Abstract
We aimed to evaluate the clinical response to platinum-based chemotherapy and treatment outcome of gastric cancer patients in the present of ERCC1, ERCC2, NBN, RAD51, and XRCC3 gene polymorphisms. A number of 415 patients of gastric cancer that received platinum-based chemotherapy were enrolled in the present study. The presence of ERCC1 rs11615 and rs2298881, ERCC2 rs1799793 and rs13181, NBN rs1805794, rs709816, and RAD51 rs1801321 and XRCC3 rs1799794 were determined using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. Conditional regression analysis identified that CC genotype of ERCC1 rs11615 and AA genotype of ERCC2 rs1799793 was associated with a better response to chemotherapy in gastric cancer patients, and the odds ratio (ORs)(95% confidence interval (CI)) were 2.70(1.33–5.70) and 3.12(1.52–6.84), respectively. By the Cox analysis, the CC genotype of ERCC1 rs11615, AA genotype of ERCC2 rs1799793, and CC genotype of NBN rs1805794 were significantly associated with a longer overall survival (OS) of gastric cancer. In conclusion, our results suggest that ERCC1 rs11615, ERCC2 rs1799793, and NBN rs1805794 polymorphisms in the DNA repair pathways may influence the response to chemotherapy and OS of gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fourth most common cancer and the second most common cause of cancer-related death all over the world, especially in East Asia [1]. It is estimated that more than 70 % of gastric cases occur in developing countries, and half of the newly occurred gastric cancer cases were in China [1, 2]. Surgery for early-stage gastric cancer is used as a primary method, but many gastric cancer cases still present local or distant recurrence even when receiving similar treatment. Platinum-based chemotherapy for gastric cancer is considered as a systemic therapy following primary surgery or as neoadjuvant chemotherapy before surgery in patients with advanced gastric cancer [3].
The prognosis of gastric cancer cases was improved significantly through chemotherapy, and the main reasons are that the efficacy and toxicity showed are greatly individual in different patients [4, 5]. Recently, increasing evidences showed that many gene polymorphisms have an important role in influencing the response to chemotherapy, such as excision repair cross-complementation group 1 (ERCC1), excision repair cross-complementation group 4 (ERCC4), X-ray repair cross-complementing group 1 (XRCC1), and vascular endothelial growth factor (VEGF) genes in gastric cancer patients [6–10]. It is reported that platinum analog has a critical role in binding DNA and forming DNA adducts, both intrastrand and interstrand cross-links (ICLs), and inhibiting DNA replication [11]. Previous studies reported that DNA repair mechanisms are key factors in determining the response to platinum-based chemotherapy [6, 10]. DNA repair pathways are complex and have a role in many different enzymes. Enzymes in nucleotide excision repair (NER) pathway can affect many helix-distorting DNA lesions and cytotoxic DNA interstrand cross-links [12], and there are two common enzymes in the NER pathway, including excision repair cross-complementation group 2 (ERCC2) gene and excision repair cross-complementation group 1 (ERCC1), which are reported to be associated with chemotherapy response and prognosis of gastric cancer [6, 10].
Homologous recombination repair (HRR) plays an important role in successfully repairing other complex DNA damage. Nibrin (NBN) is one important enzyme in the HRR pathway, and it has a role in recognizing DNA damage and RAD51 recombinase (RAD51) and X-ray complementing defective repair in Chinese hamster cells 3 (XRCC3) catalyses homologous search and strand invasion. However, previous few studies reported the association between NBN gene polymorphisms and response to chemotherapy in gastric cancer [13, 14]. Consequently, the aim of this study was to evaluate the clinical response to platinum-based chemotherapy and treatment outcome of gastric cancer patients in the present of ERCC1, ERCC2, NBN, RAD51, and XRCC3 gene polymorphisms.
Patients and methods
Patients
Four hundred fifteen patients of gastric cancer that received platinum-based chemotherapy between May 2008 and May 2011 were enrolled in the present study from the Affiliated Hospital of Inner Mongolia Medical University. Patients were newly diagnosed and histopathologically confirmed gastric cancer, and patients with a history of pregnancy, malignancy, chemotherapy or radiotherapy, serious concomitant systemic disorder unable to receive chemotherapy, and metastasis with symptoms were excluded from the present study.
