Abstract
The search for novel drug candidates is a priority goal for cancer therapy. Natural products isolated from plants are often used as valuable leads for the synthesis of analogs with simpler structure. Two synthetic α-methylene-δ-lactones with chroman-2-one skeleton, designated DL-3 and DL-5, exhibiting strong cytotoxic activity against several cancer cell lines, have been tested alone and in combination with well-known anticancer drugs, 5-fluorouracil, oxaliplatin, and taxol, in breast cancer MCF-7 cells. Parthenolide, a plant-derived α-methylene-γ-lactone, was used as a positive control. The effects on cell proliferation, DNA damage, and apoptosis induction were evaluated. Neither of the tested compounds significantly enhanced the effects produced by taxol, but a strong synergistic effect was observed with 5-fluorouracil and oxaliplatin. Only small differences between the actions of both α-methylene-δ-lactones were found. The synergistic effects produced by these compounds in MCF-7 cells were stronger as compared with parthenolide. Our findings show that simple and easy-to-obtain synthetic compounds with α-methylene-δ-lactone motif can potentiate the efficiency of anticancer drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
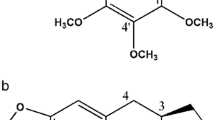
Plants have always been an excellent source of biologically active agents used in traditional medicine. An interesting group of plant-derived compounds with anticancer potential are α-methylene-γ-lactones 1 (Fig. 1), with parthenolide (PTL) (Fig. 2), isolated from feverfew (Tanacetum parthenium) being the most studied representative.
The active fragment in the PTL structure is an exocyclic methylene moiety conjugated with a carbonyl group which can react via the Michael-type reaction with mercaptyl groups in cysteine residues of enzymes, other functional proteins, and free intracellular glutathione, leading to the formation of covalent adducts [1, 2]. Such alkylation of cellular thiols may interfere with key biological processes, leading to the controlled cell death, apoptosis [3]. PTL was shown in vitro to induce apoptosis, inhibit cell cycle and proliferation, and diminish metastasis in various cancer cell lines [4–6]. At the molecular level, the cytotoxic action of this compound involves different mechanisms and is associated with the inhibition of the transcription factor nuclear factor (NF)-κB [7, 8] and MAPK signaling pathway [9], induction of oxidative stress and mitochondrial dysfunction [10, 11], modulation of DNA methylation [12], and microtubule-interfering activity [13].
In contrast to α-methylene-γ-lactones, α-methylene-δ-lactones 2 (Fig. 1) are much less abundant in nature (Fig. 1). The known examples of natural compounds with such motif are vernolepin and vernomenin isolated from Vernonia hymenolepis [14] and crassin from Pseudoplexaura porosa [15]. These compounds showed some cytotoxicity against human pharyngeal carcinoma (KB) cells, but not much is known so far about their anticancer potential. Having at hand general methodologies for the introduction of a methylene group onto the δ-lactone ring, we have synthesized a series of α-methylene-δ-lactones with chroman-2-one skeleton 3 (Fig. 1) and tested their cytotoxicity on three cancer cell lines [16]. The most potent compound of the series, 1-isopropyl-2-methylene-1,2-dihydrobenzochromen-3-one, designated DL-3 (Fig. 2), was further evaluated as a potential anticancer agent [17]. DL-3 was found to activate the intrinsic pathway of apoptosis, associated with the loss of mitochondrial membrane potential and changes in Bax/Bcl-2 ratio and suppressed cell migration and invasion.
The aim of this study was to investigate the possible synergistic effects of DL-3 and its analog, 4-isopropyl-7-methoxy-3-methylenechroman-2-one, designated DL-5 (Fig. 2), used in combination with well-known anticancer drugs, 5-fluorouracil (5-FU), taxol (Tx), and oxaliplatin (Ox). In the co-treatment experiments, the induction of apoptosis, inhibition of proliferation, and induction of DNA damage were investigated in MCF-7 cells. PTL was used as a positive control.
Materials and methods
Materials and general procedures
PTL, Ox, and Tx were purchased from Tocris Bioscience (Bristol, UK). 5-FU was from Sigma-Aldrich (St. Louis, MO, USA). The synthesis of 3-methylenechroman-2-ones (DL-3 and DL-5) was described earlier [16]. Ox was dissolved in a culture medium. 5-FU, PTL, Tx, and synthetic δ-lactones were dissolved in DMSO and further diluted in culture medium to obtain less than 0.1 % DMSO concentration. In each experiment, controls without and with 0.1 % DMSO were performed; 0.1 % DMSO had no effects on the observed parameters.
