Abstract
MicroRNAs (miRNAs) are a class of small noncoding RNAs that play important roles in tumorigenesis and tumor progression through regulation of gene expression. Earlier, miR-142-3p was shown to decreased in cervical cancer cells; here; we explore the biological functional role of miR-142-3p and underlying mechanism in cervical cancer cells. We first detected the expression of miR-142-3p in six human cervical cancer cell lines and chose HeLa and SiHa cells for functional studies. By gain and loss of function experiments, we showed that overexpression of miR142-3p resulted in downregulation of Frizzled7 receptor (FZD7) and inhibited proliferation and invasion in HeLa and SiHa cells, whereas miR142-3p inhibitor-transfected cells showed reduced FZD7 expression and increased invasion capacity. In addition, we demonstrated that FZD7 was a direct target of miR-142-3p by dual luciferase assay and Western blot analysis. Overexpression of FZD7 expression was able to reverse the inhibitory effects induced by miR-142-3p. Taken together, miR-142-3p functions tumor suppressive effects in cell proliferation and invasion in cervical cancer cells, suggesting a potential therapeutic approach for cervical cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical cancer is the second most common malignancy in women worldwide, with an estimated incidence of 530,000 new cases and 270,000 deaths per year [1]. It has been reported that more than 80 % of cervical cancers occur in developing countries, where widespread cervical screening is unavailable [2, 3]. Current treatment approaches for cervical cancer mainly include surgery, radiotherapy, and chemotherapy [4]. However, these treatments still have limitations; tumor recurrence and metastasis frequently occur in patients with advanced cancer [5]. Therefore, exploring molecules involved in the intricate process and identification of new and effective therapy targets for new treatment strategies are of great significance.
It has been known that aberrant activation of WNT signals frequently occur in malignancies [6, 7]. The canonical WNT pathway is implication in cell proliferation and differentiation, while the noncanonical WNT pathway is crucial for cellular motility, invasion, and epithelial-mesenchymal transition (EMT), which aggravate malignancy [8, 9]. The Frizzled7 receptor (FZD7), a member of WNT receptors, has been reported indispensably for activation of both canonical and noncanonical WNT pathways [10, 11]. Evidence is accumulating that high FZD7 expression existed in various cancers [12–14]. King et al. [15] reported that overexpression of FZD7 promoted tumor cell invasion and metastasis. Asad et al. [11] showed that FZD7 drives in vitro aggressiveness in ovarian cancer cells. Yang et al. demonstrated that FZD7 was critical for cell proliferation in triple negative breast cancer, indicating an oncogenic role of FZD7 in cancer, and several therapeutic strategies targeting this molecule have been described [16].
MicroRNAs (miRNAs) are conserved, small noncoding RNAs that regulate gene expression by targeting 3′-UTR of target mRNA, resulting in post-transcription repression or mRNA degradation [17–19]. Increasing evidence indicates that miRNAs are important regulators in diverse processes such as proliferation, differentiation, apoptosis, and cell mobility [20, 21], as well as carcinogenesis [22]; they exert regulatory roles by altering the targeted oncogenes or tumor suppressor expression. In a recent study, Tang et al. [23] showed that the expression level of miR-142-3p was significantly lower than the normal cervical epithelium cells, indicating a regulatory impact of miR-142-3p in cervical cancer. Through prediction with online software Target Scan (http://www.targetscan.org/) and microRNA.org (http://www.microrna.org/), we found that FZD7 was a potential target of miR-142-3p, and so far, limited information was found about the relationship between miR-142-3p and FZD7. In this study, we investigated the role of miR-142-3p in cell proliferation, migration, and invasion of cervical cancer cell lines. Concurrently, FZD7 was identified as a new potential target of miR-142-3p.
