Abstract
CD68 has been widely used as a pan-macrophage marker for tumor-associated macrophages (TAM) which always involve in carcinogenesis. But the correlations between CD68+ TAMs and prognosis of patients show to be inconsistent, which might due to lack of specific markers of TAMs. We here found that the microlocalization of CD68+ TAMs also played a unique role in prognosis of patients with oral squamous cell carcinoma (OSCC). CD68+ TAMs were identified in paraffin-embedded OSCC specimens (n = 91) by using immunohistochemistry. The number of CD68+ TAMs was remarkably increased from adjacent none-neoplasia tissues (NT) to tumor nest (TN), but tumor stroma (TS) was infiltrated with highest frequency of CD68+ TAMs (P < 0.0001). Unexpectedly, more CD68+ TAMs in TS, but not NT or TN, were associated with high tumor grade (P = 0.033), lymph node metastasis (P = 0.034), and shorter 10-year overall survival time, disease free survival. Considering TAMs was derived from monocytes in peripheral blood, we assessed the relationship between leukocytes in peripheral blood and CD68+ TAMs in OSCC and found that more CD68+ TAMs in TS were accompanied with decreased monocytes and lymphocytes in peripheral blood (P < 0.05). Although Cox regression analysis revealed that CD68+ TAMs in TS were not an independent prognostic factor for OSCC patients, we raised a possibility that the microlocalization of CD68+ TAMs was an indispensable factor for the advance of OSCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic inflammation plays a key role in the initiation of carcinoma, which including oral squamous cell carcinoma (OSCC), and eventually gives impetus to the establishment of tumor microenvironment which consists of leukocytes, extracellular matrix, endothelial cells, and fibroblasts, all of which influence the fate of cancer cells and the clinical outcome [1]. Macrophages, which derive from circulating monocytes, play an important role in innate immunity and adaptive immunity [2, 3]. Due to the phenotypic plasticity, macrophages are often simplified as classical pro-inflammatory‘M1’or alternative anti-inflammatory‘M2’by phenotype difference. Tumor-associated macrophages (TAMs), a major cellular component in the tumor microenvironment, have been thought to be M2 macrophages [4, 5]. Importantly, CD68 has been widely used as a pan-macrophage marker in over 80 % of studies [6]. However, the correlations between CD68+ TAMs and prognosis of patients show to be inconsistent. Several reports found that the presence of CD68+ TAMs was associated with decreased 5-year survival rates in several cancer including thyroid, lung, hepatocellular, and esophageal cancers [7–10]. But some studies showed that no significant associations were found between the percentage of CD68+ TAM and survival time for OSCC patients [11–13]. Therefore, researches on other more unique marker for TAMs, such as CD163, CD204, and vascular endothelial growth factor (VEGF) [14], were considered as an approach to explain the inconsistency of prognosis.
Of note, the matter of microlocalizations of CD68+ TAMs was noticed in some studies and it had been reported that the presence of CD68+ TAMs in tumor nest (TN) promoted tumor progression and was related to poor prognosis in hepatocellular cancer patients [15]. Infiltration of CD68+ macrophages in breast tumor stroma (TS) were positively associated to tumor size and were an independent prognostic factor [16]. But the impacts of microlocalizations of CD68+ TAMs on clinical outcomes of OSCC patients were limited.
Here, we focused on the microlocalizations of CD68+ TAMs in OSCC and analyzed the correlations of distinct microlocalizations of CD68+ TAM with the clinicopathological parameters and survival time including 10-year overall survival time and disease free survival. As the high infiltration of TAMs in tumor was mainly from circulating mononuclear precursors, we determined the relationship between leukocytes in peripheral blood and CD68+ TAMs in OSCC. Our results suggest that TMAs infiltration in different compartments of tumor section may play a different role in the progression of OSCC.
