Abstract
Osteosarcoma is the most common primary malignant tumor in children and young adults, and the molecular regulation of the invasion of osteosarcoma (OS) remains unknown. In this study, we found that increased expression of ALX1 was associated with the progression of osteosarcoma and that ALX1 protein levels were significantly elevated in matched distant metastases. High ALX1 levels also predict shorter overall survival of osteosarcoma patients. We investigated the therapeutic potential of targeting ALX1 expression using the technique of RNA silencing via short hairpin RNA (shRNA). Synthetic shRNA duplexes against ALX1 were introduced to downregulate the expression of ALX1 in a highly malignant osteosarcoma cell line, U2OS. The results obtained indicated that shRNA targeting of ALX1 could lead to an efficient and specific inhibition of endogenous ALX1 activity. Furthermore, we found that depletion of ALX1 caused a dramatic cell cycle arrest, followed by massive apoptotic cell death, and eventually resulted in a significant decrease in migration and invasion of the osteosarcoma cell line studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteosarcoma (OS) is the most common malignant bone tumor in children and adolescents [1–3]. Many patients experience tumor recurrence following treatment with current osteosarcoma therapy, which consists of chemotherapy and surgery. The distal femoral and proximal tibial metaphyses are the most common sites for development of osteosarcoma. Although combined chemotherapy and surgery have been applied, the 5-year survival of patients with no metastatic disease at diagnosis reaches about 70 % [4]. Nevertheless, the 5-year survival of patients with metastatic cancer becomes as low as about 20 %. However, the molecular regulation of the invasion of osteosarcoma remains elusive. To that point, there is an urgent need for studying the tumor biology of OS in order to increase our understanding so as to treat it more efficiently.
In this study, we found that Aristaless-like homeobox1 (ALX1), also known as Cart1, is important for the induction of morphologic changes in OS cells. A study on ALX1 expression levels in several OS tissue was carried out. In order to investigate the possibility of turning ALX1 into a novel therapeutic target for the treatment of osteosarcoma, U2OS was chosen to silence the expression of ALX1 with the highly specific post-transcriptional suppression of RNAi. Thereafter, proliferation, cell cycle status, and apoptosis were also studied. The results obtained suggest that targeting of ALX1 may be used as a potential and specific therapeutic tool for the treatment of human osteosarcoma.
Materials and methods
Immunohistochemistry
Primary osteosarcoma biopsies of 60 patients and normal bone tissue specimens were collected between June 2000 and December 2005 according to the regulations of the local ethical committee. Immunohistochemistry using anti-ALX1 antibodies was performed as previously described [5]. Briefly sections were deparaffinized in xylene, rehydrated in alcohol and water, antigen repaired, and blocked. Anti-ALX1 antibodies (1:150) were incubated overnight, followed by incubation with horseradish peroxidase-labeled polymer for 20 min. Sections were then stained with DAB for 5 min. All sections were counterstained with hematoxylin, dehydrated, and mounted. Scoring was performed blindly by a pathologist according to the semiquantitative seventier system developed by Allred et al. [6]. This system assesses the percentage of positive cells (none = 0, <10 % = 1, 10–50 % = 2, and >50 % = 3) and the intensity of staining (none = 0, weak = 1, intermediate = 2, and strong = 3). The intensity and percentage scores are then added to give a final immunoreactivity score ranging from 0 to 6.
Cell culture
U2OS cell line was purchased from American Type Culture Collection. Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20 % fetal bovine serum (Invitrogen, Carlsbad, CA, USA). The cells in which ALX1 had been knocked down (U2OS ALX1-shRNA) and the control cells (U2OS control-shRNA) have been described and used in previous study [7]. They were made by the stable transfection with a shRNA-expressing vector against ALX1 and a scrambled shRNA, respectively. All the cells were maintained in DMEM supplemented with 10 % FBS at 37 °C in a 5 % CO2 and humidified atmosphere.