Cell culture
The MCF-7 cell line was purchased from the European Collection of Cell Cultures (ECACC). The MCF-7 cells were cultured in Minimum Essential Medium Eagle (MEME, Sigma-Aldrich, St. Louis, MO, USA) with glutamine (2 mM) (Sigma-Aldrich, St. Louis, MO, USA), MEM Non-essential amino acid solution 100× (Sigma-Aldrich, St. Louis, MO, USA), gentamycin (5 μg/mL), and 10 % heat-inactivated fetal bovine serum (FBS) (both from Biological Industries, Beit-Haemek, Israel). Cells were maintained at 37 °C in a 5 % CO2 atmosphere and were grown until 80 % confluent.
Cell viability assay (MTT)
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed according to a known procedure [18]. The cells were grown to sub-confluent levels in the culture medium and then plated onto 24-well plates (104 cells/well) in the final volume of 1 mL of the culture medium. After 24 h, various concentrations of the tested compounds were added and the plates were incubated for 24, 48, and 72 h. Then, the cells were incubated for 2 h at 37 °C with MTT [5 mg/mL in phosphate-buffered saline (PBS, GIBCO, Invitrogen, Carlsbad, CA, USA)]. The absorbance of the blue formazan product was measured at 540 nm using an automated plate reader (iMark Bio-Rad, Hercules, CA, USA) and compared with control (untreated cells). All experiments were performed in triplicate.
Analysis of cell proliferation, apoptosis, and DNA damage by flow cytometry
Cell proliferation, DNA damage, and apoptotic cell death were determined using the “Apoptosis, DNA Damage, and Cell Proliferation Kit” (BD Bioscience), according to the manufacturer guidelines. The test kit includes all the necessary components, such as BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution; BD Perm/Wash™ Buffer; BD Cytofix/Cytoperm™ Plus Permeabilization Buffer, DAPI, (Bromodeoxyuridine) BrdU, DNase, and antibodies (PerCP-Cy™5.5 Mouse Anti-BrdU Alexa Fluor® 647 Mouse Anti-H2AX (pS139); and PE Mouse Anti-Cleaved PARP (Asp214)).
Briefly, MCF-7 cells were seeded in a 25-cm2 cell culture flask at a density 0.1 × 106/mL in 10 mL of a standard growth medium. After 24 h, the growth medium was replaced by a fresh growth medium supplemented with the tested compounds in the desired concentrations. The cells incubated without tested compounds were used as the controls. After 24 h of incubation, the culture medium was collected and the floating cells were counted and discarded. The number of discarded cells was never greater that 15 % of the cell population. The fresh growth medium supplemented with BrdU (final concentration 10 μM) was added to each flask. After 8 h of incubation, culture medium was collected and cells were washed twice with PBS (GIBCO, Invitrogen, Carlsbad, CA, USA) and harvested by trypsinization. Cells detached from the culture flask and cells floating in the culture medium were combined into one tube and centrifuged (200×g, 5 min). The total number of cells was determined. Cells were resuspended in BD Cytofix/Cytoperm Fixation/Permeabilization Solution (100 μL per tube) and incubated for 30 min on ice. Afterwards, cells were washed once with 1 mL of 1× BD Perm/Wash Buffer and centrifuged (200×g, 5 min). The supernatant was discarded. The cells were stored overnight in 500 μL of a staining buffer at 4 °C.
After washing (as previously described), cells were fixed and permeabilized according to the manufacturer’s protocol. Then, the cells were treated with 100 μL of diluted DNase (1 mg/mL in DPBS) per tube and incubated for 1 h at 37 °C in order to expose BrdU epitopes. Following this treatment, cells were simultaneously stained with fluorochrome-labeled anti-BrdU, H2AX (pS139), and cleaved PARP (Asp214) antibodies for 20 min at room temperature. Cells were resuspended in a staining buffer and analyzed by flow cytometry (Becton Dickinson Canto II). The data was visualized and quantified by constructing a dot-plot using the BD FACSDiva software.
Statistical analysis
Statistical analyses were performed using Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA). The data were expressed as means ± SEM. Statistical comparisons were assessed by a one-way ANOVA followed by a post hoc multiple comparison Student-Newman-Keuls test. A probability level of 0.05 or lower was considered statistically significant.