Materials and methods
Patients and tissue specimens
This retrospective study collected 91 patients randomly who were diagnosed with primary OSCC by Department of Pathology at Nanjing Stomatology Hospital between 2003 and 2011, and ethical approval for the use of oral squamous cell carcinoma specimens for this study was obtained from the Research Ethics Committee of Nanjing Stomatology Hospital. Among all the subjects, pregnant, lactating individuals, and patients who were diagnosed other malignant diseases or some autoimmune diseases (e.g., rheumatoid arthritis, systemic lupus erythematous, diabetes, etc.) were excluded from our experimental group, and all the patients had no long history of alcohol abuse. None of the patients underwent preoperative chemotherapy and/or radiotherapy. All patients were followed up until 1 March 2014. In this study, all the tissues of OSCC were evaluated according to WHO classifications by two pathologists.
Numbers of lymphocytes and monocytes in peripheral blood of OSCC patients were collected before surgery and measured by sysmex five classification blood cell analyzers.
Immunohistochemistry
CD68 expression was analyzed immunohistochemically on 2-μm-thick, formalin-fixed, and paraffin-embedded specimen sections. Briefly, the sections were incubated in three washes of xylene for 5 min each and were followed by two washes of 100 % ethanol for 10 min, 95 % ethanol for 10 min, and ddH2O for 5 min each. Antigen unmasking was prepared by boiling in pH 9.0, 10 mM Tris/1 mM EDTA, blocked with 3 % hydrogen peroxide for 10 min at room temperature, and washed. Then, anti-CD68 antibody (Cat No. ab955, diluted × 200, Abcam, USA), the primary antibodies used in this study, incubated the FFPE specimen sections at 4 °C overnight, and then the EnVision Detection System kit (DAKO, Denmark) was used for the DAB chromogen followed by nuclear staining using haematoxylin. Neutral gum was used to cover the sliders and dry at room temperature for counting. Positive staining controls were carried out with paraffin-embedded tonsil and breast cancer sections using CD68 antibodies.
Analysis of immunostaining
Cells stained brown under optical microscope (Olympus BX50 microscope, Southall, UK) were regarded as positive staining. For each patient tumor sample, three separated high-power fields were examined by NIS-Elements software and the mean number of was counted as CD68+ TAMs frequency within each microlocalization, including NT (normal tissue), TN, and TS, by two experienced pathologists, independently. Among the 91 OSCC patients, the number of infiltrated TAMs was sorted in ascending order and <75 % was defined as TAMs low-infiltration group and ≥75 % was defined as TAMs high-infiltration group as previously [17]. The 75th percentile of the infiltration number for NT, TN, and TS sections was 5, 36, 53, and 89 cells/HPF.
Statistical analysis
Statistical package for social sciences version 16.0 (SPSS 16.0, SPSS Inc., Chicago, IL, USA) and Prism statistical software package (version 5.0, Graphpad Software Inc.) were used for statistical analyses. Kolmogorov–Smirnov and Shapiro–Wilk tests showed that the expression of CD68 in each tumor section did not follow a normal distribution. The Mann–Whitney U test was used to compare the two groups (e.g., CD68+ TAMs in NT versus in TN), and the differences between more than two groups (e.g., tumor stages I, II, III, and IV) were analyzed by the Kruskal–Wallis test. Kaplan–Meier survival curves and the log-rank statistic were used to analyze the correlations between CD68+ TAMs numbers and 10-year survival rate. Correlations between the numbers of infiltrating CD68+ TAMs and the numbers of leukocytes in peripheral blood were analyzed by Spearman’s Rho analysis. Cox proportional hazards regression was used for univariate and multivariate analysis of overall survival according to CD68 expression. Differences were considered statistically significant with P < 0.05.
Results
Patient characteristics and distribution of CD68+ TAMs in OSCC carcinoma
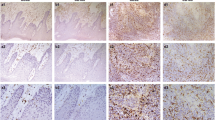
Ninety-one patients diagnosed with primary OSCC were included in the study and clinicopathologic parameters are indicated in Table 1. The mean age of these patients was 55 years with a range of 20–78 years, and the sex ratio of male-to-female approximates 0.7. TAMs were detected by CD68 antibody and positively stained cells often showed typical macrophage morphology. The positive cell number was counted by NIS-Elements software. We found few CD68+ TAMs resided in NT compartment (Fig. 1a) and sparsely present in the TN compartment (Fig. 1b) but mainly distributed in the TS compartment (Fig. 1c). In 79 of 91 (86.8 %) OSCC patients, CD68+ TAMs were found in NT compartment, TN and TS compartments. In 12 of 91 (13.2 %) patients, CD68+ TAMs were only observed in TN and TS compartments. In 3 of 91 patients (3.3 %), there were no CD68+ TAMs, no matter in NT, TN, or in TS compartments.
