohistochemical expression in the normal/non-neoplastic tissues
Studying the CDC20 expression in normal/non-neoplastic tissues was limited. We found that positive CDC20 expression was found in 19.44 % of the all examined normal/non-neoplastic tissues. Positive CDC20 expression was observed in the breast, colon, endometrium, head and neck, kidney, lung, ovary, pancreas, prostate, and testis. Previous studies reported positive CDC20 expression in the normal/non-neoplastic epithelial cells of the colon, stomach, head and neck, and pancreas (23, 26, 27, 36), while others reported that CDC20 expression was negative in normal/non-neoplastic epithelial cells of the bladder, breast, cervix, liver, and lung [24, 25, 37–39]. Our comprehensive study corroborated and expanded these observations as we found negative CDC20 expression in normal/non-neoplastic tissues from the bladder, cervix, liver, stomach, and thyroid.
CDC20 immunohistochemical expression in malignant tumors
CDC20 expression was detected in the nuclei and/or cytoplasm of the tumor cells as shown in previous studies [23, 25, 26, 36, 38, 39]. CDC20 overexpression was detected in 55.7 % while low CDC20 expression was found in 44.3 % of all examined malignant tumors.
As regarding CDC20 expression in bladder carcinoma, we found positive CDC20 expression in 60 % of the all studied cases. Positive CDC20 expression was slightly higher in SSC (66.7 %) than in TCC (56 %) but this difference did not reach a significant level. Choi et al. [25] reported that CDC20 overexpression was observed in 59 % of TCC cases. Previous studies were done on the expression of CDC20 in urothelial carcinoma but no one was done on its expression in SCC of the bladder.
In our study, breast carcinoma showed positive CDC20 expression in 60 % of cases. Positive CDC20 expression was higher in IDC (63 %) than LC (50 %) but without a statistically significant difference. Kidokoro et al. [35] found that CDC20 was overexpressed in 60 % of the breast cancer tissues while Karra et al. [37] reported that positive CDC20 expression was found in 39 % only. This increased expression could be attributed to the different method of immunohistochemical scoring system of CDC20 expression and the difference in patients’ clinicopathological data. We found a significant inverse association with ER and PR expressions and CDC20 immunostaining while no association was detected with HER2 expression and CDC20 immunostaining. Similar result was reported by Karra et al. [37].
Regarding SCC of the cervix included in this study, CDC20 overexpression was detected in 40 % of SCC of the cervix. Previous study reported that CDC20 was overexpressed in 22.3 % of SCC [38].
In colon carcinoma, we found CDC20 overexpression in 55 % of the examined cases. Wu et al. [26] reported CDC20 overexpression in 46.7 % of colon carcinoma.
In gastric carcinoma, CDC20 overexpression was detected in 56.7 % of cases. Previous study reported by Ding et al. [36] showed that CDC20 overall expression in gastric carcinoma was 51.9 % with a significant difference in expression between normal and malignant cases.
In head and neck SCC, CDC20 overexpression was found in 44 % of the examined cases. Previously, CDC20 was reported in 56.9 % of oral squamous cell carcinoma [27].
In hepatocellular carcinoma, CDC20 overexpression was detected in 62.5 % of the studied cases. Li et al. [39] reported that CDC20 expression was upregulated in HCC tissues compared to the adjacent non-tumor liver tissues and these high expression levels of CDC20 were detected in 68.18 % HCC samples.
Regarding lung carcinoma, positive CDC20 expression was present in 46.7 % of all cases. Positive CDC20 expression in ADC (33.3 %) was lower than SCC (60 %) but the difference was not statistically significant. Kato et al. [24] reported CDC20 overexpression in 19.6 % of non-small cell lung carcinoma cases with 11.5 % for ADC, 35.9 % for SCC, and 50 % for large cell carcinoma.
In ovarian carcinoma, CDC20 overexpression was found in 72.5 % of cases. CDC20 expression in mucinous carcinoma (80 %) was higher than in serous carcinoma (68 %) with no significant difference between the two types. Previous study on serous epithelial ovarian cancer reported that high CDC20 expression was associated with high tumor grade [40]. To our knowledge, no previous study was done on mucinous ovarian carcinoma.
In pancreatic ductal adenocarcinoma (PDAC), CDC20 overexpression was detected in 46.7 % of examined cases. Chang et al. [12] reported that higher CDC20 protein expression level was found in PADC tissue than in normal pancreatic tissue.
To the best of our knowledge, we reported for the first time CDC20 expression in endometrial, prostatic, renal, testicular, and thyroid carcinomas. CDC20 expression was detected in 70, 52.5, 46.7, 43.3, and 65 % of endometrial, prostatic, renal, thyroid carcinomas, and testicular seminoma, respectively. Concerning thyroid carcinoma, positive CDC20 expression was found in 45 and 40 % of papillary and follicular carcinoma cases, respectively, with no statistically difference level.
