Abstract
One purified polysaccharide protein tyrosine phosphatase (PTP) was isolated from the roots of Polygala tenuifolia. The aim of the present study is to investigate the antitumor effect of PTP on human ovarian cancer OVCAR-3 cells and explore the molecular mechanism of the action involved. The results of MTT assay and apoptosis detection assay showed that PTP inhibited cellular proliferation of OVCAR-3 cells and induced apoptotic cellular death via arresting cell circle at the G0/G1 phase. Reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analysis identified that bcl-2 gradually decreased at both transcription and protein levels after PTP treatment for 48 h in OVCAR-3 cells, while those of bax, cytochrome c, caspase-3, and caspase-9 increased. In addition, the low expression of NF-κB in PTP-treated OVCAR-3 cells would trigger the extrinsic pathway of programmed cell death signaling in tumor cells. These results together suggest that PTP may induce apoptosis of OVCAR-3 cells through a mitochondrial pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycomb protein BMI-1 is broadly overexpressed in various types of cancer cells, such as leukemia [1], mantle cell lymphoma [2], non-small-cell lung cancer [3], breast cancer [4], colorectal cancer [5], nasopharyngeal carcinoma [6], prostate cancer [7], and ovarian cancer [8], and its depletion in those cells often leads to reduced cell growth and proliferation and increased cell apoptosis [9, 10]. These observations led us to hypothesize that downregulation of BMI-1 can result in the apoptosis of cancer cells, including ovarian cancer. We previously reported the Bmi-1 protein was highly expressed in ovarian epithelial cancer tissues and that elevated expression of Bmi-1 correlated closely with increased telomerase activity [8]. We also found that the cell viability of human ovarian carcinoma OVCAR-3 cells decreased when stably transfected with antisense Bmi-1 RNA [11]. In view of the charming attraction of a natural product to fight against this disease, we purified one homogeneous polysaccharide protein tyrosine phosphatase (PTP) from the roots of Polygala tenuifolia and evaluated its antitumor effect in vitro and in vivo [12]. The results identified PTP could significantly inhibit the growth of ovarian cancer. Importantly, Bmi-1 expression was suppressed by PTP treatment at both mRNA and protein levels, together with the low telomerase activity. All these findings have thrown light on the mechanism by which PTP participates in tumor progression, but the underlying mechanisms are not yet well understood. In this context, determining the mechanism by which downregulation of BMI-1 by PTP treatment sensitizes the cancer cells to apoptosis would be important for the development of new therapeutic strategies to combat ovarian cancer. This report firstly documented that the polysaccharide PTP from the roots of P. tenuifolia induced apoptosis in human ovarian cancer OVCAR-3 cells through the mitochondrion-dependent apoptotic pathway.
Materials and methods
Materials and chemicals
P. tenuifolia was harvested from Jilin province of China in October, 2009. The roots were washed, air dried, and cut into small pieces before use. The materials were thoroughly washed with tap water, air dried at room temperature, and finely powdered for subsequent experiment. DEAE-Sephacel and Sephadex G-100 were purchased from Amersham Pharmacia Co., Sweden.
Isolation and purification of polysaccharide PTP
PTP was extracted from P. tenuifolia with a method as previously described [12]. Briefly, the dried powders of P. tenuifolia (100 g) were defatted with toluene–ethanol (1:1, v/v) for 6 h in a Soxhlet apparatus under reflux. The remaining residue was then extracted with distilled water at 80 °C for two times and 1 h each time. Subsequently, the whole combined extract was filtered, concentrated, and centrifuged and then the supernatant was precipitated with 95 % EtOH until the end concentration of 50 % (v/v) at 4 °C for 1 h. Then, 10 % CaCl2 was added and kept overnight to precipitate the tannin. The supernatant containing water-soluble polysaccharide collected by centrifugation was ultrafiltrated with membrane (MWCO: 1 k) and membrane (MWCO: 10 k) on an ultrafiltration apparatus. The ultrafiltrate (outside of membrane of 10 k) was concentrated and lyophilized to yield crude polysaccharide (cPTP). The crude polysaccharides were dissolved in distilled water and filtered through a 0.45-μm Millipore filter. The solution was subjected to DEAE-Sephacel column (4 × 60 cm) chromatography and eluted with deionized water at a flow rate of 1 mL/min. Fraction was collected and monitored with the phenol–sulfuric acid method [13]. One main fraction eluted with deionized water was further applied to Sephadex G-100 column (2.5 × 100 cm) with the same buffer at a flow rate of 1 mL/min. The eluted fractions containing a large amount of sugar were only separated into one fraction and lyophilized to afford a purified polysaccharide, named as PTP. The purified polysaccharide was kept in solid powder for further analysis.