Baseline characteristics of all of the patients were obtained using a self-designed questionnaire and medical records. An informed consent form for blood sample collection for genotyping was obtained from the patients. This study was approved by the ethics committee of the Affiliated Hospital of Inner Mongolia Medical University.
Treatment outcome
The patients in the study were followed up until May 2013. Each patient was followed up by attending clinics or telephones every 1 month until death or the end of follow-up. Tumor response to chemotherapy was evaluated based on World Health Organization (WHO) criteria [15]. Tumor responses were evaluated by contrasted computed tomography scan and/or magnetic resonance imaging every 2 cycles to document complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Response was confirmed over 4 weeks later. Overall survival (OS) was calculated from the date of chemotherapy to the date of death or last clinical follow-up.
DNA extraction and genotyping
Each patient was asked to provide a 5-ml peripheral venous blood sample after participating into our study. Genomic DNA was extracted from peripheral blood lymphocytes using TIANamp Blood DNA Kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s protocol. The presence of ERCC1 rs11615 and rs2298881, ERCC2 rs1799793 and rs13181, NBN rs1805794 and rs709816, and RAD51 rs1801321 and XRCC3 rs1799794 was determined using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). The primers and probes for the eight gene polymorphisms were designed using Assay Design 3.1 software (Sequenom Inc., San Diego, CA, USA). The PCR conditions were as follows: an initial denaturation at 95 °C for 5 min, 35 cycles of amplification with denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, and extension at 72 ° C for 30 s, followed by a final extension step of 7 min at 72 ° C. For quality control, approximately 10 % of the patients were randomly selected to repeat the genotyping procedure with different researchers. The reproducibility was 100 %.
Statistical analysis
Continuous variables were shown by mean ± SD, and categorical variables were shown by number of subjects (%). The association between response to chemotherapy and the eight gene polymorphisms was described as odds ratio (ORs) and 95 % confidence interval (CI) using unconditional logistic regression analysis. The prognostic value of eight gene polymorphisms for the OS was estimated by multivariate analysis using the Cox proportional hazards models, describing as the hazard ratio (HR) and 95 % CI. Meanwhile, baseline characteristics were adjusted in order to avoid potential confounding effects, such as age, sex, smoking and drinking, histological types, and tumor node metastasis (TNM) stage at entry. Survival curves were analyzed by the Kaplan-Meier method. A dominant genetic model was used in all statistical analyses. Two-tailed P values <0.05 were considered a statistical difference. All statistical analyses were conducted using the STATA version 9.0 statistical software.
Results
Patients’ characteristics and outcome
A total of 452 patients with gastric cancer were invited to participate in the present study, and 415 patients consented, resulting in a participation rate of 91.81 %. The demographic and clinical characteristics of the patients were shown in Table 1. Our study included 272 males (65.5 %) and 143 females (34.5 %) with a median age of 56.2 ± 15.6 years (range, 32.4–76.5 years) upon initial diagnosis. Of the 415 patients, 169 (40.7 %) were ever smokers, 247 (59.5 %) were drinkers, 179 (43.1 %) were intestinal type of gastric cancer, 189 (45.5 %) were signet ring type, and 263 (63.4 %) were III–IV stages. Our study found that patients exhibiting stages III–IV cancer and have a habit of tobacco smoking and alcohol drinking were associated with a longer OS period.
Genotypes of eight gene polymorphisms
The genotype distributions of ERCC1 rs11615 and rs2298881, ERCC2 rs1799793 and rs13181, NBN rs1805794 and rs709816, and RAD51 rs1801321 and XRCC3 rs1799794 demonstrated Hardy-Weinberg equilibrium. Conditional regression analysis identified that CC genotype of ERCC1 rs11615 and AA genotype of ERCC2 rs1799793 were associated with a better response to chemotherapy in gastric cancer patients, and the ORs(95 %CI) were 2.70(1.33–5.70) and 3.12(1.52–6.84), respectively (Table 2). However, we did not find significant association between response to chemotherapy and polymorphisms in ERCC1 rs2298881, ERCC2 rs13181, NBN rs1805794 and rs709816, and RAD51 rs1801321 and XRCC3 rs1799794.