As regards the correlation of CDC20 overexpression and tumor grade, we found that high CDC20 expression in tumor cells was associated with high grade. Most high-grade tumor cells exhibited diffuse and strong nuclear and/or cytoplasmic staining for CDC20, whereas most low-grade tumor cells showed no staining. This difference was reaching a statistically significant level in carcinomas of the bladder, cervix, colon, endometrium, kidney, liver, ovary, prostate, and stomach. In concordance with our study, previous studies were reported such results [23, 25, 26, 36–40]. However, the difference did not reach a statistically significant level as reported by [24, 27].
Regarding correlation of CDC20 overexpression and tumor stage, we noted that CDC20 overexpression was significantly associated with advanced tumor stage in carcinomas of the breast, colon, endometrium, and prostate. Similar results were noted by some of the previous cohorts [25, 26, 39]. However, other previous studies reported that CDC20 overexpression was not correlated with tumor stage [23, 24, 27, 36–38].
The present study coincided with other studies in that the increase in CDC20 expression has been reported in many human cancers. Significant associations between CDC20 expression and prognosis-related features such as histological grade and TNM stage were observed [23–27].
It was reported that CDC20 overexpression was associated with mis-separation of chromosomes at the metaphase, premature anaphase initiation, and aneuploidy in the tumor cells [40]. Based on the role of CDC20 in regulating cell growth, our findings suggested that CDC20 overexpression may have a role in tumorigenesis and permeating tumor growth.
In conclusion, CDC20 gene has had an important role in malignant tumors by serving as a useful biological marker to predict prognosis in patients with different cancers through identifying high-risk groups with high tumor grade and advanced stage. New strategy of adjuvant therapy can help those with CDC20 overexpression to improve their poor prognosis.
References
Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396(6712):643–9.
Suijkerbuijk SJ, Kops GJ. Preventing aneuploidy: the contribution of mitotic checkpoint proteins. Biochim Biophys Acta. 2008;1786(1):24–31.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Jallepalli PV, Lengauer C. Chromosome segregation and cancer: cutting through the mystery. Nat Rev Cancer. 2001;1(2):109–17.
Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432(7015):338–41.
Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23(11):2016–27.
Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274(5285):246–8.
Amon A. The spindle checkpoint. Curr Opin Genet Dev. 1999;9(1):69–75.
Iwanaga Y, Kasai T, Kibler K, Jeang KT. Characterization of regions in hsMAD1 needed for binding hsMAD2. A polymorphic change in an hsMAD1 leucine zipper affects MAD1–MAD2 interaction and spindle checkpoint function. J Biol Chem. 2002;277(34):31005–13.
Li R. Bifurcation of the mitotic checkpoint pathway in budding yeast. Proc Natl Acad Sci U S A. 1999;96(9):4989–94.
Haruki N, Saito H, Harano T, Nomoto S, Takahashi T, Osada H, et al. Molecular analysis of the mitotic checkpoint genes BUB1, BUBR1 and BUB3 in human lung cancers. Cancer Lett. 2001;16292:201–5.
Hernando E, Orlow I, Liberal V, Nohales G, Benezra R, CordonCardo C. Molecular analyses of the mitotic checkpoint components hsMAD2, hBUB1 and hBUB3 in human cancer. Int J Cancer. 2001;95(4):223–7.
Ouyang B, Knauf JA, Ain K, Nacev B, Fagin JA. Mechanisms of aneuploidy in thyroid cancer cell lines and tissues:evidence for mitotic checkpoint dysfunction without mutations in BUB1 and BUBR1. Clin Endocrinol (Oxf). 2002;56(3):341–50.
Doak SH, Jenkins GJ, Parry EM, Griffiths AP, Baxter JN, Parry JM. Differential expression of the MAD2, BUB1 and HSP27 genes in Barrett’s oesophagus—their association with aneuploidy and neoplastic progression. Mutat Res. 2004;547(1–2):133–44.
Jeong SJ, Shin HJ, Kim SJ, Ha GH, Cho BI, Baek KH, et al. Transcriptional abnormality of the hsMAD2 mitotic checkpoint gene is a potential link to hepatocellular carcinogenesis. Cancer Res. 2004;64(23):8666–73.
Sze KM, Ching YP, Jin DY, Ng IO. Association of MAD2 expression with mitotic checkpoint competence in hepatoma cells. J Biomed Sci. 2004;11(6):920–7.
Kienitz A, Vogel C, Morales I, Muller R, Bastians H. Partial downregulation of MAD1 causes spindle checkpoint inactivation and aneuploidy, but does not confer resistance towards taxol. Oncogene. 2004;24(26):4301–10.