Cell line and culture
The human ovarian carinoma cell line OVCAR-3 was obtained from Shanghai Institute for Biological Sciences, Chinese Academy of Science and was cultured in RPMI 1640 containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 % fetal bovine serum (FBS) and were maintained at 37 °C in a humidified atmosphere with 5 % CO2.
Cell viability assay
Cytotoxicities were assessed using MTT assays [14]. Briefly, cells were harvested during logarithmic growth phase and seeded into flat-bottomed 96-well plates at a density of 5 × 103 cells per well and incubated for 12 h. PTP ranging from 0.1 to 1.6 mg/mL was added, and plates were incubated for additional 24, 48, and 72 h. Subsequently, culture medium was removed, and 20 μL MTT solution (5 mg/mL) was added to each well and incubated for 4 h at 37 °C. Then, the supernatant was discarded, and 100 μL anhydrous DMSO was added to terminate the experiments. Cell viability was measured by Bio-Rad Model 680 Microplate reader at 570 nm. Experiments were carried out at least thrice. Cell survival was calculated with the following formula: survival (%) = (mean experimental absorbance / mean control absorbance) × 100 % [15].
Apoptosis assay
After OVCAR-3 cells were exposed to the indicated concentrations of PTP (0.4, 0.8, and 1.6 mg/mL) for 48 h, the cells were harvested and washed twice with cold phosphate-buffered saline (PBS). Subsequently the cells were stained with annexin V-FITC and propidium iodide (PI) at room temperature for 15 min in the dark. The samples were analyzed immediately after staining using a flow cytometer (Becton Dickinson, USA) and analyzed by the CellQuest software. For each measurement, at least 20,000 cells were counted. Apoptotic cells were defined as annexin V-FITC-positive cells. The apoptosis rate (%) = (the number of apoptotic cells / the number of total cells observed) × 100 % [15].
Cell cycle analysis
Cell cycle was analyzed by flow cytometry analysis [16]. Briefly, the cells (5 × 105) were seeded in six-well plates and treated with various concentrations of PTP (0.4, 0.8, and 1.6 mg/mL) for 48 h. After treatment, the cells were collected by trypsinization, fixed in 70 % ethanol, washed with cold phosphate-buffered saline (PBS, pH 7.4) and then stained with PI solution (50 μg/mL of propidium iodide, 100 μg/mL RNase, and 0.1 % Triton X-100 in PBS) in the dark for 30 min at room temperature. The stained cells were analyzed for cell cycle phase distribution by flow cytometry (FACSCalibur, BD Bioscience). More than 1 × 104 cells were collected and analyzed with CellQuest™ software (Becton Dickinson).
ELISA for DNA assessment during apoptosis
Cells were cultured with PTP (0.4, 0.8, and 1.6 mg/mL) for 48 h. The optical density (OD) value of DNA fragments was assayed using an apoptosis testing kit (Roche, USA). The results were calculated as the OD value of the experimental sample/the OD value of the control.
Reverse transcription-polymerase chain reaction (RT-PCR) for mRNA analysis of bcl-2, bax, cytochrome c, caspase-3, and caspase-9
OVCAR-3 cells were treated with PTP at various concentrations of 0.4, 0.8, and 1.6 mg/mL for 48 h. Total RNA was isolated from solvent control and treated SW-480 cells using the TRIzol™ reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol and kept at −80 °C until use. And the first strand of cDNA was synthesized. The primers for PCR used in this experiment were as follows: bcl-2 (304 bp): sense primer 5′-GTGGAGGA GCTCTTCAGGGA-3′ and antisense primer: 5′-AGGCACCC AGGGTGATGCAA-3′; bax (477 bp): sense primer 5′-GGCCCACCAGCTCTGAGCAGA-3′ and antisense primer 5′-GCCACGTGGGCGTCCCAAAGT-3′; cytochrome c (204 bp): sense primer 5′-TGAAGCCGCTCGCAAGACTCC-3′ and antisense primer 5′-GGCTGTCAAAAGGGGCGGTCT-3′; caspase-9 (273 bp): sense primer 5′- GCTTAGGGT CGCTAATGCTG-3′ and antisense primer 5′-TGTCGTCAATC TGGAAGCTG-3′; caspase-3 (297 bp): sense primer 5′-ATGGAAGCGAATCAATGGAC-3′ and antisense primer: 5′-GGCTCAGAAGCACACAAACA-3′; β-actin (353 bp): sense primer 5′-GCTCGTCGTCGACAACGGCTC-3′ and antisense primer 5′-CAAACATGATCTGGGTCATCT TCTC-3′. PCR conditions were as follows: 30 s at 95 °C, 35 s at 56 °C, 34 s at 72 °C for 28 cycles, followed by 8 min at 72 °C for different primers. PCR product (5 μL) was loaded for 1 % gel electrophoresis (Kaiji Biotechnology Company, Nanjing, China). The expression of apoptotic genes was normalized by the expression of the internal reference gene β-actin. The results were semi-quantified using the ultrasonic vibration potential (UVP) gel photograph analyzing system (Bio-Rad) and Quantity One software (Bio-Rad).