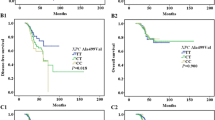
The association between eight gene polymorphisms and survival with gastric cancer is shown in Table 3. The median duration of the follow-up was 28.6 ± 6.2 months (range 3–60 months). Among the 415 patients, 191 patients succumbed due to cancer during the follow-up period, providing a 5-year survival rate of 53.98 %. The CC genotype of ERCC1 rs11615 and AA genotype of ERCC2 rs1799793 were significantly associated with a longer OS (for ERCC1 rs11615, adjusted HR, 2.30; 95 %CI, 1.18–4.58; for ERCC2 rs1799793, adjusted HR, 2.45; 95 %CI, 1.25–4.95) (Table 3). Moreover, CC genotype of NBN rs1805794 was associated with longer OS of gastric cancer (adjusted HR, 2.24; 95 %CI, 1.20–4.25). However, ERCC1 rs2298881, ERCC2 rs13181, NBN rs709816, RAD51 rs1801321, and XRCC3 rs1799794 polymorphisms were not significantly associated with OS of gastric cancer patients. Examination of Kaplan-Meier curves for the ERCC1 rs11615, ERCC2 rs1799793, and NBN rs1805794 genotypes showed that the three gene polymorphisms are associated with the overall survival of gastric cancer patients (Figs. 1, 2, and 3).
Moreover, we assess the association between ERCC1 rs11615, ERCC2 rs1799793, and NBN rs1805794 polymorphisms and characteristics of gastric cancer, including tobacco smoking, alcohol drinking, and tumor stage of gastric cancer. However, we did not find a significant interaction between the three gene polymorphisms and tobacco smoking, alcohol drinking, and tumor stage of gastric cancer.
Discussion
Accumulated evidence shows that genetic polymorphisms including drug metabolizing enzymes, drug transporters, and drug targets are linked to interindividual differences in the efficacy and toxicity for many medications. A pharmacogenetic analysis is thought as a promising tool to develop specific chemotherapy plans. In this study, we investigated the polymorphisms of ERCC1 rs11615 and rs2298881, ERCC2 rs1799793 and rs13181, NBN rs1805794 and rs709816, and RAD51 rs1801321 and XRCC3 rs1799794 as potential biomarkers of activity to platinum-based chemotherapy and the role of the eight gene polymorphisms in the overall survival in gastric cancer patients. Our results indicate that CC genotype of ERCC1 rs11615 and AA genotype of ERCC2 rs1799793 are associated with a better response to chemotherapy in gastric cancer patients. Moreover, in the multivariable Cox regression, the CC genotype of ERCC1 rs11615, AA genotype of ERCC2 rs1799793, and CC genotype of NBN rs1805794 are significantly associated with a longer OS.
We have shown that ERCC1 rs11615 and ERCC2 rs1799793 are important factors influencing both response to platinum-based chemotherapy and OS of gastric cancer patients, and the association with chemotherapy and OS remains significant after adjusting for multiple comparisons. Previously published studies have observed associations of ERCC1 rs11615 and ERCC2 rs1799793 with response to chemotherapy and clinical outcome of gastric cancer [16–21], but the results are inconsistent. Li et al. conducted a cohort study with 231 patients and showed that CC genotype of ERCC1 rs11615 polymorphism was associated with a better response to chemotherapy and a longer OS of gastric cancer [16]. Chen et al. conducted a cohort study with 255 patients and found that AA genotype of ERCC1 rs11615 was associated with lower risk of death from gastric cancer [17]. However, two studies found that the AA genotype of ERCC1 rs11615 could increase the risk of death from gastric cancer [18, 19]. Moreover, another study did not find that ERCC1 rs11615 was associated with OS of gastric cancer [20]. These inconsistent results might be due to differences in ethnicities, source of patients, disease stages, sample size, and by chance.
