Abstract
Transforming growth factor-beta1 (TGF-beta1) plays an important role in the pathogenesis of multiple malignancies, and also, its expression strongly affects the outcomes of cancer patients. The objective of this study was to determine the clinical significance of the serum levels of TGF-beta1 in gastric cancer patients. A total of 63 patients with a pathologically confirmed diagnosis of gastric cancer were enrolled into this study. Serum TGF-beta1 concentrations were determined by the solid-phase sandwich ELISA method. Thirty healthy age- and sex-matched controls were included in the analysis. The median age at diagnosis was 62 years, range 28 to 82 years. There was no significant difference in baseline serum TGF-beta1 levels between gastric cancer patients and the healthy control group (p = 0.08). The known clinical variables including age of patient, gender, site of lesion, histology, histological grade, stage of disease, and serum levels of lactate dehydrogenase (LDH), CEA, and carbohydrate antigen (CA) 19.9 were not found to be correlated with serum TGF-beta1 concentrations (p > 0.05). However, the chemotherapy-responsive patients had higher serum TGF-beta1 levels compared with chemotherapy-unresponsive ones (median values 330.50 v 49.54 pg/mL, respectively, p = 0.01). Moreover, patients with elevated serum TGF-beta1 concentrations had significantly favorable overall survival compared with those with lower levels (median 71.1 v 39.9 weeks, respectively, p = 0.04). In conclusion, serum levels of TGF-beta1 may have predictive and prognostic roles in patients with gastric cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transforming growth factor-beta (TGF-beta) plays a complex role during carcinogenesis. It is a potent inhibitor of epithelial cell proliferation during early stages of carcinogenesis and may also exert tumor promoter activities at later stages of carcinogenesis [1–14]. Most human tumors secrete large amounts of TGF-beta, which promotes cellular activities, such as proliferation, differentiation, migration, and survival [1–14]. It also contributes to the development of angiogenesis and is a negative regulator of immune functions.
Gastric cancer displays multifactorial etiology, and its genetic and immunological background has not yet been fully elucidated. In vitro trials showed that cultured gastric cancer cell lines produce excessive levels of cytokines and growth factors with pleiotropic biological activities [1–8]. Among them, TGF-beta functions as an autocrine and paracrine factor that drives many cellular processes such as tumor growth, invasion, angiogenesis, and metastasis.
Increased expression and secretion of TGF-beta isoforms in gastric cancer cells have been documented by several trials when compared with normal gastric cells [1–8]. In normal gastric cells, TGF-beta acts as a potent inhibitor of proliferation and differentiation. Cell autonomous activation of the TGF-beta pathway in gastric cell lines has been well documented. Among them, only TGF-beta1 isoform as a major secreted isoform in vitro was expressed both in normal gastric cells and gastric cancer cells and secreted high quantities to plasma [6–14].
TGF-beta1 shows biphasic effects on contributing to gastric tumorigenesis and progression. It may play a negative or tumor suppressor role by inhibiting cellular proliferation or by promoting cellular differentiation and apoptosis in the initial stage of gastric carcinogenesis [13]. However, as cancer develops, the paracrine TGF-beta1 produced by the cancer cells and the stromal cells stimulates angiogenesis and suppresses immune response, finally leading to progressive invasion and metastasis. Therefore, plasma levels of TGF-beta1 are found elevated in gastric cancer patients in concordance with metastatic progression [8–10, 12, 13].
Although majority of available findings were provided from preclinical trials, so far, there are only a few clinical studies to investigate the clinical significance of TGF-beta1 isoform in serum or plasma in gastric cancer patients [6–14]. Both conflicting and concordant results were provided by these trials. Thus, the significance of the serological levels of TGF-beta1 in patients with gastric cancer is not known yet.
Therefore, we evaluated the soluble serum levels of TGF-beta1 in gastric cancer patients and assessed associations with the prognosis, various known clinical variables, and response to chemotherapy, in order to examine whether these are potential new biomarkers, for use in the treatment of gastric cancer in this study.