Methods
Cell culture
Six human cervical cancer cell lines (ME-180, Hela, CaSki, MS751, SiHa, C-33A) were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). ME-180 cells were cultured in McCoy’s 5A medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10 % fetal bovine serum (FBS; Hyclone, Logan, UT, USA). CaSki cells were cultured in RPMI-1640 (Gibco, Grand Island, NY, USA), while other cell lines were cultured in DMEM (Gibco) containing 10 % FBS. The cells maintained t at 37 °C in a humidified atmosphere of 5 % CO2.
Transfection
Pre-MiR-142-3p, miR-142-3p inhibitor, and the scrambled control oligonucleotides (control) were purchased from GenePharma (Shanghai, China). In brief, 100 pmol of synthesized oligonucleotide were transiently transfected into cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. To obtain FZD7 overexpressed cells, the pEGFP-N1 plasmids containing FZD7-coding sequences or empty pEGFP-N1 plasmids were transfected into cells using Lipofectamine 2000 reagent (Invitrogen).
MTT assay
Cell proliferation was determined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Transfected/nontransfected HeLa and SiHa cells were seeded in 96-well microplate at a density of 5 × 103 cells/well. MTT solution (0.2 mg/ml) was added to each well at different time point (12, 24, 48, or 72 h). After incubation at 37 °C for 4 h, the cell-free supernatant was removed and the resulting formazan crystals were dissolved in 200 μl DMSO. The optical density (OD) at 490 nm was determined with a microplate reader (BioTek, Vermont, USA).
Real-time PCR
Total RNA was extracted from cultured cells using RNA simple Total RNA Kit (TIANGEN Co., Beijing, China); RNA was converted into complementary DNA (cDNA) using Super MMLV Reverse Transcriptase (BioTeke, Beijing, China). Expression of miR-142-3p was measured using a TapMan miRNA assay (Applied Biosystems) according to the manufacturer’s instructions. The expression of miR-142-3p was normalized to U6 small nuclear RNA. Expression of FZD7 was detected using ExicyclerTM 96 real-time (RT) PCR machine (Bioneer, Daejeon, Korea), with the specific primers as follows: FZD7, 5′-GCCTCGACGCTCTTTACCG-3′ (forward) and 5′-GCAGCCCTCCTTCTTGGTG-3′ (reverse) and β-actin, 5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ (forward) and 5′-CTGTCACCTTCACCGTTCCAGTTT-3′ (reverse). The PCR reaction was performed for 10 min at 95 °C followed by 40 cycles of 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 30 s.
Western blot analysis
Total proteins from the cultured cells were extracted using NP-40 lysis buffer (Beyotime Institute of Biotechnology, Haimen, China); the protein concentration was measured by bicinchoninic acid (BCA) kits. A total amount of 40 μg proteins from each group were separated by SDS-PAGE and electro-transferred to a PVDF membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5 % non-fat milk room temperature for 1 h and incubated with a specific primary antibody (anti-FZD7, 1:500, anti-vimentin, 1:1000, Bioss, Beijing, China; anti-snail, 1:200, Santa Cruz, CA, USA; anti-E-cadherin and anti-β-actin, 1:1000, Boster, Wuhan, China) overnight at 4 °C. After washing with TBST three times, the membrane was incubated with corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (Beyotime) for 1 h. Targeted proteins were visualized using electrochemiluminescence (ECL) reagents (7Sea Biotech, Shanghai, China); the scanned images were analyzed with Gel-Pro-Analyzer software.
Matrigel invasion assay
The invasion capacities of HeLa and SiHa cells were assessed using Transwell membrane filter inserts (8-μm pore size, Corning, NY, USA) coated with Matrigel (BD Biosciences, San Jose, CA, USA). Briefly, cells were re-suspended with serum-free medium and then added into the upper chambers (2 × 104 cells); the bottom chambers of the Transwell were filled with cell growth medium containing 20 % FBS. After 24-h incubation, noninvasive cells in the upper chamber were removed and the invasive cells on the lower surface were fixed and stained, and photographed. The mean number of invaded cells in ten different microscopic fields per membrane was counted in triplicate.