Results
Cytotoxic activity
The cytotoxicity of PTL and the synthetic α-methylene-δ-lactones DL-3 and DL-5 was studied using the MTT assay. The viability of MCF-7 cells, following 24, 48, and 72 h of incubation with the tested compounds in increasing concentrations, was assessed. PTL and both δ-lactones showed high cytotoxic activity which increased with time (Fig. 3). After 24 h of incubation, the inhibition concentration (IC)50 values, which represent concentration of a compound required to inhibit metabolic activity of the cells by 50 %, were 3.54 ± 0.76, 4.25 ± 0.3, and 9.5 ± 0.7 for DL-3, DL-5, and PTL, respectively. These concentrations were used in all further experiments.
The effects of the synthetic compounds and anticancer drugs used separately on cell proliferation, DNA damage, and apoptotic cell death
BrdU incorporation into DNA of MCF-7 cells was used as an index of cell proliferation. The proliferation was assessed by flow cytometry, using PerCP-Cy™5.5 Mouse Anti-BrdU antibodies.
The DNA damage in MCF-7 cells was evaluated by flow cytometry, using Alexa Fluor 647 Mouse Anti-H2AX (pS139) antibodies, directed against the phosphorylated form of human H2AX protein at the pS139 residue. The induction of apoptosis in MCF-7 cells was assessed by flow cytometry using the fluorochrome-labeled PE Anti-Cleaved PARP (Asp214) antibodies.
MCF-7 cells were incubated for 24 h with increasing concentrations of anticancer drugs or δ-lactones. The concentration range for 5-FU, Tx, and Ox was chosen based on the literature data [19–23]. For δ-lactones, concentrations equal to 0.5× IC50, 1× IC50, and 2× IC50 were used.
5-FU, Tx, and Ox inhibited cell proliferation and induced DNA damage and apoptosis in a dose-dependent manner (Fig. 4a–c). These tests allowed us to choose the appropriate concentrations for the combination experiments which were 25 μM, 30 nM, and 35 μM for 5-FU, Tx, and Ox, respectively.
Inhibition of cell proliferation (BrdU test) (a), induction of DNA damage (H2AX test) (b), and induction of apoptosis (PARP test) (c) by anticancer drugs 5-FU, Tx, and Ox in MCF-7 cells. After 24 h of incubation with tested compounds, the medium was changed, fresh medium with BrdU was added, and cells were incubated for additional 8 h. The cells were stained with fluorochrome-labeled anti-BrdU, anti-cleaved PARP (Asp214) and anti-H2AX (pS139) antibodies for 20 min at room temperature, and analyzed by flow cytometry. Data represent mean ± SEM of three independent experiments. Statistical significance was assessed using one-way ANOVA and a post hoc multiple comparison Student-Newman-Keuls test. *p < 0.05, **p < 0.01, and ***p < 0.001 were considered as significantly different from untreated cells regarded as control (c), 100 %
Both δ-lactones, DL-3 and DL-5, as well as PTL also dose-dependently inhibited cell proliferation. However, in DNA damage and apoptosis induction experiments, a significant effect was not observed at the lowest concentration (0.5× IC50) (Fig. 5a–c). Therefore, the IC50 concentrations were chosen for the combination experiments.
Inhibition of cell proliferation (BrdU test) (a), induction of DNA damage (H2AX test) (b) and induction of apoptosis (PARP test) (c) by PTL and synthetic δ-lactones, DL-3 and DL-5, in MCF-7 cells. After 24 h of incubation with tested compounds medium was changed, fresh medium with BrdU was added and cells were incubated for additional 8 h. The cells were stained with fluorochrome-labeled anti-BrdU, anti-cleaved PARP (Asp214) and anti-H2AX (pS139) antibodies for 20 min at room temperature and analyzed by flow cytometry. Data represent mean ± SEM of three independent experiments. Statistical significance was assessed using one-way ANOVA and a post hoc multiple comparison Student-Newman-Keuls test. **p < 0.01 and ***p < 0.001 were considered as significantly different from untreated cells regarded as control (c), 100 %
Combination experiments with anticancer drugs and synthetic α-methylene-δ-lactones
Synergism in drugs is defined as joint action of two drugs in such a manner that one enhances the action of the other to produce an effect greater than that which may be obtained with either one alone.