Numbers of CD68+ TAMs in NT, TN, and TS compartments were calculated separately (Fig. 2). Under the high-power field (HPF), the mean number of CD68+ cells in NT, TN, and TS was 6.4 ± 5.5 cells/HPF, 34.3 ± 13.9 cells/HPF, and 56.7 ± 24.9 cells/HPF, respectively. Moreover, the mean number of CD68+ TAMs in TS was the highest among all compartments (P < 0.0001). The number of CD68+ TAMs in NT compartment was lowest and was no more than 22 cells/HPF in the whole tissue sections (P < 0.0001). Hence, the majority of tumors were infiltrated with CD68+ TAMs and mainly in TS and TN compartments in OSCC.
Correlations of tumor infiltrating CD68+ TAMs in NT, TN, and TS with clinicopathologic characteristics
Next, we examined the correlations between the presence of CD68+ TAMs in NT, TN, and TS compartments and clinicopathologic characteristics, including patient age, gender, tumor stage, tumor differentiation, and lymph node positive/negative status. Data were shown in Table 1, the mean number of CD68+ TAMs in each of tumor samples was similar when compared between gender, age, tumor size, and tumor differentiation, respectively (P > 0.05). Numbers of CD68+ TAMs in TS within smoking group were lower than no-smoking group (P < 0.05). In contrast, there was significantly higher infiltration of CD68+ TAMs with TNM stages III–IV in TS compartments than it with TNM stages I–II (P < 0.05) patients. Furthermore, OSCC patients with lymph node metastasis had higher infiltration of TAMs in TS areas when compared to LNM negative patients (P < 0.05) which indicated that, for OSCC patients, high grade TNM stage and status of LNM were accompanied with more CD68+ TAMs infiltration in TS.
Correlation of the different microlocalization of CD68+ TAMs with survival time
During the follow-up period, two patients had disease recurrence and ten patients died, clinical and follow-up data were obtained from clinical records. All the 91 OSCC patients were divided into two groups consisted of TAM high-infiltration group and TAM low-infiltration group. Then the correlation between the overall survival time (OS), disease free survival time (DFS), and the CD68+ TAMs in different microlocalization including NT (Fig. 3 a, d), TN (Fig. 3 b, e), and TS (Fig. 3 c, f) was analyzed. The results demonstrated that cumulative survivals of the patients with high-infiltration group of TAMs in TS compartments correlated with poor OS and DFS (P < 0.05). However, there was no significant difference between the 10-year overall survival or disease free survival and the CD68+ TAMs infiltration in NT or TN compartment (P > 0.05).
Numbers of TAMs in TS were negatively correlated with the numbers of monocytes and lymphocytes in peripheral blood
As TAMs derived from monocytes in peripheral blood, so we raise a hypothesis that the infiltrating TAMs were associated with the change of the numbers of leukocytes in peripheral blood and found that monocytes number in peripheral blood was negatively related to the number of CD68+ TAMs in NT (P = 0.01, r = −0.28) (Fig. 4a), TS compartments (P = 0.02, r = −0.24) (Fig. 4c). However, there was no significant difference between the number of monocytes in peripheral blood and the TAMs infiltration in TN compartment (P = 0.59, r = 0.06) (Fig. 4b).
The relationship between CD68+ TAM infiltration in a normal tissue, b tumor nest, or c tumor stroma and the numbers of monocytes in peripheral blood. Besides, the relationship between the numbers of lymphocytes in peripheral blood and CD68+ TAM infiltration in d normal tissue, e tumor nest, and f tumor stroma was assessed
Moreover, correlations of TAMs numbers in NT, TN, and TS compartments with the number of lymphocytes in peripheral blood were assessed. Results demonstrated that there was a significantly negative correlation between lymphocytes numbers and the TAM numbers in NT, TS compartments (P = 0.019, r = −0.25; P = 0.049, r = −0.21 (Fig. 4d, f) but not in TN compartment (P = 0.07, r = 0.20) (Fig. 4e).