Luciferase reporter assay
Luciferase reporter assay was used to test whether the miR-142-3p bound directly to the 3′-UTR region of FZD7. The 3′-UTR of FZD7 with a miR-142-3p targeting sequence was cloned into the pmirGLO luciferase reporter vector. The sequences used to amplify FZD7 3′-UTR were 5′-ACAGCTAGCGAGGCGATCAGCAGATACCA-3′ (forward) and 5′-CCTCGGTCGACACAGTACCCTCTATGATTGGC-3′ (reverse). The sequences used to amplify FZD7 3′-UTR mutant were 5′-CTTTGAGTGAACCCTCCAATCTT-3′ (forward) and 5′-AGGGTTCACTCAAAGGTGGGAT-3′ (reverse). HeLa and SiHa cells were cotransfected with the pmirGLO vectors containing FZD7 3′-UTR with wild-type (WT) or mutant (Mut) sequences and miR-142-3p precursor, or negative control (NC) using Lipofectamine 2000 (Invitrogen). Luciferase activity was measured 48 h after transfection using the Dual-Luciferase Reporter Assay System (Promega). All experiments were performed in triplicate.
Statistical analysis
All experiments were performed independently at least three times. Data were expressed as the mean ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA or Student’s t test. Values of p < 0.05 were considered significant. GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA) was used for all data analysis.
Results
Expression level of FZD7 correlates inversely with miR-142-3p expression in cervical cancer cell lines
To investigate the role of miR-142-3p and its correlation with FZD7 in CC, we first examined the expression levels of miR-142-3p in six cervical cancer cell lines; as shown in Fig. 1a, HeLa and SiHa cells appear to have low levels of miR-142-3p among the cell lines tested and ME-180 cells with high expression of miR-143-3p as determined by miRNA-RT-PCR (Fig. 1a). Interestingly, the expression of FZD7 inversely correlated with miR-142-3p, as indicated by high expression of FZD7 in HeLa and SiHa cells while low levels in ME-180 cells at both mRNA and protein levels (Fig. 1b, c). These initial results indicate that miR-142-3p expression negatively correlates with FZD7 in cervical cancer cells.
MiR-142-3p downregulates FZD7 expression, inhibits proliferation, and invasion in HeLa and SiHa cells
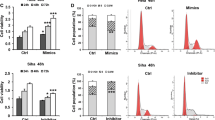
To further explore the functional role of miR-142-3p in cervical cancer and its correlation with FZD7, the pre-miR-142-3p and miR-142-3p inhibitor were used to increase or reduce miR-142-3p expression, respectively. In gain of function experiment, the expression level of miR-142-3p was increased by three times of the NC cells as determined by RT-PCR (p < 0.01, Fig. 2a). Interestingly, overexpression of miR-142-3p resulted in significant decrease in FZD7 mRNA and protein levels (p < 0.05 or p < 0.01, Fig. 2b–d), as well as suppressed proliferation and invasion of HeLa and SiHa cells as compared with NC cells (p < 0.05 or p < 0.01, Fig. 2e, f). In loss of function experiment, the miR-142-3p expression level decreased to 56 and 63 %, respectively, in miR-142-3p-transfected HeLa and SiHa cells compared with the NC cells (p < 0.01, Fig. 3a). Inversely, decreased miR-142-3p expression led to upregulation of FZD7 and invasive capacity of HeLa and SiHa cells (p < 0.01, Fig. 3b–f). In summary, these data suggest that miR-142-3p inversely correlates with FZD7 expression and inhibits proliferation and invasion in cervical cancer cells.