As mentioned before, the main goal of the present study was to investigate possible synergism in activity of selected anticancer drugs and synthetic α-methylene-δ-lactones in MCF-7 cells. Cells were co-incubated with PTL or synthetic compounds (DL-3 or DL-5) and anticancer drugs for 24 h. Effect of combination treatment on cell proliferation, DNA damage, and apoptosis was evaluated and compared to the effect produced by these substances alone. The statistical significance of combined treatment vs 5-FU, Tx, and Ox is shown in Fig. 6a–c. Whenever the statistical significance was found also vs DL-3 or DL-5, additional bars for respective δ-lactones were placed on the graphs. We considered the effect synergistic only when there was a statistically significant change vs both the anticancer drug and α-methylene-δ-lactone.
Combined effect of PTL or synthetic δ-lactones, DL-3 or DL-5, used at their IC50 concentrations and 5-FU (25 μM), Tx (30 nM), or Ox (35 μM) on inhibition of cell proliferation (BrdU test) (a), induction of DNA damage (H2AX test) (b), and induction of apoptosis (PARP test) (c) in MCF-7 cells. Cells were co-incubated with PTL or synthetic δ-lactones (DL-3, DL-5) and anticancer drugs for 24 h. Then medium was changed, fresh medium with BrdU was added, and cells were incubated for additional 8 h. The cells were stained with fluorochrome-labeled anti-BrdU anti-cleaved PARP (Asp214) and anti-H2AX (pS139) antibodies for 20 min at room temperature and analyzed by flow cytometry. Data represent mean ± SEM of three independent experiments. Statistical significance was assessed using one-way ANOVA and a post hoc multiple comparison Student-Newman-Keuls test. *p < 0.05, **p < 0.01, ***p < 0.001, in comparison to respective anticancer drug alone; # p < 0.05, ## p < 0.01, ### p < 0.001, in comparison to respective synthetic δ-lactone alone
Inhibition of proliferation produced by 5-FU was greatly enhanced by DL-5, but not by DL-3 (Fig. 6a). Both δ-lactones produced significant synergistic effect with 5-FU, enhancing DNA damage (Fig. 6b). Neither of the tested compounds complemented the action of Tx in the performed tests (Fig. 6a–c). We have demonstrated that the combination of Ox with DL-3 or DL-5 decreased the MCF-7 cell proliferation (Fig. 6a) and increased the number of apoptotic cells, from 14 % for Ox alone to 30 and 20 % for combination of Ox with DL-3 and DL-5, respectively (Fig. 6c). Ox alone in 35 μM concentration was able to induce DNA damage in about 27 % of cell population, and this high value was not further increased in the combination experiments.
PTL, used as a positive control, showed combined effects with 5-FU and Ox, enhancing inhibition of MCF-7 cell proliferation (Fig. 6a). Also, DNA damage induced by Tx was strongly increased in co-treatment with PTL (Fig. 6b).
Discussion
Cancer is the leading cause of death in developed countries. Currently used chemotherapy is primarily based on the cytotoxic drugs. High toxicity of such therapy and resistance of cancer cells to administered medications is a driving force for the development of new strategies for cancer treatment. A new promising paradigm for anticancer therapy is combination chemotherapy, involving at least two drugs that work by different mechanisms, thereby decreasing the likelihood that resistant cancer cells will develop. Moreover, the use of drugs targeting multiple signaling pathways increases the efficacy of such treatment, allowing to use each drug at its optimal dose which reduces side effects. The need for novel strategies with high-grade antitumor specificity prompts the researchers to examine new substances that, in combination with already known antineoplastic agents, could enhance response to chemotherapy.
PTL was shown to synergistically enhance action of various known anticancer drugs that act through different mechanisms, improving their therapeutic profiles in vitro on cancer cells, or in animal models [24].
