Abstract
The platelet count, as an inflammation marker, is involved in the progress of tumor invasion. However, the prognostic value of platelet counts and the platelet-to-lymphocyte ratio (PLR) has not been investigated in patients with advanced hepatocellular carcinoma (HCC). This study aimed to determine the prognostic value of platelet counts and PLR in HCC patients. A total of 243 ethnic Chinese advanced HCC patients from two major hospitals, not receiving systemic sorafenib, were analyzed retrospectively. The prognostic value of differential blood cell counts and PLR for overall survival (OS) was determined by integrating the Cancer of the Liver Italian Program (CLIP) score system and model for end-stage liver disease by using a stepwise model of multivariate Cox regression. The Kaplan–Meier method and receiver operating characteristic (ROC) curves were utilized accordingly. PLR was confirmed to be an independent predictor for OS (p < 0.01), while the remaining parameters had no predictive value. Then, advanced HCC patients were dichotomized into two groups based on the PLR value (≤111.23 or >111.23), according to ROC analysis. Patients with a high PLR had a lower 3-month survival rate (37.6 vs. 57.6 %) compared with patients with a low PLR. PLR was associated with aggressive malignant behavior, characterized by distant metastasis and portal vein thrombosis. Additionally, PLR was not associated with the CLIP score and Child–Pugh grade. PLR was identified as an independent prognostic factor for advanced HCC patients not receiving systemic sorafenib; the predictive ability of PLR partially relies on its association with the aggressive nature of HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patient selection has become one of the top issues for clinical trials testing targeted therapies, especially for patients with advanced hepatocellular carcinoma (HCC) [1–3]. Unfortunately, except for the advanced liver cancer prognostic system (ALCPS), prognostic score systems designed specifically for advanced HCC patients are few [2]. According to our previous studies, ALCPS had a similar prognostic performance as the Cancer of the Liver Italian Program (CLIP) score system, which was much more simplified than the ALCPS that consists of 27 complicated parameters [3]. The limitation of ALCPS considering its simplicity restricted its wide usage. Therefore, it is essential to identify novel prognostic factors for advanced HCC patients.
Recently, inflammation triggered by tumor necrosis or local antitumor immunological responses within the tumor has been confirmed to be unfavorable, considering the survival of cancer patients [4, 5]. Among the clinically available indicators of inflammation, C-reactive protein levels [6], white blood cell counts, platelet counts [7, 8], and blood neutrophil counts [9] have been the most investigated. However, these indicators may not be suitable for patients with advanced HCC who are vulnerable to bacterial infections [10]. The platelet count, as a marker of inflammation, has been under recent intensive investigation and was reported to be involved in the progress of tumor invasion [11, 12]. Given the multiple roles of platelets in tumor biology, it is a promising clinical parameter for patient selection and prognosis prediction. However, the prognostic value of the platelet count and the platelet-to-lymphocyte ratio (PLR) has not yet been identified in patients with advanced HCC. Therefore, we aimed to assess the role of the PLR and platelet count in predicting the overall survival (OS) of patients with advanced HCC based on a two-center study.
Methods
Patients
We retrospectively identified 243 patients diagnosed with advanced HCC between November 2008 and April 2010, who presented to the Third Affiliated Hospital of Sun Yat-sen University and the Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China. The diagnosis of HCC was confirmed based on the pathological analysis or the American Association for the Study of Liver Diseases radiological criteria by using either computed tomography or magnetic resonance imaging. Advanced HCC was defined as Barcelona Clinic Liver Cancer (BCLC) stage C and D that was not amendable to locoregional therapy, with the baseline as the time of the first evaluation for advanced HCC. None of the patients received sorafenib as systemic treatment. Patients with substantial missing data or with previous and/or secondary cancers were excluded. This study was approved by the Clinical Ethics Review Board at both the Third Affiliated Hospital of Sun Yat-sen University and the Third Affiliated Hospital of Guangzhou Medical University. A written informed consent was obtained from all the patients at the time of admission.