Overexpression of miR-142-3p reduces FZD7 expression and inhibits proliferation and invasion in HeLa and SiHa cells. Real-time PCR detected the expression of a miR-142-3p and b FZD7 after transfection with pre-miR-142-3p. c, d Western blot analysis of the FZD7 expression in HeLa and SiHa cells, with β-actin an internal reference. e The MTT assay was used to measure cell proliferation capacity in HeLa and SiHa cells treated with NC or pre-miR-142-3p. f A transwell invasion assay was performed to detect the invasive capacity of HeLa and SiHa cells. The invaded cells were counted under the microscope after 24-h incubation. Data are expressed as the mean ± SD of triplicate experiments. Compared with the NC group, *p < 0.05; **p < 0.01
Downregulation of miR-142-3p increases FZD7 expression and invasion in HeLa and SiHa cells. Real-time PCR detected the expression level of a miR-142-3p and b FZD7 after transfection with miR-142-3p inhibitor. c, d Western blot analysis of FZD7 expression in HeLa and SiHa cells, with β-actin an internal reference. e The MTT assay was used to measure cell proliferation capacity in HeLa and SiHa cells treated with NC or miR-142-3p inhibitor. f A transwell invasion assay was performed to detect the invasive capacity of HeLa and SiHa cells. The invaded cells were counted under the microscope after 24-h incubation. Data are expressed as the mean ± SD of triplicate experiments. Compared with the NC group, *p < 0.05; **p < 0.01
MiR-142-3p upregulates E-cadherin expression and downregulates snail expression in HeLa and SiHa cells
Epithelial-mesenchymal transition (EMT) is an essential step for malignancy [24]. The EMT process is characterized by loss of epithelial markers like E-cadherin, attended by increased mesenchymal markers such as vimentin and snail [25]. We previously showed that downregulation of FZD7 increased epithelial marker E-cadherin expression in HeLa and SiHa cells [26]. In this experiment, Western blot results showed that E-cadherin expression in pre-miR-142-3p-transfected HeLa and SiHa cells was significantly increased compared to the control (p < 0.01, Fig. 4a, b), while the expression levels of mesenchymal markers vimentin and snail were downregulated after pre-miR-142-3p transfection. The results indicate that miR-142-3p negatively regulates the EMT process in cervical cancer cells.
MiR-142-3p upregulates E-cadherin expression and downregulates snail expression in HeLa and SiHa cells. a HeLa and b SiHa cells were transfected with pre-miR-142-3p or NC; the expression of E-cadherin, vimentin, and snail was determined by Western blot analysis. β-actin was used as an internal reference. Data are expressed as the mean ± SD of triplicate experiments. Compared with the NC group, **p < 0.01
MiR-142-3p downregulates FZD7 expression by targeting the 3′-UTR region of FZD7
To investigate whether FZD7 is a direct target of miR-142-3p, we cotransfected the pre-miR-142-3p or NC, and pmiriGLO-FZD7-3′-UTR reporter or mutant into HeLa and SiHa cells. Western blot results showed that the expression level of FZD in cells cotransfected with pre-miR-142-3p and pmiriGLO-FZD7-3′-UTR reporter significantly decreased compared with the NC cells (Fig. 5a, b). Furthermore, relative luciferase activity in cells cotransfected with pre-miR-142-3p and wild-type FZD7 3′-UTR luciferase reporter decreased significantly to 28.5 ± 4.2 % in HeLa and 33.3 ± 5 % in SiHa cells (p < 0.01, Fig. 5d, e). However, cotransfection with the pre-miR-142-3p did not significantly alter mutant FZD7 3′-UTR reporter activity in both cells (Fig. 5d, e). These results suggest that miR-142-3p directly binds to the 3′-UTR of FZD7 mRNA; FZD7 acts as a direct target of miR-142-3p.