Material and methods
Patients
This study included 63 consecutive patients admitted to the Institute of Oncology, Istanbul University. All patients had histologically confirmed gastric cancer and had not received chemotherapy or chemoradiation in the last 6 months. The staging was determined according to the AJCC (American Joint Committee on Cancer) and UICC (International Union against Cancer) staging systems. The pretreatment evaluation included assessment of detailed clinical history and physical examination with a series of biochemistry tests including lactate dehydrogenase (LDH), complete blood cell counts including thrombocytes (PLTs), leukocytes (WBCs), hemoglobin (Hb), and serum tumor markers, CEA and carbohydrate antigen (CA) 19.9. Those with Eastern Cooperative Oncology Group (ECOG) performance status 2 or less and appropriate blood chemistry tests received chemotherapy on an outpatient basis that included different combinations of fluorouracil, folinic acid, capecitabine, docetaxel, cisplatin, and epirubicin, with/without radiotherapy depending on the stage of disease. Follow-up programs included clinical, laboratory, and radiological assessments performed at 8-week intervals during chemotherapy or every 12 weeks for no anticancer treatment. Response to treatment was determined according to the revised RECIST criteria version 1.1.
For comparison of serum levels of TGF-beta1, 30 healthy controls (age- and sex-matched) were included in the analysis. Informed consent was obtained from all patients, and the study was reviewed and approved by our local ethical committee.
Measurement of serum TGF-beta1 levels
Serum samples were obtained on first admission before any adjuvant and metastatic treatment was given or follow-up patients. Blood samples were obtained from patients with gastric cancer and healthy controls by venipuncture and clotted at room temperature. The sera were collected following centrifugation and frozen immediately at −20 °C until analysis.
TGF-beta1 ELISA (Invitrogen Corporation, USA) uses a double-antibody sandwich enzyme-linked immunosorbent assay to determine the level of human TGF-beta1 in samples. Serum samples, standards, and biotin conjugate were added to the wells to incubate for 3 h. Unbound material was washed away. TGF-beta1 combined with streptavidin-HRP was added to form an immune complex and then allowed to incubate for 30 min. Unbound material was washed away. Chromogen solution was added and incubated for 30 min (protected from light) for the conversion of the colorless solution to a blue solution, the intensity of which was proportional to the amount of TGF-beta1 in the sample. As the effect of the acidic stop solution, the color has become yellow. The colored reaction product was measured using an automated ELISA reader (Rayto, RT-1904C Chemistry Analyzer, Atlanta, GA, USA) at 450 nm. The results were expressed as picograms per milliliter.
Statistical analysis
Continuous variables were categorized using median values as cutoff point. Assessment of relationships, comparisons between various clinical/laboratory parameters, and serum levels of TGF-beta1 assay were accomplished using Mann-Whitney U test. Survival was calculated from the date of the first admission to hospital to death resulting from any cause or to last contact with the patient or any family member. Kaplan-Meier method was used for estimation of survival of patients, and differences in survivals were assessed by the log-rank statistics. A p value <0.05 was considered significant. Statistical analysis was carried out using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
A total of 63 patients with diagnosis of gastric cancer were enrolled in this study. The baseline histopathological characteristics and the demographic characteristics of the patients are listed in Table 1. The median age at diagnosis was 62 years, range 28 to 82 years.
There was no significant difference in baseline serum TGF-beta1 levels between gastric cancer patients and the healthy control group (median values 211.67 v 79.11 pg/mL, respectively, p = 0.08) (Table 2). The known clinical variables including age of patient, gender, site of lesion, histology, histological grade, stage of disease, and serum levels of LDH, CEA, and CA 19.9 were not found to be correlated with serum TGF-beta1 concentrations (p > 0.05) (Table 3). However, the chemotherapy-responsive patients had higher serum TGF-beta1 levels compared with chemotherapy-unresponsive ones (median values 330.50 v 49.54 pg/mL, respectively, p = 0.01).
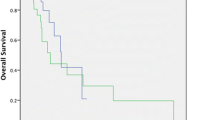
The median follow-up time was 25 weeks (range 1 to 164 weeks). At the end of the observation period, 35 patients (55.6 %) were dead. The median survival for all patients was 42.0 ± 4.2 weeks (95 % confidence interval (CI) = 33.8–50.2). The 1-year survival rates were 42.2 % (95 % CI = 28.3–56.1). The presence of metastasis (M1) (p = 0.03), antrum localization (p = 0.04), elevated erythrocyte sedimentation rate (p = 0.02), higher serum CEA levels (p = 0.01), elevated serum CA 19.9 concentrations (p = 0.04), and unresponsiveness to chemotherapy (p = 0.05) had statistically significant worse survival variables (Table 4). Moreover, patients with elevated serum TGF-beta1 concentrations had significantly favorable overall survival compared with those with lower levels (median 71.1 v 39.9 weeks, respectively, p = 0.04) (Table 4 and Fig. 1).