Stable knockdown cell line generation
A vector-based RNAi approach was used to generate the stable ALX1 knockdown cell line. Briefly, according to the corresponding RNAi sequences used in transient experiment, the double-stranded short hairpin RNA (shRNA) template for both the negative control and ALX1 were designed and cloned into the Hind III/Bgl II sites of the pSUPER.retro.neo+GFP vector (Oligoengine Corporation), respectively. The recombinant RNAi constructs (pSUPER-Control-shRNA and pSUPER-ALX1-shRNA) were confirmed by direct sequencing and transfected into U2OS cells by Lipofectamine2000 (Invitrogen), respectively. The transfected cells were then subcultured and selected in the presence of G418 for generating the negative control and ALX1 stable knockdown cells, designated as pControl-sh and pALX1-sh, respectively.
Migration assay
Cell motility was tested in 8-μm-pore polycarbonate membrane Transwell chambers (Corning) essentially as described previously [8]. Cells were resuspended in DMEM/F12 without fetal bovine serum, and 75,000 cells were added to the top chamber of the Transwell chambers. DMEM/F12 containing 10 % FBS was added to the bottom chamber, and cells were allowed to migrate for 24 h. Nonmigrated cells were scraped off the top of the membrane. Migrated cells were fixed in 4 % formaldehyde and stained in Giemsa. Cells were counted under a microscope in five different fields in duplicate wells, in at least three independent experiments.
Apoptosis assay
Apoptosis was assayed using the Annexin V-FITC Apoptosis Kit (keygen, China) according to the manufacturer’s instructions. Briefly, the cells were harvested and washed twice with PBS, followed by resuspension in Annexin-V binding buffer, and then FITC-conjugated Annexin V and PI were added. After incubation for 10 min at room temperature in the dark, another binding buffer was added, and the samples were immediately analyzed using FCM.
Quantitative real-time PCR
Total RNA was extracted from cultured cells using EZNA Total Rna Kit (OMEGA Bio-tek, USA), and complementary DNA (cDNA) was generated using PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Otsu, Japan). Quantitative real-time PCR was performed using the SYBR Premix ExTaq II (TLiRNaseH Plus) (TaKaRa, Otsu, Japan) with a CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA).
Western blot analysis
Cells were lysed with RIPA buffer (Beyotime, China) and boiled for 5 min. The protein concentration of each lysate was measured using the BCA method (Beyotime, China). Equal quantities of protein from each cell lysate were separated on SDS-polyacrylamide electrophoresis gels and transferred to PVDF membranes (Millipore, Billerica, MA). The membranes were blocked with 5 % skimmed milk, incubated with each primary antibody overnight at 4 °C, washed with TBS-T buffer (10 mM Tris–HCl pH 7.4, 150 mM NaCl, 0.05 % Tween20) and incubated with secondary antibodies. The proteins were visualized using enhanced chemiluminescence (GE Healthcare Biosciences)
Statistical analysis
All variables between groups were compared using the Pearson X 2 test or Student’s t test. P < 0.05 was considered statistically significant. Numerical data were calculated using Microsoft Excel and analyzed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
ALX1 is over-expressed in human osteosarcoma
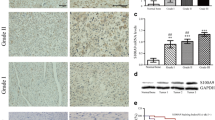
ALX1 immunohistochemistry was performed as outlined in “Materials and methods” on an osteosarcoma tissue, consisting of tissue cores of primary tumors collected from 60 patients with a mean age of 22 years (range 5 to 66 years) (Fig. 1a, b). Twenty-two patients had metastatic disease, and five of them presented with metastases at diagnosis. A Kaplan–Meier survival analysis showed that, irrespective of metastatic or local disease, 37 patients with immunohistochemically detectable expression of ALX1 in tumor tissue had a significantly (p = 0.012) shorter overall survival of 35 ± 9 (mean ± SE) months than 23 patients with nondetectable ALX1 expression and a mean overall survival of 69 ± 13 months (Fig. 1c).