The 3′-UTR region of FZD7 is a target for miR-142-3p. a, b Western blot analysis of FZD7 expression in HeLa and SiHa cells cotransfected with pmirGLO reporter containing wild or mutated binding site of FZD7, and pre-miR-142-3p or NC; the expression level of FZD7 was normalized to β-actin. c The sequences of miR-142-3p binding sites within the 3′-UTR of FZD7 and the mutated binding site are presented. d, e Dual luciferase assays were performed in HeLa and SiHa cells cotransfected with the wild or mutated reporter, and pre-miR-142-3p or NC. Firefly luciferase activity was normalized to the Renilla luciferase activity. Data are expressed as the mean ± SD of triplicate experiments. Compared with the NC + FZD7-WT group, **p < 0.01
The inhibition of cell invasiveness by miR-142-3p is rescued by overexpression of FZD7
To prove direct evidence that miR-142-3p inhibits the invasive capacities of cervical cancer cells via targeting FZD7, we cotransfected HeLa and SiHa cells with miR-142-3p, and pEGFP-N1-FZD7, or pEGFP-N1-vector. Western blot results (Fig. 6a, b) confirmed that FZD7 and miR-142-3p have been conducted into the cells, as evidenced by low expression level of FZD7 in miR-142-3p-transfected cells (p < 0.05 vs vector group), but high expression in FZD7 + miR-142-3p cells (p < 0.01 vs vector + miR-142-3p group). Transwell invasion assay (Fig. 6c) showed that miR-142-3p transfection inhibited cell invasion in both cell types as compared with the vector groups (p < 0.01), while the number of invaded cells was elevated in FZD7 + miR-142-3p group compared with that in miR-142-3p group (p < 0.01). Data indicate that overexpression of FZD7 could effectively rescue the invasive capacity of HeLa and SiHa cells treated with miR-142-3p.
Overexpression of FZD7 reverses the effect of miR-142-3p on the inhibition of cell invasiveness. a, b Western blot analysis of FZD7 expression in HeLa and SiHa cells after cotransfection, with β-actin as the internal reference. c Transwell invasion assay was performed to detect the invasive capacity of HeLa and SiHa cells. Data are expressed as the mean ± SD of triplicate experiments. Compared with the vector group, *p < 0.05; **p < 0.01. Compared with the vector + miR-142-3p group, ## p < 0.01
Discussion
miRNAs have been demonstrated to play important roles in the development and progression of cancer [27, 28]. They can act as oncogenes or tumor suppressors by regulating targeted genes expression. Thereby, identification of cancer-specific miRNAs and their targets is critical for understanding their role in tumorigenesis and might be important for defining novel therapeutic targets [22]. Previously, miR-142-3p has been shown to function tumor suppressive effects in hepatocellular carcinoma [29] and pancreatic cancer [30], while it served as oncogenic biomarker for esophageal squamous cell carcinoma and T cell acute lymphoblastic leukemia [31, 32]. These data indicate that miR-142-3p may have distinct functions in different cells or tissues that attribute to distinct targets. Here, we demonstrated that miR-142-3p inhibits cell proliferation and invasion of cervical cancer cells, as well as reversely correlated with FZD7 expression. We provide the first evidence that miR-142-3p acts as a tumor suppressor in cervical cancer cells by directly regulating FZD7 expression, which may provide new insights about its role and value in cervical carcinogenesis.
MiR-142-3p has been reported as being involved in tumor cell proliferation and metastasis in many cancer types. For example, forced miR-142-3p expression inhibited cell proliferation in colon cancer [33], osteosarcoma cells [34], and pancreatic cancer [30]; miR-142-3p overexpression inhibited the migratory and invasive capacities of hepatocellular carcinoma cells [29]. In accordance with these findings, we found that miR-142-3p exerted tumor suppressive effects in cervical cancer cells. Overexpression of miR-142-3p repressed cell proliferation and invasive capacity of cervical cancer cells, whereas downregulation of this molecule resulted in increased invasion and proliferation. Moreover, we found that miR-142-3p decreased EMT process, as indicated by downregulation of mesenchymal markers including vimentin and snail while upregulation of epithelial protein E-cadherin. Our results were consistent with a recent study, which reported decreased miR-142-3p expression in cervical cancer cell lines and high levels in normal cervical epithelium cells [23]. These results indicate that miR-142-3p acts as a tumor suppressor in cervical cancer.