For ERCC2 rs1799793, a previous study showed that the non-synonymous ERCC2 rs1799793 was associated with lower DNA repair capacity [22]. Our results are in concordance with the proposed biological effect of ERCC2 rs1799793, as a lower repair capacity may lead to increased DNA damage and therefore to better efficacy. Only two studies reported the association between ERCC2 rs1799793 polymorphism and response to chemotherapy and OS of gastric cancer [20, 21]. Li et al. reported that individuals carrying AA genotype of ERCC2 rs1799793 were associated with significantly poorer overall survival and significantly higher risk of death [21]. However, Chu et al. did not find that ERCC2 rs1799793 polymorphism could play different roles in the OS of gastric cancer [20]. Future studies are greatly needed to confirm the association between ERCC2 rs1799793 and response to chemotherapy and OS of gastric cancer.
Compared to NER gene polymorphisms, less is known about gene polymorphisms in HRR pathway and their influence on platinum-based chemotherapy, and no study have evaluated their roles in gastric cancer. Our study showed that polymorphic NBN rs1805794 also tended to be associated with better response. Genetic variability of NBN thus seems to be important for its DNA repair capacity. These observations are also in agreement with our previous results indicating that NBN rs1805794 genotypes confer to differences in levels of DNA damage and cancer risk [23, 24]. The role of NBN gene polymorphisms in response to platinum-based chemotherapy is greatly needed to be explained in further studies.
The present study has three limitations. First, the study was conducted in a single hospital in China; thus, it may not be representative of the general population. Selection bias may exist in this study. Second, the number of cases analyzed in the present study was relatively small, which may reduce the statistical power to detect differences between the various DNA repair gene groups. As the sample size is limited, this may limit the statistical power to find differences between groups. Therefore, further studies using a large multicenter cohort are required to investigate the association between DNA repaired genes and response to chemotherapy and OS of gastric cancer.
In conclusion, our results suggest that ERCC1 rs11615 and ERCC2 rs1799793 polymorphisms in the DNA repair pathways may influence the response to chemotherapy and OS of gastric cancer, and NBN rs1805794 polymorphism could influence the OS of gastric cancer. Moreover, the three gene polymorphisms could contribute to identification of patients to achieve better response to platinum-based chemotherapy. Further studies with a larger sample size are warranted to confirm our findings.
References
Stomach Cancer. Estimated incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx, 2014.
International Agency for Research on Cancer. IARC working group on the evaluation of carcinogenic risks to humans, schistosomes, liver flukes, Helicobacter pylori. IARC monographs on the evaluation of carcinogenic risks to humans, vol. 61. Lyon: International Agency for Research on Cancer; 1994. p. 1–241.
Macdonald JS. Clinical overview: adjuvant therapy of gastrointestinal cancer. Cancer Chemother Pharmacol. 2004;54 Suppl 1:S4–11.
Alberts DS, Garcia D, Mason-Liddil N. Cisplatin in advanced cancer of the cervix: an update. Semin Oncol. 1991;18(1 Suppl 3):11–24.
Zhou F, Yu Z, Jiang T, Lv H, Yao R, Liang J. Genetic polymorphisms of GSTP1 and XRCC1: prediction of clinical outcome of platinum-based chemotherapy in advanced non-small cell lung cancer (NSCLC) patients. Swiss Med Wkly. 2011;141:w13275.
Liu L, Li CH, Jin TF, Xu DY. Study on the ERCC1 gene polymorphism response to chemotherapy and prognosis of gastric cancer. Genet Mol Res. 2014;13(4):8722–8.
Zhang X, Bai Z, Chen B, Feng J, Yan F, Jiang Z, et al. Polymorphism of methylenetetrahydrofolate reductase gene is associated with response to fluorouracil-based chemotherapy in Chinese patients with gastric cancer. Chin Med J (Engl). 2014;127(20):3562–7.
Oh SY, Kwon HC, Kim SH, Lee S, Lee JH, Hwang JA, et al. The relationship of vascular endothelial growth factor gene polymorphisms and clinical outcome in advanced gastric cancer patients treated with FOLFOX: VEGF polymorphism in gastric cancer. BMC Cancer. 2013;13:43.