Expression of ALX1 in human osteosarcoma correlates with poor patient survival. a ALX1 immunostaining of a tissue microarray consisting of control normal bone tissue and specimens from osteosarcoma biopsies. b ALX1 immunostaining of a randomly selected representative tumor specimen (below) and nonspecific control staining of a corresponding tissue specimen in the absence of primary ALX1 antibodies (up). c Kaplan–Meier plots of ALX1 expression in 20 cases of osteosarcoma patients. Overall survival rate was performed by log-rank test
ALX1 depletion inhibits the migration and invasiveness of U2OS cells
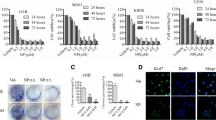
To explore the role of ALX1 in maintaining the migratory and invasive traits in OS cells, we then examined the effects of ALX1 depletion in the cell lines with high ALX1 expression using a monolayer wound-healing assay and a Matrigel-based Boyden chamber invasion assay. Firstly, quantitative RT-PCR and Western blot analysis demonstrated that ALX1 expression was significantly inhibited at both mRNA and protein levels 48 h after transfection, whereas the expression of actin was unchanged (Fig. 2a). Figure 2b shows a representative photograph of cell migration, and Fig. 2c shows a statistical analysis of the effects of depletion of ALX1 on the migration. The percentage of relative migration distance in the ALX1-shRNA cell clone whose ALX1 had been silenced, when compared with the percentage of relative migration distance in parent (control-shRNA, 54 ± 6.6), is reduced. The data indicate that silencing ALX1 decreased the cell migration, suggesting increased expression of ALX1 in U2OS contributes to the elevated migration of these cells. Figure 2a shows a representative photograph of the cell invasion, and Fig. 2b shows the statistical analysis of the effects of silencing ALX1 on cell invasion. Numbers of invasive cells in the ALX1-shRNA cell clone, when compared with numbers of invasive cells in parent (U2OS: 410 ± 21.1) and transfection control (control-shRNA: 422 ± 21.1). The data indicate that silencing ALX1 decreased the cell invasion, suggesting that high expression of ALX1 in U2OS contributes to the elevated cell invasion.
ALX1 depletion inhibits migration and invasion in osteosarcoma. U2OS cells were transiently transfected with the control shRNA and ALX1 shRNA, respectively. Following shRNA transfection, cells were subjected to would healing assay (a), transwell migration (b), and Matrigel invasion assay (c), respectively. *P < 0.05
ALX1 depletion inhibits osteosarcoma cells tumorigenesis in vivo
To examine whether depletion of ALX1 expression could inhibit the tumorigenicity in vivo, the stable ALX1 knockdown and the control cells were injected subcutaneously into nude mice. As shown in Fig. 3, silencing of ALX1 expression remarkably inhibited the tumor growth in both weight and size in nude mice. Mice were sacrificed 36 days after tumor cell injection and the tumor weight was determined, tumor weight and tumor size of ALX1 knockdown group (0.12 ± 0.04 g, 86 ± 39 mm3) was only 9 and 12 % of the control group (1.3 ± 0.4 g, 720 ± 212 mm3) (Fig. 3c).
ALX1 knockdown inhibits osteosarcoma cells tumorigenicity in vivo. a ALX1 knockdown cells (shCtrl) and control cells (shALX1) were injected subcutaneously into the dorsal flanks of nude mice, respectively. b The tumor size was measured about 5 days for tumor growth curve construction. c The tumor weight was measured at the end of experimental.*P < 0.05
ALX1 depletion enhanced the apoptosis of U2OS cells
Furthermore, we determined whether or not ALX1 depletion resulted in apoptosis in osteosarcoma cells because ALX1 depletion has been shown to induce apoptosis in breast cancer cells. Flow cytometry analysis indicated that the cells with DNA content increased dramatically at later stages after transfection, suggesting that ALX1-depleted cells undergo apoptosis (Fig. 4a). About 14–76 % of ALX1-depleted cells displayed G1 DNA 72 h after transfection, whereas only 3–5 % of control cells had this phenotype.