The Wnt signaling pathways have been demonstrated to play critical roles in cancer processes, such as proliferation, cell cycle, invasion, and EMT [35, 36]. Aberrant activation of WNT signals frequently occur in malignancies [37]. Thus, targeted regulation of WNT pathways could be potential therapeutic interventions for cancer. Previously, miR-142-3p has been reported to implicate in WNT pathway regulation. For instance, Carraro G et al. [38] showed that miR-142-3p balanced proliferation and differentiation in the embryonic lung mesenchyme through modulating WNT signaling by directly targeting Apc expression. Shen W et al. [33] demonstrated that miR-142-3p inhibited colon cancer cell proliferation through negative regulation of CD133, Lgr5, and ABCG2 expression, in which Lgr5 was a part of the canonical WNT signaling pathway. In the present study, we found that miR-142-3p not only regulated cell proliferation but also affected invasive capacity of HeLa and SiHa cells. Furthermore, we demonstrated that FZD7 was a direct target of miR-142-3p in cervical cancer cells by online software prediction, luciferase reporter assay, and Western blot analyses. Overexpression of miR-142-3p repressed FZD7 expression at both mRNA and protein levels and inhibited proliferation and invasion of HeLa and SiHa cells. Whereas inhibition of miR-142-3p led to increased FZD7 expression and enhanced invasion. More over, upregulation of FZD7 could partially rescue the invasion inhibition induced by miR-142-3p in HeLa and SiHa cells. These results were in line with our previous report that FZD7 enhanced invasion, migration, and EMT in cervical cancer cells [26]. Taken together, our data indicate that miR-142-3p functions as a tumor suppressor in cervical cancer partially through targeting FZD7.
In summary, our data indicate that miR-142-3p inhibits proliferation and invasion of cervical cells by directly regulating FZD7 expression. MiR-142-3p could be a potential therapeutic target for cervical cancer.
References
Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol Off J Eur Soc Med Oncol/ESMO. 2011;22:2675–86.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer J Int Cancer. 2010;127:2893–917.
Wang X, Tang S, Le SY, Lu R, Rader JS, Meyers C, et al. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS ONE. 2008;3:e2557.
Duenas-Gonzalez A, Cetina L, Mariscal I, de la Garza J. Modern management of locally advanced cervical carcinoma. Cancer Treat Rev. 2003;29:389–99.
Glick SB, Clarke AR, Blanchard A, Whitaker AK. Cervical cancer screening, diagnosis and treatment interventions for racial and ethnic minorities: a systematic review. J Gen Intern Med. 2012;27:1016–32.
Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50.
Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–98.
Dissanayake SK, Wade M, Johnson CE, O’Connell MP, Leotlela PD, French AD, et al. The Wnt5a/protein kinase c pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282:17259–71.
Katoh M. WNT/PCP signaling pathway and human cancer (review). Oncol Rep. 2005;14:1583–8.
Abu-Elmagd M, Garcia-Morales C, Wheeler GN. Frizzled7 mediates canonical Wnt signaling in neural crest induction. Dev Biol. 2006;298:285–98.
Asad M, Wong MK, Tan TZ, Choolani M, Low J, Mori S, et al. FZD7 drives in vitro aggressiveness in stem-a subtype of ovarian cancer via regulation of non-canonical Wnt/PCP pathway. Cell Death Dis. 2014;5:e1346.
Kirikoshi H, Sekihara H, Katoh M. Up-regulation of frizzled-7 (FZD7) in human gastric cancer. Int J Oncol. 2001;19:111–5.
Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene. 2011;30:4437–46.
Merle P, Kim M, Herrmann M, Gupte A, Lefrancois L, Califano S, et al. Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol. 2005;43:854–62.
King TD, Zhang W, Suto MJ, Li Y. Frizzled7 as an emerging target for cancer therapy. Cell Signal. 2012;24:846–51.