Microlocalization of CD68+ TAMs was not an independent risk factor for overall survival in OSCC
Univariate analyses revealed that age, smoke, TNM, differentiation, LNM, tumor size, and leukocytes counts in blood were not significant associated with overall survival (P > 0.05), but CD68+ TAMs infiltration in TS showed to be a risk factor for the overall survival of OSCC patients (P = 0.033). However, multivariate analyses implicated that microlocalization of CD68+ TAMs in TS was not an independent risk factor for overall survival in OSCC (Table 2).
Discussion
Different microlocalization of infiltrating immune cells renders distinct interaction with tumor and stromal cells. Therefore, the complex cancer cellular ecology makes it necessary to investigate specific function of immune cell infiltration in the anatomically different compartments. Our studies demonstrated higher numbers of CD68+ TAMs in TS were correlated with high tumor grade and lymph node metastasis. More infiltration of TAMs in TS was correlated with short OS and DFS of OSCC patients. For the first time, we found that the microlocalization of CD68+ TAMs in TS predicted poor clinical outcomes and was negatively correlated with monocytes and lymphocytes in PBMCs of OSCC patients.
More than 300,000 new cases worldwide are diagnosed with OSCC each year [18]. The progression of OSCC involved with abnormal proliferation of oral keratinocytes by HPV infection, smoking, alcohol, and chewing betel quid. Such stimulators in oral lesion sites for a long-term would induce inflammatory environment via the recruitment of various immunocytes and promote tumor growth, progression, and invasion. Currently, there still lack valid therapeutic target and effective prognosis or diagnosis biomarkers for OSCC in early clinical test.
As a pan-macrophage marker, CD68 was widely used as a marker for TAMs and recently studies have found that different localization of TAM was linked to different prognosis of patients. In the current study, we found that brown staining of TAM presented both in NT, TS, and TN compartments among 79 patients (86.8 %), but three patients (3.3 %) were totally CD68 negative in NT, TN, and TS areas. This was consistent with previous research on OSCC [19–21]. When compared with the numbers of CD68+ TAMs to clinicopathological parameters, we found TNM stage and lymph node metastasis correlated to high frequency of CD68+ TAMs infiltration in TS, although tumor size and differentiation seemed to be unrelated to the numbers of infiltrated TAM in this study. Vilas Boˆas et al. have also found that tumor differentiation was not significantly related to CD68+ TAMs inflammatory infiltration in OSCC (n = 27) [19]. In addition, another independent research on evaluating the distribution of cancer-associated fibroblasts (CAFs) and TAMs in OSCC (n = 98) indicated that there was no relationship between TAMs and differentiation but the tumor size was positively related to high-expression CD68 TAMs [21]. A study from Liu et al. showed that elevated TAM infiltration inside and around tumor section was observed, and there was a significant correlation between CD68+ TAMs and tumor size and LNM (P < 0.05) in OSCC (n = 112) [22]. These differences may be caused by the different study cohort and analysis method, they have not pay attention to impact of the microlocalization of TAMs on OSCC progression. Besides, methods to acquire the cut-off value of numbers of CD68+ TAMs were different.
Although a reduced survival rate had been found to be associated with high frequency of TAMs in the majority of solid cancers, such as breast cancer, prostate cancer, gastric, and esophageal squamous cell carcinoma [8, 23–25], the prognosis value of CD68+ TAMs infiltration in OSCC was poorly known and few study found that the CD68 expression in the whole tumor tissue was not a prognostic indicator for OSCC but the CD163 was [21]. However, our study showed that high infiltration of CD68+ TAMs in TS, but not in NT or TN, correlated with poor OS or DFS of OSCC patients (P < 0.05).