Wasch R, Engelbert D. Anaphase-promoting complex dependent proteolysis of cell cycle regulators and genomic instability of cancer cells. Oncogene. 2005;24(1):1–10.
Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8(5):379–93.
Kim S, Yu H. Mutual regulation between the spindle checkpoint and APC/C. Semin Cell Dev Biol. 2011;22(6):551–8.
Sudakin V, Chan GK, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 2001;154(5):925–36.
Howell BJ, Moree B, Farrar EM, Stewart S, Fang G, Salmon ED. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr Biol. 2004;14(11):953–64.
Chang DZ, Ma Y, Ji B, Liu Y, Hwu P, Abbruzzese JL, et al. Increased CDC20 expression is associated with pancreatic ductal adenocarcinoma differentiation and progression. J Hematol Oncol. 2012;4:5–15.
Kato T, Daigo Y, Aragaki M, Ishikawa K, Sato M, Kaji M. Overexpression of CDC20 predicts poor prognosis in primary non small cell lung cancer patients. J Surg Oncol. 2012;106(4):423–30.
Choi JW, Kim Y, Lee JH, Kim YS. High expression of spindle assembly checkpoint proteins CDC20 and MAD2 is associated with poor prognosis in urothelial bladder cancer. Virchows Arch. 2013;463(5):681–7.
Wu WJ, Hu KS, Wang DS, Zeng ZL, Zhang DS, Chen DL, et al. CDC20 overexpression predicts a poor prognosis for patients with colorectal cancer. J Transl Med. 2013;11:142.
Moura IM, Delgado ML, Silva PM, Lopes CA, do Amaral JB, Monteiro LS. High CDC20 expression is associated with poor prognosis in oral squamous cell carcinoma. J Oral Pathol Med. 2014;43(3):225–31.
Izawa D, Pines J. The mitotic checkpoint complex binds a second CDC20 to inhibit activate APC/C. Nature. 2015;517(7536):631–4.
Hongtao Y. Cdc20. A WD40 activator for a cell cycle degradation machine. Mol Cell. 2007;27(1):3–16.
Mondal G, Sengupta S, Panda CK, Gollin SM, Saunders WS, Roychoudhury S. Overexpression of CDC20 leads to impairment of the spindle assembly checkpoint and aneuploidization in oral cancer. Carcinogenesis. 2007;28(1):81–92.
Clarke DJ, Segal M, Andrews CA, Rudyak SG, Jensen S, Smith K, et al. S-phase checkpoint controls mitosis via an APC-independent CDC20p function. Nat Cell Biol. 2003;5(10):928–35.
Shi J, Orth JD, Mitchison T. Cell type variation in responses to antimitotic drugs that target microtubules and kinesin-5. Cancer Res. 2008;68(9):3269–76.
Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14(2):111–22.
Huang HC, Shi J, Orth JD, Mitchison TJ. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 2009;16(4):347–58.
Kidokoro T, Tanikawa C, Furukawa Y, Katagiri T, Nakamura Y, Matsuda K. CDC20, a potential cancer therapeutic target, is negatively regulated by p53. Oncogene. 2008;27(11):1562–71.
Ding ZY, Wu HR, Zhang JM, Huang GR, Ji DD. Expression characteristics of CDC20 in gastric cancer and its correlation with poor prognosis. Int J Clin Exp Pathol. 2014;7(2):722–7.
Karra H, Repo H, Ahonen I, Löyttyniemi E, Pitkänen R, Lintunen M, et al. CDC20 and securin overexpression predict short-term breast cancer survival. Br J Cancer. 2014;110(12):2905–13.
Kim Y, Choi JW, Lee JH, Kim YS. MAD2 and CDC20 are upregulated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the uterine cervix. Int J Gynecol Pathol. 2014;33(5):517–23.
Li J, Gao JZ, Du JL, Huang ZX, Wei LX. Increased CDC20 expression is associated with development and progression of hepatocellular carcinoma. Int J Oncol. 2014;45(4):1547–55.
Ouellet VGM, Le Page C, Filali-Mouhim A, Lussier C, Tonin PN, Provencher DM, et al. Tissue array analysis of expression microarray candidates identifies markers associated with tumor grade and outcome in serous epithelial ovarian cancer. Int J Cancer. 2006;119(3):599–607.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Mariana F. Gayyed and Nehad M. R. Abd El-Maqsoud contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gayyed, M.F., El-Maqsoud, N.M.R.A., Tawfiek, E.R. et al. A comprehensive analysis of CDC20 overexpression in common malignant tumors from multiple organs: its correlation with tumor grade and stage. Tumor Biol. 37, 749–762 (2016). https://doi.org/10.1007/s13277-015-3808-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3808-1