Nambotin SB, Lefrancois L, Sainsily X, Berthillon P, Kim M, Wands JR, et al. Pharmacological inhibition of frizzled-7 displays anti-tumor properties in hepatocellular carcinoma. J Hepatol. 2011;54:288–99.
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97.
Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by micrornas: are the answers in sight? Nat Rev Genet. 2008;9:102–14.
Croce CM, Calin GA. Mirnas, cancer, and stem cell division. Cell. 2005;122:6–7.
Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–33.
Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–61.
Tang T, Wong HK, Gu W, Yu MY, To KF, Wang CC, et al. MicroRNA-182 plays an onco-miRNA role in cervical cancer. Gynecol Oncol. 2013;129:199–208.
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–54.
Qureshi R, Arora H, Rizvi MA. EMT in cervical cancer: its role in tumour progression and response to therapy. Cancer Lett. 2015;356:321–31.
Deng B, Zhang S, Miao Y, Zhang Y, Wen F, Guo K. Down-regulation of frizzled-7 expression inhibits migration, invasion, and epithelial-mesenchymal transition of cervical cancer cell lines. Med Oncol. 2015;32:102.
Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol. 2005;7:719–23.
Kent OA, Mendell JT. A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene. 2006;25:6188–96.
Wu L, Cai C, Wang X, Liu M, Li X, Tang H. MicroRNA-142-3p, a new regulator of RAC1, suppresses the migration and invasion of hepatocellular carcinoma cells. FEBS Lett. 2011;585:1322–30.
MacKenzie TN, Mujumdar N, Banerjee S, Sangwan V, Sarver A, Vickers S, et al. Triptolide induces the expression of miR-142-3p: a negative regulator of heat shock protein 70 and pancreatic cancer cell proliferation. Mol Cancer Ther. 2013;12:1266–75.
Lv M, Zhang X, Jia H, Li D, Zhang B, Zhang H, et al. An oncogenic role of miR-142-3p in human T-cell acute lymphoblastic leukemia (T-ALL) by targeting glucocorticoid receptor-alpha and cAMP/PKA pathways. Leukemia. 2012;26:769–77.
Lin RJ, Xiao DW, Liao LD, Chen T, Xie ZF, Huang WZ, et al. MiR-142-3p as a potential prognostic biomarker for esophageal squamous cell carcinoma. J Surg Oncol. 2012;105:175–82.
Shen WW, Zeng Z, Zhu WX, Fu GH. MiR-142-3p functions as a tumor suppressor by targeting CD133, ABCG2, and Lgr5 in colon cancer cells. J Mol Med (Berl). 2013;91:989–1000.
Yang YQ, Qi J, Xu JQ, Hao P. MicroRNA-142-3p, a novel target of tumor suppressor menin, inhibits osteosarcoma cell proliferation by down-regulation of FASN. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2014;35:10287–93.
Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014.
Menezes ME. The Wnt/β-catenin signaling pathway in epithelial mesenchymal transition. J Postdoctoral Res. 2014;1:12.
Manzo-Merino J, Contreras-Paredes A, Vazquez-Ulloa E, Rocha-Zavaleta L, Fuentes-Gonzalez AM, Lizano M. The role of signaling pathways in cervical cancer and molecular therapeutic targets. Arch Med Res. 2014;45:525–39.
Carraro G, Shrestha A, Rostkovius J, Contreras A, Chao CM, El Agha E, et al. MiR-142-3p balances proliferation and differentiation of mesenchymal cells during lung development. Development. 2014;141:1272–81.
Acknowledgments
This study was supported by a grant from The First Affiliated Hospital of China Medical University (No. FSFH1214).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deng, B., Zhang, Y., Zhang, S. et al. MicroRNA-142-3p inhibits cell proliferation and invasion of cervical cancer cells by targeting FZD7. Tumor Biol. 36, 8065–8073 (2015). https://doi.org/10.1007/s13277-015-3483-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3483-2