Discussion
TGF-beta1 is secreted by normal gastric cells and gastric tumor cells at various stages. Despite some discrepancies, many studies showed that an increase in TGF-beta1 expression levels correlates with tumor progression and is associated with poor prognosis [1–4], whereas its expression may only have a minor influence on survival [5]. In these studies, positive staining for TGF-beta1 correlated with lesion progression and increased in advanced stages compared with early stages and normal tissues [5–7]. Moreover, the expression rates were frequent in differentiated [3] and undifferentiated histology [8], diffuse type adenocarcinoma [5], and intestinal type adenocarcinoma [2].
So far, this isoform was studied in serum or plasma of gastric cancer patients in only a few trials. The results revealed that serum concentrations of TGF-beta1 were higher in patients with gastric cancer than in healthy controls [7, 10–13]. Likewise, we also found similar result in this study. However, this finding could not reach a statistically significant level (p = 0.08).
However, the relationship between serum concentrations of TGF-beta1 and clinicopathological characteristics was found to be controversial. Some previous studies found that serum levels of TGF-beta1 in gastric cancer patients were positively correlated to tumor mass, venous invasion, metastasis, and clinical stage [8–10, 12, 13]. Contrarily, others have reported no differences in serum TGF-beta1 concentrations in terms of serosal invasion, lymph node involvement, vascular invasion, distant metastasis, tumor size, or histopathological grades in gastric cancer [6, 7, 11, 12]. Moreover, no significant differences in serum TGF-beta1 levels were found in gastric cancer patients with different ages, genders, tumor localizations, and histological grades [12–14]. The present study demonstrated that none of the clinicopathological variables such as age, sex, localization of tumor, histology, grade of histology, stage of disease, and tumor markers including CEA and CA 19.9 was correlated with serum levels of TGF-beta1 in patients with gastric cancer.
Serum levels of TGF-beta1 have been examined firstly in patients with gastric cancer as a possible indicator of prognosis by Saito et al. [9]. In this study, preoperative serum levels of TGF-beta1 were measured in 111 patients with gastric cancer by ELISA. An elevated level of TGF-beta1 was significantly correlated with lymph node metastasis and poor prognosis. Moreover, the preoperative serum levels of TGF-beta1 in patients with peritoneal recurrence were significantly higher than in patients both with lymph node recurrence and without relapse. Authors concluded that serum levels of TGF-beta1 might be useful for predicting recurrence patterns and prognosis in patients with gastric cancer. Similarly, we found that the concentration of serum TGF-beta1 constitutes an independent prognostic factor correlated with prognosis. Patients with elevated serum TGF-beta1 concentrations had significantly favorable overall survival compared to those with lower levels (p = 0.04).
To date, none of the trials that studied serum levels of TGF-beta1 in gastric cancer investigated and presented its predictive value to chemotherapy. Therefore, there exists no information about a possible influence of serum TGF-beta1 concentration on response to chemotherapy. According to us, this is the first study to examine the association between the serum level of TGF-beta1 and its predictive role in patients with gastric cancer. In addition to its prognostic value, serum TGF-beta1 levels had also a predictive role in chemotherapy. In our study, the chemotherapy-responsive patients had higher serum TGF-beta1 levels compared with chemotherapy-unresponsive ones (p = 0.01). This finding gives us useful opportunity for selecting the patients responsive to chemotherapy.
The differences in the findings with TGF-beta1 probably reflect the differences in source, kinetics of expression or destruction, and possibly signals including their expression and release. Additionally, these contradicting results of these studies might be attributable to several other factors. So far, no consensus exists on which tumors and methods should be used for testing TGF-beta1 expressions. During recent decades, immunohistochemistry (IHC) has become a useful adjunctive method in diagnostic histopathology. There are a number of drawbacks with IHC, the most important of which are lack of assay standardization and variance in the interpretation of the IHC staining. Additionally, each of the studies was performed on a relatively small sample size, which may have been insufficient to show significant differences. A standardized method remains to be established and validated in larger series of patients in prospective studies.