Data collection
Institutional review board approval was obtained. The electronic charts were used to retrieve data regarding the potential prognostic factors, including age; sex; Karnofsky performance status (KPS); pre-therapy data; and laboratory counts of white blood cells, neutrophils, lymphocytes, and platelets. Other parameters included were the Child–Pugh score, CLIP score, model for end-stage liver disease (MELD) score, and Seventh Edition American Joint Committee on Cancer tumor–node–metastasis staging as well as the survival time. All patients underwent regular follow-up at either hospital. KPS of the patients had not been routinely registered in our database and was retrospectively assessed from the clinical records regarding the physical status, cancer-related symptoms, and self-maintenance scale.
Statistical analysis
The OS and 3-month survival were the main endpoints of this study. Survival was defined as the time interval between HCC diagnosis and death or the last follow-up. Multivariate analysis using a stepwise Cox proportional hazards model was used to test for independent significance of baseline characteristics and explanatory variables. The performance of staging systems and relevant parameters was assessed by using the Kaplan–Meier method, and differences in survival between groups were compared by using the log-rank test. In addition, the receiver operating characteristic (ROC) curves of PLR for predicting the 3-month survival were plotted. The sensitivity–probability of the score less than the cutoff point indicated death within 3 months, and the specificity–probability of the score greater than the cutoff point indicated survival beyond 3 months, both of which could be evaluated for each possible cutoff point. The cutoff point representing the highest Youden Index (specificity + specificity − 1) was selected as the optimal threshold value. The area under the ROC curve was calculated and compared to represent the performance of each system by using the methods described by Hanley and McNeil. The linear trend χ 2 test was used to evaluate the discriminatory ability of the 3-month survival of gradients of parameters. The neutrophil-to-lymphocyte ratio (NLR) and PLR were integrated into the CLIP score system or MELD to test their independent prognostic value. The criterion for statistical significance was set at an α of 0.01, and all p values were based on two-sided tests. All the statistical analyses were performed using SPSS v 20.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patients’ characteristics
All 243 patients included in this study were ethnic Chinese people; 21 patients were excluded for fundamental data loss. Most of the patients (87.2 %) were hepatitis B virus carriers. None of the patients received antitumor treatment that was recommended in the National Comprehensive Cancer Network guidelines and BCLC system. Ten patients had received chemotherapy outside a clinical trial. The median follow-up time was 81 days (range, 2–1345 days), and 208 (85.6 %) patients died before the end of this study. The 3-month survival rate was 47.3 % (Table 1).
Prognostic value of PLR
The median count of white blood cells, neutrophil granulocytes, lymphocytes, and platelets was 6.51 × 109/L (range, 1.8 × 109/L to 36.1 × 109/L), 4.24 × 109/L (range, 0.50 × 109/L to 30.6 × 109/L), 1.17 × 109/L (range, 0.32 × 109/L to 14.00 × 109/L), and 141 × 109/L (range, 4 × 109/L to 503 × 109/L), respectively. The median PLR was 111.69 (range, 5.29–583.02) (Table 1 and Fig. 1a).
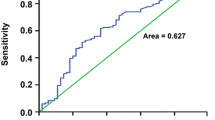
a Distribution of PLR in patients with advanced HCC (n = 243). b Receiver operating characteristic curves for PLR to predict 3-month survival. c Kaplan–Meier survival curves are shown for overall survival of advanced HCC patients with different level of PLR. HCC hepatocellular carcinoma, PLR platelet-to-lymphocyte ratio
Univariate analysis by Cox regression revealed that the white blood cell count, neutrophil granulocyte count, NLR, and platelet count were associated with unfavorable OS. In addition, the lymphocyte count was not associated with the OS. Importantly, a higher PLR indicated a significantly unfavorable prognosis (Table 2). The CLIP score system has been the best prognostic system for advanced HCC patients up to now, based on its capacity and simplicity [3]. Thus, in order to eliminate any potential bias due to limited sample size, we used the CLIP score system and MELD to test the independence of NLR and PLR as predictors for OS by using a multivariate Cox regression model. As a result, PLR was the only independent predictor among all the inflammation markers for OS of advanced HCC patients (p = 0.003 and p = 0.000 compared with CLIP and MELD, respectively). NLR did not reach a significant p value of 0.01 compared with the CLIP score or MELD (Table 3).