Western blotting analysis
The total cell lysates and cytosolic and mitochondrial fractions were isolated from the cells as previously described [17]. After culturing with PTP (0.4, 0.8, and 1.6 mg/mL) for 48 h, cells were collected and the proteins were extracted using RIPA lysis buffer. The protein concentration was measured using the BCA method. Equal amounts of protein were separated by 10 % SDS-PAGE electrophoresis and then transferred to polyvinylidene difluoride membranes (0.45-μm pore size). The membranes with proteins were incubated at 4 °C overnight with the primary antibody (mouse anti-human bcl-2, bax, cytochrome c, NF-kB, caspase-3, or caspase-9 antibodies) at a 1:500 dilution and then incubated with the HRP-conjugating secondary antibody in a final dilution of 1:5000 for 1 h. The blots were developed, and the proteins were detected using the Odyssey infrared imaging system (LI-COR) according to the manufacturer’s instructions. The densitometry of the band was analyzed using a UVP gel photograph analyzing system, and the relative expression levels of bcl-2, bax, cytochrome c, caspase-3, and caspase-9 were calculated.
Statistical analysis
Data in all experiments were shown as mean ± SD. Statistical significance was compared between each treated cell group and control cells using Student’s t test. Values with P < 0.05 were considered significantly different from untreated control values.
Results
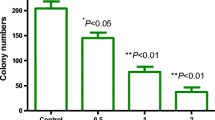
Effect of PTP on cell proliferation
The cytotoxic activity of water-soluble polysaccharide PTP from the roots of P. tenuifolia on OSCC human ovarian carcinoma OVCAR-3 cells was evaluated by MTT assay. The percentage of growth inhibition of PTP at various concentrations on OVCAR-3 cells was determined as the percentage of viable treated cells in comparison with viable cells of untreated controls, the viability of which was assumed to be 100 %. As shown in Fig. 1, after treatment with PTP at the concentrations ranging from 0 to 1.6 mg/mL for 24, 48, and 71 h, the cell viability of OVCAR-3 cells was significantly decreased in a dose- and time-dependent manner. When the culture time was over 48 h, there was only a small decrease of cell viability on the same concentration of PTP along with the time increasing, and cell viability arrived at the lower value especially at the high concentration in the range of 0.4 to 1.6 mg/mL. Therefore, we choose the concentrations of 0.4, 0.8, and 1.6 mg/mL and 48 h of incubation time for the following experiments.
Effects of PTP on the cell cycle and apoptosis in OVCAR-3 cells
Since PTP exerted an antiproliferation effect, cell apoptosis and cell cycle distribution were assayed by flow cytometry to further elucidate the potential mechanism through which PTP inhibits cell growth. Representative histograms for cell apoptosis and cell cycle distribution in OVCAR-3 cells following exposure to different concentrations of PTP were shown in Fig. 2a, b. After treatment with PTP for 48 h, annexin V-positive cells were observed, and the percentage of apoptotic cells induced by 0.4, 0.8, or 1.6 mg/mL of PTP was increased to 23.5, 51.2, and 75.6 %, respectively. Accordingly, PTP treatment resulted in the increase of G0/G1 phase compared to media control. From the result, 76.68, 82.21, and 85.34 % of cells were arrested at G0/G1 phase after treatment with 0.4, 0.8, and 1.6 mg/mL of PTP, respectively, whereas in the untreated group, 69.73 % of OVCAR-3 cells were in G0/G1 phase. This dose-dependent increase at G0/G1 phase was coupled with the decreased percentage of cells in S phase, which indicated that PTP inhibited the cellular proliferation of OVCAR-3 cells via arresting the cell cycle at G0/G1 phase.