In conclusion, TGF-beta1 is closely related to the invasion, metastasis, and prognosis of gastric cancer, and production of TGF-beta1 in the tumor contributes to the total amount of TGF-beta1 in the blood circulation. We showed that serum levels of TGF-beta1 may have predictive and prognostic roles in patients with gastric cancer. However, the small sample size and short follow-up time of our study could be considered as significant limitation and might have influenced these results. However, our study contributes to the literature, because we performed it that contained all stages group of disease preliminarily in literature. Further studies in a larger patient population are necessary to determine the potential clinical significance of these assays in patients with gastric cancer.
References
Vagenas K, Spyropoulos C, Gavala V, Tsamandas AC. TGFbeta1, TGFbeta2, and TGFbeta3 protein expression in gastric carcinomas: correlation with prognostic factors and patient survival. J Surg Res. 2007;139:182–8.
Ananiev J, Gulubova M, Tchernev G, Penkova M, Miteva R, Julianov A, et al. Relation between transforming growth factor-β1 expression, its receptor and clinicopathological factors and survival in HER-2 negative gastric cancers. Wien Klin Wochenschr. 2011;123:668–73.
Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, et al. The expression of transforming growth factor-1 is significantly correlated with the expression of vascular endothelial growth factor and poor prognosis of patients with advanced gastric carcinoma. Cancer. 1999;86:1455–62.
Hawinkels LJ, Verspaget HW, van Duijn W, van der Zon JM, Zuidwijk K, Kubben FJ, et al. Tissue level, activation and cellular localization of TGF-beta1 and association with survival in gastric cancer patients. Br J Cancer. 2007;97:398–404.
Kim JY, Jeon TJ, Bae BN, Kwon JE, Kim HJ, Park K, et al. The prognostic significance of growth factors and growth factor receptors in gastric adenocarcinoma. APMIS. 2012;121:95–104.
Maehara Y, Kakeji Y, Habashima A, Emi Y, Watanabe A, Akazawa K, et al. Role of transforming growth factor-β in invasion and metastasis in gastric carcinoma. J Clin Oncol. 1999;17:607–14.
Ma GF, Miao Q, Zeng XQ, Luo TC, Ma LL, Liu YM, et al. Transforming growth factor-β1 and -β2 in gastric precancer and cancer and roles in tumor-cell interactions with peripheral blood mononuclear cells in vitro. PLoS One. 2013;8:e54249.
Suda A, Saito N, Seshimo A, Kameoka S, Kobayashi M. Examination of transforming growth factor beta1 expression in the serum and tumor tissue of gastric cancer. Int Surg. 2009;94:182–8.
Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, et al. An elevated serum level of transforming growth factor-beta 1 (TGF-beta 1) significantly correlated with lymph node metastasis and poor prognosis in patients with gastric carcinoma. Anticancer Res. 2000;20:4489–93.
Niki M, Okajima K, Isozaki H, Toyoda M, Ichinona T, Nomura E, et al. Measurement of the plasma transforming growth factor-beta 1(TGF-beta 1) level in patients of gastric carcinoma-compared with the serum IAP level and the lymphocyte subsets (CD3, CD4, CD8). Nippon Shokakibyo Gakkai Zasshi. 1996;93:303–11.
Coban S, Yuksel O, Kockar MC, Koklu S, Basar O, Tutkak H, et al. The significance of serum transforming growth factor beta 1 in detecting of gastric and colon cancers. Hepatogastroenterology. 2007;54:1472–6.
Lin Y, Kikuchi S, Obata Y, Yagyu K. Serum levels of transforming growth factor β1 are significantly correlated with venous invasion in patients with gastric cancer. J Gastroenterol Hepatol. 2006;21:432–7.
Li X, Yue ZC, Zhang YY, Bai J, Meng X, Geng JS, et al. Elevated serum level and gene polymorphisms of TGF-β1 in gastric cancer. J Clin Lab Anal. 2008;22:164–71.
Yatsuya H, Toyoshima H, Tamakoshi K, Tamakoshi A, Kondo T, Hayakawa N, et al. Serum levels of insulin-like growth factor I, II, and binding protein 3, transforming growth factor beta-1, soluble fas ligand and superoxide dismutase activity in stomach cancer cases and their controls in the JACC Study. J Epidemiol. 2005;15 Suppl 2:S120–5.
Conflicts of interest
None
Role of the funding source
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tas, F., Yasasever, C.T., Karabulut, S. et al. Serum transforming growth factor-beta1 levels may have predictive and prognostic roles in patients with gastric cancer. Tumor Biol. 36, 2097–2103 (2015). https://doi.org/10.1007/s13277-014-2817-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2817-9