Comparison of patients with high and low PLR
ROC analysis presented similar results with Cox regression and revealed that the PLR was a significant predictor of 3-month survival (Fig. 1b). The cutoff point was 111.23 with the highest predictive performance for both specificity and sensitivity. Thus, the PLR value of ≤111.23 was defined as a low level while a PLR value of >111.23 was defined as a high level, and patients were dichotomized into two groups accordingly. The differences in baseline characteristics were subsequently compared between patients with different PLRs. Patients with a high PLR had a significantly lower 3-month survival rate (37.6 vs. 57.6 %) compared with patients with a low PLR (Fig. 1c and Table 4). Importantly, a high PLR was associated with aggressive malignant features, including higher clinical stages, distant metastasis, and portal vein thrombosis. Besides, a high PLR was associated with unfavorable prognostic factors such as a higher alkaline phosphatase level and a higher MELD score. Meanwhile, a high PLR was associated with increased counts of white blood cells, neutrophil granulocytes, and platelets, but with decreased lymphocyte counts. However, patients with a high PLR presented with a better international normalized ratio than those with a low PLR. In addition, the PLR was not associated with the CLIP score and Child–Pugh grade (Table 4).
Discussion
In this era of targeted therapy, few targeted therapy generated a favorable result for advanced HCC patients except for sorafenib [1]. The causes were believed to be poor prognosis of advanced HCC patients [2, 3] and shortage of specificity in patient selection. Thus, patient selection for advanced HCC clinical trials is critical for future clinical trials. The platelet count, as an inflammation marker, has been under recent intensive investigation and was reported to be involved in the progression of aggressive tumor behavior [11, 12]. The multiple functions of platelets in tumor development make it a promising clinical factor for patient selection and prognosis prediction. This study is the first investigation to identify the prognostic value of PLR in advanced HCC patients. We found that the PLR, but not the platelet count, was an independent predictor for OS in patients with advanced HCC. Advanced HCC patients with a PLR >111.23 had a decreased 3-month survival than those with a PLR <111.23 (37.6 vs. 57.6 %), which makes PLR a preferable criterion for patient selection in future clinical trials.
The PLR was identified as an independent prognosis factor for advanced HCC patients in our study, and this result is comparable to its value identified in other types of cancers. A higher PLR was reported to indicate poor prognosis in patients with non-metastatic non-small-cell lung cancer [13], resectable small cell carcinoma of esophagus [14], squamous cell carcinoma of the vulva [15], resectable epithelial ovarian cancer [16], colon cancer [17, 18], and advanced gastric cancer [19]. In addition, PLR has an intermediate statistical ability to predict HCC recurrence after liver transplantation [20]. The predictive capability of PLR partially relies on its association with aggressive behavior of malignancies, including distant metastasis and portal vein thrombosis in our study. A higher PLR was reported to be associated with higher stages or tumor recurrence in HCC [20], ovarian cancer [16], and vulva carcinoma [15]. The aggressive progression of tumors based on injury to the vessel endothelium, which was one of the causes of increased platelet levels [11], gave it an enhanced capability in predicting the prognosis of cancer patients.
Increasing experimental evidence demonstrates that platelets promote tumor metastasis. Activated platelets guard tumor cells from immune elimination by promoting their arrest at the endothelium, thus supporting the establishment of secondary lesions [11, 12]. Platelet surface molecules and released factors, such as αIIbβ3 [21], platelet-derived growth factor, basic fibroblast growth factor, and hepatocyte growth factor [22], have also been shown to be involved in tumor metastasis. Platelet-derived transforming growth factor-beta as well as the direct contact between platelets and tumor cells synergistically promote transition of cancer cells to an invasive mesenchymal-like phenotype, thus enhancing metastasis [23]. In addition, platelets and neutrophil granulocytes are sequentially recruited to disseminated tumor cells to form “early metastatic niches” that promote metastatic progression [24]. On the other hand, lymphocyte depletion was the likely reflection of an impaired T lymphocyte-mediated antitumor response [25], and a decreasing T4/T8 ratio results in a weaker lymphocyte-mediated immune response to tumors [26]. Thus, because of an increasing interest in therapies targeting platelet-involved biological processes, PLR may be a potential criterion for patient selection in future clinical trials.