Effect of PTP on DNA fragmentation in OVCAR-3 cells
As shown in Fig. 3, PTP was found to increase the DNA fragmentation compared to the negative control, especially at the concentrations of 0.8 and 1.6 mg/mL (P < 0.05). In accordance with quantification of apoptosis by flow cytometry, these results further suggested the apoptotic activity of PTP in OVCAR-3 cells.
Effect of PTP on mRNA expression of bcl-2, bax, cytochrome c, caspase-3, and caspase-9 in OVCAR-3 cells
The Bcl-2 family, composed of both antiapoptotic and proapoptotic proteins, regulates cell death by controlling mitochondrial membrane permeability during apoptosis [18]. Thus, it is a mediator of the mitochondrial apoptosis pathway. To investigate the role of the Bcl-2 family in PTP-induced apoptosis, we first analyzed the changes in the levels of antiapoptotic Bcl-2 and proapoptotic Bax in treatment and control cells. RT-PCR and Western blot analysis showed that treatment of OVCAR-3 cells with PTP dose-dependently increased the expression of the proapoptotic Bax in OVCAR-3 cells. On the other hand, the expression of the antiapoptotic Bcl-2 was significantly decreased after being treated with the same condition (Fig. 4). Thus, the ratio of Bax to Bcl-2 was significantly increased after treatment with PTP, and this change would promote cell apoptosis. Our results suggested that PTP could induce apoptosis through changing the Bax/Bcl-2 ratio and, therefore, increasing the mitochondrial membrane permeability to trigger the whole cascade of apoptotic reactions. Accordingly, PTP-treated tumor cells showed a dose-dependent release of cytochrome c into the cytosol, which was well correlated with this conclusion (Fig. 4).
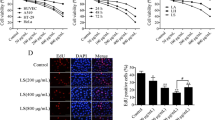
Effect of PTP on mRNA and protein expression of bcl-2, bax, cytochrome c, caspase-3, and caspase-9 in OVCAR-3 cells. a Protein expression of bcl-2, bax, cytochrome c, caspase-3, and caspase-9 in OVCAR-3 cells was confirmed by Western blot, and β-actin was used as a loading control. b mRNA expression of bcl-2, bax, cytochrome c, caspase-3, and caspase-9 in OVCAR-3 cells were confirmed by RT-PCR and β-actin as an internal reference. c Quantitative analysis of protein expression of bcl-2, bax, cytochrome c, caspase-3, and caspase-9 in OVCAR-3 cells. d Quantitative analysis of mRNA expression of bcl-2, bax, cytochrome c, caspase-3, and caspase-9 in OVCAR-3 cells. Data represent the mean ± SD (n = 3). *P < 0.05 when compared to control cells
Because the caspase family plays a central role in the apoptotic process, we next examined the effect of PTP on caspase activation. As shown in Fig 4, PTP treatment could significantly increase the transcription and protein expression of caspase-3 and caspase-9 in OVCAR-3 cells. These results indicated that caspase-3 and caspase-9 were involved in the apoptotic effects induced by PTP.
Effect of PTP on the NF-кB protein expression in OVCAR-3 cells
To investigate whether PTP inhibits NF-kB in human ovarian carcinoma OVCAR-3 cells, we perform Western blots to examine NF-κB expression at the protein levels. As shown in Fig. 5, PTP inhibited NF-κB expression of OVCAR-3 cells after treating for 48 h at all concentrations, and the inhibitory action was much more obvious as concentrations increased. Those results suggested that the effect of NF-kB was abolished by PTP.