ALX1 depletion induces apoptosis and affects cell cycle progression in osteosarcoma. a Cells were harvested and then stained with Annexin/PI for apoptosis detection. The basal level of apoptosis in was 4.94 %, in the shCtrl and shALX1 were 3.08 and 11.34 %. Significant differences in cell apoptosis were noticed among between groups (*P < 0.05). b The effect of ALX1 depletion on cell cycle distribution as shown by FCM. Cells were collected and then stained with propidium iodide (PI). ALX1 silencing induced an obvious increase in the number of cells at G0/G1 phase and reduction in S phase; there were significant differences between shCtrl and shALX1 (*P < 0.05). c Western blot analysis of cyclin D1 and p21 in shCtrl and shALX1 U2OS
ALX1 depletion induces mitotic cell cycle arrest
Next, we analyzed the effect of ALX1 depletion on cell cycle progression using flow cytometry. ALX1 depletion induced an obvious increase in the number of cells at G0/G1 phase and reduction in S phase, as 77.10 % of the U2OS cell in shALX1 were noticed at G0/G1 phase, compared to 63.75 and 64.84 % cells in the control and shCtrl, respectively (Fig. 4b). Western blot results clearly showed a reduction in the expression of cyclin D1 and an increase of p21 in shALX1 compared to the blank control and shCtrl (Fig. 4c).
Discussion
The analysis of novel potential cancer-associated genes is of importance for developing diagnostic, preventive, and therapeutic strategies for cancer treatment and management. Osteosarcoma is the most common primary malignant bone tumor in children and young adults with a high propensity for metastasis, predominantly to the lung, and consequently is associated with poor prognosis [9–12]. Osteosarcoma treatment has undergone dramatic changes over the past 20 years, whereas the survival rate shows limited improvement. Thus so far, the 5-year survival rate is approximately 20 % with surgical treatment alone. Moreover, half of the patients exhibit pulmonary metastasis, which results in high patient mortality [2, 13]. Thus, chemotherapy is typically employed in an adjuvant basis for improving the prognosis and long-term survival. However, recurrence frequently manifests as pulmonary metastasis or, less frequently, metastasis to distant bones or as a local recurrence [14–17]. Thus, a novel strategy that would effectively inhibit metastasis, particularly to the lungs, from the primary osteosarcoma site is highly desirable [18–21].
These in vitro and vivo study were an effort to investigate if ALX1 could be exploited as a novel therapeutic target for the treatment of human osteosarcoma cancer. We have found that the specific shRNA-mediated depletion of ALX1 leads to a significant decrease in cell migration, with mitotic arrest followed by massive apoptosis in the human osteosarcoma cancer cell line studied. Moreover, we also found that depletion of ALX1 reduced tumorigenicity in nude mice in vivo. These findings indicated that ALX1 played an important role in tumorigenesis. Our data strongly demonstrated that, apart from being of diagnostic value in osteosarcoma, inhibition of ALX1 in osteosarcoma may additionally serve to be of therapeutic value.
In summary, our present study strongly demonstrated that the specific shRNA-mediated silencing of ALX1 resulted in the elimination of osteosarcoma cells via the inactivation of p21/cyclin B1-mediated mitotic cell cycle arrest followed by massive apoptotic cell death. Therefore, ALX1 may serve as a potential target in the treatment of human osteosarcoma. Consequently and conceivably, gene therapeutic approaches and/or pharmacological small molecule inhibitors aimed at ALX1 may be developed for the management of osteosarcoma.
References
Siclari VA, Qin L. Targeting the osteosarcoma cancer stem cell. J Orthop Surg Res. 2010;5:78.
Gorlick R, Meyers PA. Osteosarcoma necrosis following chemotherapy: innate biology versus treatment-specific. J Pediatr Hematol Oncol. 2003;25:840–1.