Xu J, Ma J, Zong HT, Wang SY, Zhou JW. Pharmacogenetic role of XRCC1 polymorphisms on the clinical outcome of gastric cancer patients with platinum-based chemotherapy: a systematic review and meta-analysis. Genet Mol Res. 2014;13(1):1438–46.
Lu ZM, Luo TH, Nie MM, Fang GE, Ma LY, Xue XC, et al. Influence of ERCC1 and ERCC4 polymorphisms on response to prognosis in gastric cancer treated with FOLFOX-based chemotherapy. Tumour Biol. 2014;35(4):2941–8.
Marsh S, McLeod H, Dolan E, Shukla SJ, Rabik CA, Gong L, et al. Platinum pathway. Pharmacogenet Genomics. 2009 Jul;19(7):563–4.
Enoiu M, Jiricny J, Scharer OD. Repair of cisplatin-induced DNA interstrand crosslinks by a replication-independent pathway involving transcription-coupled repair and translesion synthesis. Nucleic Acids Res. 2012;40(18):8953–64.
Altan B, Yokobori T, Mochiki E, Ohno T, Ogata K, Ogawa A, et al. Nuclear karyopherin-α2 expression in primary lesions and metastatic lymph nodes was associated with poor prognosis and progression in gastric cancer. Carcinogenesis. 2013;34(10):2314–21.
Abuzeid WM, Jiang X, Shi G, Wang H, Paulson D, Araki K, et al. Molecular disruption of RAD50 sensitizes human tumor cells to cisplatin-based chemotherapy. J Clin Invest. 2009;119(7):1974–85.
Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–14.
Liu L, Li CH, Jin TF, Xu DY. Study on the ERCC1 gene polymorphism response to chemotherapy and prognosis of gastric cancer. Genet Mol Res. 2014;13(4):8722–8.
Chen ZH, Wang L, Luo LP. Association of DNA repair gene polymorphisms with response to chemotherapy and prognosis of gastric cancer. Genet Mol Res. 2014;13(3):7484–91.
Li J, Zuo X, Lv X, Kong F, Xu W, Yang S. Association of DNA repair gene polymorphisms with response to chemotherapy and prognosis of gastric cancer in a Chinese population. Tumour Biol. 2014;35(8):7569–74.
Lu ZM, Luo TH, Nie MM, Fang GE, Ma LY, Xue XC, et al. Influence of ERCC1 and ERCC4 polymorphisms on response to prognosis in gastric cancer treated with FOLFOX-based chemotherapy. Tumour Biol. 2014;35(4):2941–8.
Chu H, Gu D, Xu M, Xu Z, Gong Y, Gong W, et al. A genetic variant in ERCC2 is associated with gastric cancer prognosis in a Chinese population. Mutagenesis. 2013;28(4):441–6.
Li Y, Liu Z, Liu H, Wang LE, Tan D, Ajani JA, et al. ERCC1 and ERCC2 variants predict survival in gastric cancer patients. PLoS One. 2013;8(9):e71994.
Spitz MR, Wu X, Wang Y, Wang LE, Shete S, Amos CI, et al. Modulation of nucleotide excision repair capacity by XPD polymorphisms in lung cancer patients. Cancer Res. 2001;61(4):1354–7.
Goricar K, Erculj N, Zadel M, Dolzan V. Genetic polymorphisms in homologous recombination repair genes in healthy Slovenian population and their influence on DNA damage. Radiol Oncol. 2012;46(1):46–53.
Goričar K, Kovač V, Jazbec J, Zakotnik B, Lamovec J, Dolžan V. Influence of the folate pathway and transporter polymorphisms on methotrexate treatment outcome in osteosarcoma. Pharmacogenet Genomics. 2014;24(10):514–21.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, J., Liu, Zy., Li, Cb. et al. Genetic polymorphisms of DNA repair pathways influence the response to chemotherapy and overall survival of gastric cancer. Tumor Biol. 36, 3017–3023 (2015). https://doi.org/10.1007/s13277-014-2936-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2936-3