Effect of PTP on NF-кB protein expression in OVCAR-3 cells. a Protein expression of NF-кB in OVCAR-3 cells were confirmed by Western blot, and β-actin was used as a loading control. b Quantitative analysis of protein expression of NF-кB in OVCAR-3 cells. Data represent the mean ± SD (n = 3). *P < 0.05 when compared to control cells
Discussion and conclusions
Cancer is one of the serious diseases threatening human health. At present, it has not been entirely overcome. Currently, most chemotherapeutic agents in clinical use are effective in combating cancer cells but they are also associated with toxicity, and particularly, the occurrence of resistance constraints their effectiveness. In cancer research, the focus is on the development of novel drugs that could induce apoptosis with high efficiency and minimal side effects. Apoptosis causes specific cell death via an intrinsic ‘suicide’ mechanism and is believed to play a key role in cancer progression [19]. The mechanisms of apoptosis mainly involve two signaling pathways: the mitochondrial pathway and the cell death receptor pathway [20]. A large amount of reports have shown that alterations of mitochondrial structure and function are closely associated with apoptosis [21]. Bcl-2 family proteins, such as Bax and Bcl-2, induce mitochondrial membrane permeabilization to cause the release of cytochrome c into the cytosol, which is thought to be the key factor in apoptosis [22]. Once cytochrome c is released to the cytosol, cytochrome c together with apoptotic protease activating factor 1 (Apaf-1) activates initiator caspases (caspase-2, caspase-8, caspase-9, and caspase-10), and the latter then leads to effector caspases activation (caspase-3, caspase-6, and caspase-7) [23–25]. These effector caspases are responsible for the cleavage of various proteins leading to biochemical and morphological features characteristic of apoptosis [26]. There is accumulating evidence that naturally occurring compounds and many chemotherapeutic agents with antitumor effects can trigger the apoptosis of cancer cells [27–29]. Thus, the study presented here focused on the apoptotic mechanism of the polysaccharide PTP.
In the present study, we have reported the anticancer potential of PTP in vitro on a human ovarian carcinoma OVCAR-3 cell line and have elucidated the possible underlying mechanism. We found that PTP treatment induced not only apoptosis but also cell cycle G0/G1 arrest in OVCAR-3 cells thus leading to the low survival rate of OVCAR-3 cells. This data was further supported by results from DNA fragmentation assay, which demonstrated statistically significant increase of DNA fragmentation in PTP-treated cells compared to the untreated control cells. Moreover, the expression of bcl-2, bax, cytochrome c, caspase-3, and caspase-9 in PTP-treated OVCAR-3 cells were also detected at both protein and mRNA levels. RT-PCR and Western blot analysis identified that the expression of bcl-2 gradually decreased after PTP treatment for 48 h in OVCAR-3 cells, while that of bax, cytochrome c, caspase-3, and caspase-9 increased in a concentration-dependent fashion. Additionally, PTP could significantly suppress NF-κB protein expression in VCAR-3 cells. Based on the results of the present study, we can conclude that PTP might be useful for integrative and complementary medicine by promoting apoptotic cell death in human ovarian carcinoma OVCAR-3 cells via the mitochondria-dependent pathway.
References
Sawa M, Yamamoto K, Yokozawa T, Kiyoi H, Hishida A, Kajiguchi T, et al. BMI-1 is highly expressed in M0-subtype acute myeloid leukemia. Int J Hematol. 2005;82:42–7.
Beà S, Tort F, Pinyol M, Puig X, Hernández L, Hernández S, et al. BMI-1 gene amplification and overexpression in hematological malignancies occur mainly in mantle cell lymphomas. Cancer Res. 2001;61:2409–12.
Becker M, Korn C, Sienerth AR, Voswinckel R, Luetkenhaus K, Ceteci F, et al. Polycomb group protein Bmi1 is required for growth of RAF driven non-small-cell lung cancer. PLoS One. 2009;4:e4230.
Kim JH, Yoon SY, Jeong SH, Kim SY, Moon SK, Joo JH. Overexpression of Bmi-1 oncoprotein correlates with axillary lymph node metastases in invasive ductal breast cancer. Breast. 2004;13:383–8.
Kim JH, Yoon SY, Kim CN, Joo JH, Moon SK, Choe IS, et al. The Bmi-1 oncoprotein is overexpressed in human colorectal cancer and correlates with the reduced p16INK4a/p14ARF proteins. Cancer Lett. 2004;203:217–24.
Song LB, Zeng MS, Liao WT, Zhang L, Mo HY, Liu WL, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66:6225–32.
Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death from cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–21.
Zhang F, Sui L, Xin T. Correlations of BMI-1 expression and telomerase activity in ovarian cancer tissues. Exp Oncol. 2008;30:70–4.
Hannon GJ. RNA interference. Nature. 2002;418:244–51.
Liu L, Andrews LG, Tollefsbol TO. Loss of the human polycomb group protein BMI1 promotes cancer-specific cell death. Oncogene. 2006;25:4370–5.