In the present study, we investigated the possible synergistic effects of two synthetic α-methylene-δ-lactones, DL-3 and DL-5, used in combination with well-known anticancer drugs: 5-FU, Tx, and Ox. A nucleoside analog, 5-FU, is a classical chemotherapeutic agent, often used in the treatment of colorectal cancer. 5-FU is known to block DNA synthesis and generate DNA damage, including double-stranded DNA breaks (DSBs) via the collapse of stalled replication forks [25]. Furthermore, 5-FU is known to induce apoptosis, by changing Bax/Bcl-2 or Bcl-xl ratio. However, severe side effects, as well as often observed development of resistance to that drug, limit its usefulness. New chemotherapy combinations are being sought to enhance 5-FU anticancer effect and overcome the resistance problem. Recently, it has been reported that combined treatment of 5-FU with PTL exerted a synergistic effect and induced apoptosis in vitro and in in vivo models of colorectal cancer [19]. In the current study, we have shown that the co-treatment of MCF-7 cells with 5-FU and DL-5 effectively suppressed cell proliferation. The analysis of H2AX phosphorylation revealed that the combination of 5-FU with DL-3 or DL-5 enhanced DNA damage. The observed effect was about eight times stronger than that observed for 5-FU alone. On the other hand, co-incubation of MCF-7 cells with δ-lactones and 5-FU did not synergistically induce apoptosis or increase caspase-3 levels.
Ox is a platinum-containing compound which, similarly to cisplatin and carboplatin, exerts its cytotoxic effect via the inhibition of DNA synthesis in cancer cells. Ox functions by forming both inter- and intra-strand cross links with DNA, preventing replication and transcription. Fang et al. [22] showed that PTL markedly enhanced sensitivity of human lung cancer A549 cells to low doses of Ox. They demonstrated that, compared to PTL or Ox alone, significant improvements in cell apoptosis and growth inhibition indexes were observed in the combined treatment.
In our study, we have shown that the proliferation inhibition and apoptosis induction caused by Ox were synergistically enhanced by DL-3 and DL-5. The combined effect was not observed in the case of DNA damage induction, since the effect of Ox alone was very strong.
Tx is a cancer chemotherapeutic isolated from the bark of the Pacific yew tree, Taxus brevifolia. It works as a mitotic inhibitor that stabilizes microtubules and, as a result, interferes with their normal breakdown during cell division. Tx is used to treat patients with different types of cancer. Unfortunately, the development of resistance is often the main limitation of Tx therapy. To overcome the chemoresistance problem, several drug combinations were investigated. The co-administration of Tx and PTL in non-small lung cancer A549 cells enhanced the inhibitory effect of Tx on cell viability [21]. PTL was also shown to increase sensitivity of breast cancer MDA-MB-231 and HBL-100 cells to Tx by increasing c-Jun N-terminal kinase (JNK) activity and decreasing the level of NF-κB in cell-type-specific manner [26]. In our experiments, PTL acted synergistically with Tx, generating DNA damage in MCF-7 cells but did not enhance cell proliferation and apoptosis. Neither one of our synthetic δ-lactones sensitized MCF-7 cells to Tx.
In conclusion, we have shown that simple, easy-to-synthesize compounds containing an exo-methylene group in the δ-lactone ring can enhance anticancer activity of 5-FU and Ox. The observed differences in the action of DL-3 and DL-5 indicate that methylene group conjugated with a carbonyl function is not the only structural element important for the action of these compounds. Further functionalization of these δ-lactones is possible and may enable selective modulation of their reactivity.
References
Pati HN, Das U, Sharma RK, Dimmock JR. Cytotoxic thiol alkylators. Mini Rev Med Chem. 2007;7(2):131–9.
Lee KH, Hall IH, Mar EC, Starnes CO, ElGebaly SA, Waddell TG, et al. Sesquiterpene antitumor agents: inhibitors of cellular metabolism. Science. 1977;196:533–6.
Zhang S, Ong CN, Shen HM. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004;208:143–53.
Janecka A, Wyrębska A, Gach K, Fichna J, Janecki T. Natural and synthetic α-methylenelactones and α- methylenelactams with anticancer potential. Drug Discov Today. 2012;17:561–72.
Zhang S, Won YK, Ong CN, Shen HM. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr Med Chem Anticancer Agents. 2005;5:239–49.
Koprowska K, Czyz M. Molecular mechanisms of parthenolide’s action: old drug with a new face. Postepy Hig Med Dosw (Online). 2010;64:100–14.
García-Piñeres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, et al. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276:39713–20.
Rüngeler P, Castro V, Mora G, Gören N, Vichnewski W, Pahl HL, et al. Inhibition of transcription factor NF-kappaB by sesquiterpene lactones: a proposed molecular mechanism of action. Bioorg Med Chem. 1999;7:2343–52.
Hung TM, Hung TM, Na M, Dat NT, Ngoc TM, Youn U, et al. Cholinesterase inhibitory and anti-amnesic activity of alkaloids from Corydalis turtschaninovii. J Ethnopharmacol. 2008;119:74–80.
Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis: the anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem. 2002;277:38954–64.
Skalska J, Brookes PS, Nadtochiy SM, Hilchey SP, Jordan CT, Guzman ML, et al. Modulation of cell surface protein free thiols: a potential novel mechanism of action of the sesquiterpene lactone parthenolide. PLoS One. 2009;4:e8115.
Liu Z, Liu S, Xie Z, Pavlovicz RE, Wu J, Chen P, et al. Modulation of DNA methylation by a sesquiterpene lactone parthenolide. J Pharmacol Exp Ther. 2009;329:505–14.
Fonrose X, Ausseil, Soleilhac E, Masson V, David B, Pouny I, et al. Parthenolide inhibits tubulin carboxypeptidase activity. Cancer Res. 2007;67:3371–8.
Kupchan SM, Hemingway RJ, Werner D, Karim A, McPhail AT, Sim GA. Vernolepin, a novel elemanolide dilactone tumor inhibitor from Vernonia hymenolepis. J Am Chem Soc. 1968;90:3596–7.
Weinheimer AJ, Chang CWJ, Matson JA. Naturally occurring cembranes. Fortschr Chem Org Naturst. 1979;36:285–387.
Modranka J, Albrecht A, Jakubowski R, Krawczyk H, Różalski M, Krajewska U, et al. Synthesis and biological evaluation of α-methylidene-δ-lactones with 3,4-dihydrocoumarin skeleton. Bioorg Med Chem. 2012;20:5017–26.
Wyrębska A, Gach K, Lewandowska U, Szewczyk K, Hrabec E, Modranka J, et al. Anticancer activity of new synthetic α-methylene-δ-lactones on two breast cancer cell lines. Basic Clin Pharmacol Toxicol. 2013;113:391–400.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Kim SL, Kim SH, Trang KT, Kim IH, Lee SO, Lee ST, et al. Synergistic antitumor effect of 5-fluorouracil in combination with parthenolide in human colorectal cancer. Cancer Lett. 2013;335:479–86.
Patel NM, Nozaki S, Shortle NH, Bhat-Nakshatri P, Newton TR, Rice S, et al. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-kappaB is enhanced by IkappaBalpha super-repressor and parthenolide. Oncogene. 2000;19:4159–69.
Zhang D, Qiu L, Jin X, Guo Z, Guo C. Nuclear factor-kappaB inhibition by parthenolide potentiates the efficacy of Taxol in non-small cell lung cancer in vitro and in vivo. Mol Cancer Res. 2009;7:1139–49.
Fang LJ, Shao XT, Wang S, Lu GH, Xu T, Zhou JY. Sesquiterpene lactone parthenolide markedly enhances sensitivity of human A549 cells to low-dose oxaliplatin via inhibition of NF-kappaB activation and induction of apoptosis. Planta Med. 2010;76:258–64.
Marchetti P, Galla DA, Russo FP, Ricevuto E, Flati V, Porzio G, et al. Apoptosis induced by oxaliplatin in human colon cancer HCT15 cell line. Anticancer Res. 2004;24:219–26.
Wyrębska A, Gach K, Janecka A. Combined effect of parthenolide and various anti-cancer drugs or anti-cancer candidate substances on malignant cells in vitro and in vivo. Mini Rev Med Chem. 2014;14:222–8.
Aoki Y, Sakogawa K, Hihara J, Emi M, Hamai Y, Kono K, et al. Involvement of ribonucleotide reductase-M1 in 5-fluorouracil-induced DNA damage in esophageal cancer cell lines. Int J Oncol. 2013;42:1951–60.
Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y, et al. The sesquiterpene lactone parthenolide in combination with docetaxel reduces metastasis and improves survival in a xenograft model of breast cancer. Mol Cancer Ther. 2005;4:1004–12.
Acknowledgments
This work was financed by the Medical University of Lodz (No. 502-14-191 to KG and No. 503/1-156-02/503-01) and by the Ministry of Science and Higher Education (Project No. N N204 005736).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gach, K., Szymański, J., Pomorska, D. et al. Combined effects of anticancer drugs and new synthetic α-methylene-δ-lactones on MCF-7 cells. Tumor Biol. 36, 5971–5977 (2015). https://doi.org/10.1007/s13277-015-3273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3273-x