Once the body sensed the red flag from infection or inflammation, bone marrow progenitors would enter peripheral circulation system and differentiate into monocytes which would migrate into the inflammation compartment and differentiate into macrophage [2, 15]. M-CSF and VEGF secreted by tumor cells promoted the transduction and phenotypic conversion of macrophages, these macrophages turned into M2-like macrophages and in turn produced IL-10 and prostaglandin E2 (PGE2) to support the tumor progression and immunosuppression which was associated with the change of leukocytes ratio in peripheral blood [8]. For the first time, we demonstrated a negative relationship between CD68 stain in NT, TS, and the monocytes or lymphocytes numbers in OSCC patients (P < 0.05).
Cox regression analyses revealed that CD68 in TS or TN was not independent risk factor for OS in OSCC, but in TS of breast cancer it was an independent risk factor for BCSS (breast cancer-specific survival) and RFS (recurrence free survival), but it was not an independent risk factor for OS which was in accordance with the previous reports on renal cell carcinoma and prostate cancer [23, 26]. Another research on breast cancer showed that the number of CD68+ TAMs in TS was an independent predictor of survival rate for breast cancer patients which were in accordance with the previous reports on gastric cancer [25]. Therefore, a large cohort of samples should be included for further investigation on the prognostic factor of TAMs in different microlocalization.
In conclusion, we have revealed that high infiltration of CD68+ TAMs in TS was linked to higher TNM stage, LNM, and short OS or DFS. Besides, a reduced numbers of monocytes and lymphocytes correlated to high infiltrated CD68+ TAMs in TS, although CD68 in TS was not independent risk factor for OSCC patients. Our results suggest that microlocalization of CD68+ TAMs in tumor stroma predicted poor clinical outcome for OSCC patients.
References
Rao SK, Pavicevic Z, Du ZY, Kim JG, Fan MY, Jiao Y, et al. Pro-inflammatory genes as biomarkers and therapeutic targets in oral squamous cell carcinoma. J Biol Chem. 2010;285(42):32512–21. doi:10.1074/jbc.M110.150490.
Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–74. doi:10.1038/Nri3070.
Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–44. doi:10.1146/annurev.immunol.23.021704.115816.
Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109(8):2796–801. doi:10.1073/pnas.1104303108.
Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–27. doi:10.1016/j.ejca.2006.01.003.
Gottfried E, Kunz-Schughart LA, Weber A, Rehli M, Peuker A, Muller A, et al. Expression of CD68 in non-myeloid cell types. Scand J Immunol. 2008;67(5):453–63. doi:10.1111/j.1365-3083.2008.02091.x.
Chen JJW, Lin YC, Yao PL, Yuan A, Chen HY, Shun CT, et al. Tumor-associated macrophages: the double-edged sword in cancer progression. J Clin Oncol. 2005;23(5):953–64. doi:10.1200/Jco.2005.12.172. 23.
Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol. 2004;99(9):1667–74. doi:10.1111/j.1572-0241.2004.30733.x.
Ryder M, Ghossein RA, Ricarte JCM, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endoc-Relat Cancer. 2008;15(4):1069–74. doi:10.1677/Erc-08-0036.
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26(16):2707–16. doi:10.1200/Jco.2007.15.6521.
Costa NL, Valadares MC, Souza PP, Mendonca EF, Oliveira JC, Silva TA, et al. Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. 2013;49(3):216–23. doi:10.1016/j.oraloncology.2012.09.012.
He KF, Zhang L, Huang CF, Ma SR, Wang YF, Wang WM, et al. CD163+ tumor-associated macrophages correlated with poor prognosis and cancer stem cells in oral squamous cell carcinoma. Bio Med Research Int. 2014;2014:838632. doi:10.1155/2014/838632.
Boas DS, Takiya CM, Gurgel CA, Cabral MG, Santos JN. Tumor-infiltrating macrophage and microvessel density in oral squamous cell carcinoma. Braz Dent J. 2013;24(3):194–9. doi:10.1590/0103-6440201302049.
Cook J, Hagemann T. Tumour-associated macrophages and cancer. Curr Opin Pharmacol. 2013;13(4):595–601. doi:10.1016/j.coph.2013.05.017.