Xin T, Zhang FB, Sui GJ, Jin XM. Bmi-1 siRNA inhibited ovarian cancer cell line growth and decreased telomerase activity. Br J Biomed Sci. 2012;6:62–6.
Xin T, Zhang F, Jiang Q, Chen C, Huang D, Li Y, et al. Extraction, purification and antitumor activity of a water-soluble polysaccharide from the roots of Polygala tenuifolia. Carbohydr Polym. 2012;90:1127–31.
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–6.
Xin T, Zhang F, Jiang Q, Chen C, Huang D, Li Y, et al. The inhibitory effect of a polysaccharide from Codonopsis pilosula on tumor growth and metastasis in vitro. Int J Biol Macromol. 2012;51:788–93.
Yan YY, Su XD, Liang YJ, Zhang JY, Shi CJ, Lu Y, et al. Emodin azide methyl anthraquinone derivative triggers mitochondrial-dependent cell apoptosis involving in caspase-8-mediated Bid cleavage. Mol Cancer Ther. 2008;7:1688–97.
Liang M, Li SC, Shen B, Cai JP, Li C, Wang ZY, et al. Anti-hepatocarcinoma effects of Aconitum coreanum polysaccharides. Carbohydr Polym. 2012;88:973–6.
Yang SF, Chen MK, Hsieh YS, Yang JS, Zavras AI, Hsieh YH, et al. Antimetastatic effects of Terminalia catappa L. on oral cancer via a down-regulation of metastasis-associated proteases. Food Chem Toxicol. 2010;48:1052–8.
Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2012;7:1182–91.
Micoud F, Mandrand B, Malcus-Vocanson C. Comparison of several techniques for the detection of apoptotic astrocytes in vitro. Cell Prolif. 2001;34:99–113.
Lee EO, Lee JR, Kim KH, Baek NI, Lee SJ, Lee BH, et al. The methylene chloride fraction of Trichosanthis Fructus induces apoptosis in U937 cells through the mitochondrial pathway. Biol Pharm Bull. 2006;29:21–5.
Liu J, Shen HM, Ong CN. Role of intracellular thiol depletion, mitochondrial dysfunction and reactive oxygen species in Salvia miltiorrhiza-induced apoptosis in human hepatoma HepG2 cells. Life Sci. 2001;69:1833–50.
Kim KC, Kim JS, Son JK, Kim IG. Enhanced induction of mitochondrial damage and apoptosis in human leukemia HL-60 cells by the Ganoderma lucidum and Duchesnea chrysantha extracts. Cancer Lett. 2007;246:210–7.
Tang XL, Yang XY, Jung HJ, Kim SY, Jung SY, Choi DY, et al. Asiatic acid induces colon cancer cell growth inhibition and apoptosis through mitochondrial death cascade. Biol Pharm Bull. 2009;32:1399–405.
Liu MJ, Wang Z, Li HX, Wu RC, Liu YZ, Wu QY. Mitochondrial dysfunction as an early event in the process of apoptosis induced by woodfordin I in human leukemia K562 cells. Toxicol Appl Pharmacol. 2004;194:141–55.
Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12.
Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–6.
Kumar MA, Nair M, Hema PS, Mohan J, Santhoshkumar TR. Pinocembrin triggers Bax-dependent mitochondrial apoptosis in colon cancer cells. Mol Carcinog. 2007;46:231–41.
Yang S, Evens AM, Prachand S, Singh AT, Bhalla S, David K, et al. Mitochondrial-mediated apoptosis in lymphoma cells by the diterpenoid lactone andrographolide, the active component of Andrographis paniculata. Clin Cancer Res. 2010;16:4755–68.
Chan CK, Goh BH, Kamarudin MN, Kadir HA. Aqueous fraction of Nephelium ramboutan-ake rind induces mitochondrial-mediated apoptosis in HT-29 human colorectal adenocarcinoma cells. Molecules. 2012;17:6633–57.
Acknowledgments
This research is funded by the National Natural Science Foundation of China (no. 81373904) and the Doctoral Research Fund of the Second Affiliated Hospital of Harbin Medical University (Harbin, China).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, F., Song, X., Li, L. et al. Polygala tenuifolia polysaccharide PTP induced apoptosis in ovarian cancer cells via a mitochondrial pathway. Tumor Biol. 36, 2913–2919 (2015). https://doi.org/10.1007/s13277-014-2921-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2921-x