Pakos EE, Nearchou AD, Grimer RJ, Koumoullis HD, Abudu A, Bramer JA, et al. Prognostic factors and outcomes for osteosarcoma: an international collaboration. Eur J Cancer. 2009;45:2367–75.
Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol. 2006;125:555–81.
Simmons LW, Lovegrove M and Almbro M. Female effects, but no intrinsic male effects on paternity outcome in crickets. J Evol Biol 2014.
Allred DC, Clark GM, Elledge R, Fuqua SA, Brown RW, Chamness GC, et al. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993;85:200–6.
Yuan H, Kajiyama H, Ito S, Yoshikawa N, Hyodo T, Asano E, et al. ALX1 induces snail expression to promote epithelial-to-mesenchymal transition and invasion of ovarian cancer cells. Cancer Res. 2013;73:1581–90.
Li H, Chen X, Gao Y, Wu J, Zeng F, Song F. XBP1 induces snail expression to promote epithelial-to-mesenchymal transition and invasion of breast cancer cells. Cell Signal. 2015;27:82–9.
Wang Q, Cai J, Wang J, Xiong C and Zhao J. MiR-143 inhibits EGFR-signaling-dependent osteosarcoma invasion. Tumour Biol 2014.
Savage SA, Mirabello L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma. 2011;2011:548151.
Nathan SS, Pereira BP, Zhou YF, Gupta A, Dombrowski C, Soong R, et al. Elevated expression of Runx2 as a key parameter in the etiology of osteosarcoma. Mol Biol Rep. 2009;36:153–8.
Li H, Song F, Chen X, Li Y, Fan J, Wu X. Bmi-1 regulates epithelial-to-mesenchymal transition to promote migration and invasion of breast cancer cells. Int J Clin Exp Pathol. 2014;7:3057–64.
Li H, Chen X, Gao Y, Wu J, Zeng F, Song F. XBP1 induces snail expression to promote epithelial- to-mesenchymal transition and invasion of breast cancer cells. Cell Signal. 2015;27:82–9.
Endo-Munoz L, Evdokiou A, Saunders NA. The role of osteoclasts and tumour-associated macrophages in osteosarcoma metastasis. Biochim Biophys Acta. 1826;2012:434–42.
Perbal B, Zuntini M, Zambelli D, Serra M, Sciandra M, Cantiani L, et al. Prognostic value of CCN3 in osteosarcoma. Clin Cancer Res. 2008;14:701–9.
Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–83.
Mann KK, Wallner B, Lossos IS, Miller Jr WH. Darinaparsin: a novel organic arsenical with promising anticancer activity. Expert Opin Investig Drugs. 2009;18:1727–34.
Mei Q, Li F, Quan H, Liu Y, Xu H. Busulfan inhibits growth of human osteosarcoma through miR-200 family microRNAs in vitro and in vivo. Cancer Sci. 2014;105:755–62.
Liu Y, Wang W, Xu J, Li L, Dong Q, Shi Q, et al. Dihydroartemisinin inhibits tumor growth of human osteosarcoma cells by suppressing Wnt/beta-catenin signaling. Oncol Rep. 2013;30:1723–30.
Zhao Q, Wang C, Zhu J, Wang L, Dong S, Zhang G, et al. RNAi-mediated knockdown of cyclooxygenase2 inhibits the growth, invasion and migration of SaOS2 human osteosarcoma cells: a case control study. J Exp Clin Cancer Res. 2011;30:26.
Wu YF, Liang XJ, Liu YY, Gong W, Liu JX, Wang XP, et al. +Antisense oligonucleotide targeting survivin inhibits growth by inducing apoptosis in human osteosarcoma cells MG-63. Neoplasma. 2010;57:501–6.
Conflicts of interest
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, M., Pan, Y. & Zhou, Y. Depletion of ALX1 causes inhibition of migration and induction of apoptosis in human osteosarcoma. Tumor Biol. 36, 5965–5970 (2015). https://doi.org/10.1007/s13277-015-3271-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3271-z