Zhou J, Ding T, Pan WD, Zhu LY, Li L, Zheng LM. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125(7):1640–8. doi:10.1002/Ijc.24556.
Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi:10.1186/1471-2407-12-306.
Sandel MH, Dadabayev AR, Menon AG, Morreau H, Melief CJ, Offringa R, et al. Prognostic value of tumor-infiltrating dendritic cells in colorectal cancer: role of maturation status and intratumoral localization. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11(7):2576–82. doi:10.1158/1078-0432.CCR-04-1448.
Jemal A, Siegel R, Ward E, Murray T, Xu JQ, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–30.
Boas DSV, Takiya CM, Coelho-Sampaio TL, Moncao-Ribeiro LC, Ramos EAG, Cabral MG, et al. Immunohistochemical detection of Ki-67 is not associated with tumor-infiltrating macrophages and cyclooxygenase-2 in oral squamous cell carcinoma. J Oral Pathol Med. 2010;39(7):565–70. doi:10.1111/j.1600-0714.2010.00883.x.
El-Rouby DH. Association of macrophages with angiogenesis in oral verrucous and squamous cell carcinomas. J Oral Pathol Med. 2010;39(7):559–64. doi:10.1111/j.1600-0714.2010.00879.x.
Fujii N, Shomori K, Shiomi T, Nakabayashi M, Takeda C, Ryoke K, et al. Cancer-associated fibroblasts and CD163-positive macrophages in oral squamous cell carcinoma: their clinicopathological and prognostic significance. J Oral Pathol Med. 2012;41(6):444–51. doi:10.1111/j.1600-0714.2012.01127.x.
Liu SY, Chang LC, Pan LF, Hung YJ, Lee CH, Shieh YS. Clinicopathologic significance of tumor cell-lined vessel and microenvironment in oral squamous cell carcinoma. Oral Oncol. 2008;44(3):277–85. doi:10.1016/j.oraloncology.2007.02.007.
Gollapudi K, Galet C, Grogan T, Zhang H, Said JW, Huang J, et al. Association between tumor-associated macrophage infiltration, high grade prostate cancer, and biochemical recurrence after radical prostatectomy. Am J Cancer Res. 2013;3(5):523–9.
Mahmoud SMA, Lee AHS, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65(2):159–63. doi:10.1136/jclinpath-2011-200355.
Pantano F, Berti P, Guida FM, Perrone G, Vincenzi B, Amato MMC, et al. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. J Cell Mol Med. 2013;17(11):1415–21. doi:10.1111/Jcmm.12109.
Xu L, Zhu Y, Chen L, An H, Zhang W, Wang G, et al. Prognostic value of diametrically polarized tumor-associated macrophages in renal cell carcinoma. Ann Surg Oncol. 2014. doi:10.1245/s10434-014-3601-1.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 81402238, 81072213, 81271698), the Nanjing Medical Science & Research Project (No. YKK13145), Nanjing Medical Young engineer (QRX113311), National Key Disciplines Constructional Project Funding (Since 2011), Jiangsu Provincial Clinical Medicine of Science and Technology project (Grant No BL2012017), Nanjing Municipal Key Medical Laboratory Constructional Project Funding (Since 2012), Center of Nanjing Clinical Medicine of tumor project (Since 2014).
Conflict of interest
The authors have no conflict of interest related to this publication.
Authors’ Contributions
YH. N and L.D participated in the design of experimental protocols and drafted the manuscript. L.D. and XF.H. participated in the data collection and immunohistochemical staining and data collection. L.D.,YH.N., and YC.D. performed the statistical analysis and participated in manuscript preparation. QG H and YH N conceived of this idea, outlined study design, and helped manuscript preparation. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ni, YH., Ding, L., Huang, XF. et al. Microlocalization of CD68+ tumor-associated macrophages in tumor stroma correlated with poor clinical outcomes in oral squamous cell carcinoma patients. Tumor Biol. 36, 5291–5298 (2015). https://doi.org/10.1007/s13277-015-3189-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3189-5