In summary, the present study is the first to identify PLR as an independent prognostic factor in advanced HCC patients not receiving systemic sorafenib. A subgroup of advanced HCC patients with a lower PLR had better 3-month survival rates, and these patients are ideal candidates for further clinical studies of targeted therapies.
References
Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844–55.
Yau T, Yao TJ, Chan P, Ng K, Fan ST, Poon RT. A new prognostic score system in patients with advanced hepatocellular carcinoma not amendable to locoregional therapy: implication for patient selection in systemic therapy trials. Cancer. 2008;113:2742–51.
Li X, Dong M, Lin Q, Chen ZH, Ma XK, Xing YF, et al. Comparison of current staging systems for advanced hepatocellular carcinoma not amendable to locoregional therapy as inclusion criteria for clinical trials. Asia Pac J Clin Oncol. 2013;9:86–92.
Bugada D, Allegri M, Lavand’homme P, De Kock M, Fanelli G. Inflammation-based scores: a new method for patient-targeted strategies and improved perioperative outcome in cancer patients. Biomed Res Int. 2014;2014:142425.
Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–91.
Nozoe T, Iguchi T, Adachi E, Matsukuma A, Ezaki T. Preoperative elevation of serum c-reactive protein as an independent prognostic indicator for gastric cancer. Surg Today. 2011;41:510–3.
Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–72.
Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via p2y2 receptor. Cancer Cell. 2013;24:130–7.
Grosse-Steffen T, Giese T, Giese N, Longerich T, Schirmacher P, Hansch GM, et al. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin Dev Immunol. 2012;2012:720768.
Llovet JM, Moitinho E, Sala M, Bataller R, Rodriguez-Iglesias P, Castells A, et al. Prevalence and prognostic value of hepatocellular carcinoma in cirrhotic patients presenting with spontaneous bacterial peritonitis. J Hepatol. 2000;33:423–9.
Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–34.
Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130:2747–60.
Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev. 2013;14:5237–42.
Feng JF, Huang Y, Zhao Q, Chen QX. Clinical significance of preoperative neutrophil lymphocyte ratio versus platelet lymphocyte ratio in patients with small cell carcinoma of the esophagus. Sci World J. 2013;2013:504365.
Ertas IE, Gungorduk K, Akman L, Ozdemir A, Terek MC, Ozsaran A, et al. Can preoperative neutrophil: lymphocyte and platelet: lymphocyte ratios be used as predictive markers for lymph node metastasis in squamous cell carcinoma of the vulva? Eur J Obstet Gynecol Reprod Biol. 2013;171:138–42.
Raungkaewmanee S, Tangjitgamol S, Manusirivithaya S, Srijaipracharoen S, Thavaramara T. Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer. J Gynecol Oncol. 2012;23:265–73.
Szkandera J, Pichler M, Absenger G, Stotz M, Arminger F, Weissmueller M, et al. The elevated preoperative platelet to lymphocyte ratio predicts decreased time to recurrence in colon cancer patients. Am J Surg. 2014;208(2):210–4.
He W, Yin C, Guo G, Jiang C, Wang F, Qiu H, et al. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol. 2013;30:439.
Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim KH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with folfox chemotherapy. BMC Cancer. 2013;13:350.
Lai Q, Castro Santa E, Rico Juri JM, Pinheiro RS, Lerut J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int. 2014;27:32–41.
Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012–9.
Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost. 2004;30:95–108.
Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–90.
Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci U S A. 2014;111(30):E3053–61.
Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, et al. Foxp3+ regulatory t cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–11.
Pinato DJ, Stebbing J, Ishizuka M, Khan SA, Wasan HS, North BV, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol. 2012;57:1013–20.
Conflicts of interest
None
Financial support
This work was supported by grants from the National Natural Science Foundation of China (No. 81372374), the Combination Project of Production, Education and Research from Guangdong Province and Ministry of Education (No. 2012B091100460), and the Science and Technology Planning Project of Guangdong Province 2011B031800076 (to Q. Lin), 2011B031800014 (to M. Dong) and 2012B031800259 (to J. Wen).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xing Li, Zhan-Hong Chen, and Yan-Fang Xing contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, X., Chen, ZH., Xing, YF. et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumor Biol. 36, 2263–2269 (2015). https://doi.org/10.1007/s13277-014-2833-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2833-9