Abstract
Cyclophilin A (CypA) was shown to be upregulated in human cholangiocarcinoma (CCA) tissues. Suppression of intracellular CypA (inCypA) significantly reduces cell proliferation in vitro and tumor growth in nude mice. In the present study, the effect and potential mechanism of secreted CypA (sCypA) on cell proliferation of CCA cell lines were further investigated. CCA cells were treated with sCypA-containing conditioned media (CM) or with purified recombinant human CypA (rhCypA). Cell proliferation, cell cycle, ERK1/2, p38 MAPK, NF-κB, and STAT3 activities were examined by MTS assay, flow cytometry, and Western blot. sCypA was detected in CM from MMNK1 (an immortalized human cholangiocyte cell line) and six CCA cell lines. The sCypA levels corresponded to the inCypA levels indicating the intracellular origin of sCypA. Both sCypA-containing CM and rhCypA significantly increased proliferation of CCA cells. CD147 depletion by shRNA-knockdown or neutralizing with a CD147-monoclonal antibody significantly reduced sCypA-, and rhCypA-mediated cell proliferation. Upon rhCypA treatment, ERK1/2 was rapidly phosphorylated; whereas neutralizing CD147 inhibited ERK1/2 phosphorylation. Cell cycle analysis showed a significant increase in S phase and decrease in G1 population in rhCypA-treated cells. The expression levels of cyclin D1 and phosphorylated-retinoblastoma protein in the rhCypA-treated cells were increased compared with those in the non-treated control cells. p38 MAPK pathway was shown to be suppressed in siCypA-treated cells. In summary, CypA is secreted from CCA cells and enhances cell proliferation in an autocrine/paracrine manner, at least via direct binding with CD147, which may activate the ERK1/2 and p38 MAPK signaling pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world’s highest incidence of cholangiocarcinoma (CCA) is reported in Northeastern Thailand [1] where the liver fluke, Opisthorchis viverrini, is endemic. Chronic infection with this liver fluke has been shown to be the strong risk factor for CCA in this region [2]. Patients with CCA are usually diagnosed at late stages due to the lack of early diagnostic markers and, hence, few patients are candidates for potentially curative surgical resection. Chemotherapy is the backbone of treatment for those whom surgery is not possible. Systemic genotoxic chemotherapy and molecular-targeted monotherapy, however, do not produce long-term survival benefits [3, 4]. Therefore, multi-targeted and combinational treatment strategies may be a new hope for patients with advanced CCA.
Cyclophilin A (CypA), peptidyl-prolyl cis-trans isomerase, plays an important role in cellular processes. It acts as an acceleration factor in protein folding and assembly [5], facilitates intracellular trafficking [6], signal transduction, and transcriptional regulation [7]. CypA is an intracellular protein [8] which expresses in all tissue/cell lineages in mammals. The secreted form of CypA (sCypA), however, is reported in some pathological conditions. sCypA is secreted by a variety of cell types in response to inflammatory stimuli [9, 10]. In rheumatoid arthritis, sCypA exhibits potential chemotactic effects on leukocytes [11, 12], upregulates matrix metalloproteinases via the mitogen-activated protein kinases (MAPK) and the nuclear factor-κB signaling pathway [13, 14]. On the other hand, sCypA enhances cell proliferation in lung and pancreatic cancer cells via activation of extracellular signal-regulated kinases 1/2 (ERK1/2) [15] and p38-MAPK pathways [16]. sCypA exerts these functions via a signaling receptor, CD147 [17]. Many studies showed that CypA confers growth advantage and correlates with poor clinical outcomes in various cancers [18]. At present, however, the exact mechanisms of the mitogenic activity of sCypA and its role in pathogenesis of cancer remain unclear.
Recently, it has been demonstrated that CypA was upregulated in the majority of CCA patients’ tissues. It was clearly shown that CypA confers a growth advantage on CCA cells both in vitro and in vivo [19]. Moreover, CD147, a known receptor of CypA, was also over-expressed in CCA cell lines [20]. In the present study, it was further demonstrated that CCA cells do secrete CypA, which significantly enhances cell proliferation. Moreover, sCypA exerts its mitogenic activity via CD147. The downstream signaling of sCypA action was explored.
Materials and methods
Cell lines
All CCA cell lines used in this study were established from primary tumors of histologically proven CCA patients as previously described [19, 21]. An immortalized human cholangiocytes cell line, MMNK1, was a gift from Dr. Kobayashi N [22]. Stable cells expressing shCypA (TI309388, OriGene), shCD147 (GI357710, OriGene), and shV plasmids (TR20003, OriGene) were established using retroviral vectors in KKU-M139 and KKU-M213 cells, according to the manufacturer’s instructions [19]. Stable cell lines were selected with the media containing 0.5 μg/mL puromycin and cultured for at least 2 weeks. Expression levels of the specified genes were confirmed by real-time PCR and Western blot. All cell lines were maintained at 37 °C with 5 % CO2 in RPMI 1640 medium supplemented with 10 % fetal bovine serum and 1 % antibiotics/anti-mycotics.
Antibodies
Anti-CypA (Millipore), anti-β-actin (Sigma Aldrich), anti-CD147 (Abcam), anti-ERK1/2, anti-pERK1/2, anti-Cyclin D1, anti-RB, anti-p-RB, anti-STAT3, anti-pSTAT3, anti-p38, anti-p-p38, anti-NF-κB-p50, -p52, -p65, and anti-histone H1 (Santa Cruz Biotechnology) were used in this study. Purified monoclonal antibody (clone M6-1B9) [23] against the extracellular part of CD147 was used for CD147 blocking experiment in this study.
Conditioned medium (CM)
CM was collected from CCA cells cultured in RPMI 1640 medium without FBS for 48 h. Protein degradation was avoided by adding protease inhibitor cocktail (Roche Diagnostics). CM was concentrated to 20-fold by Vivaspin 20 with a 3 kDa molecular weight cutoff according to the manufacturer (GE Healthcare) and kept at −20 °C until use. Total protein was determined using the Quick Start™ Bradford protein assay kit (Bio-Rad Laboratories Inc.).
Western blot analysis
Cells were lyzed with NP40 lysis buffer for 30 min on ice. Cell lysates were collected, and protein concentration was determined using the Quick Start™ Bradford protein assay kit and kept at −20 °C until use. Total protein (20 μg) was separated on a 12 % SDS-polyacrylamide gel electrophoresis and transferred to a Hybond™-P PVDF membrane (GE Healthcare). The membrane was probed with each primary antibody at 4 °C overnight and 1:20,000 HRP-conjugated secondary antibody (Invitrogen) for 1 h at room temperature. The immunoactive bands were detected using an enhanced chemiluminescence prime Western blotting detection reagent kit (GE Healthcare).
Transient knockdown CypA using siRNA
Cells (2 × 105 cells/well) were seeded in a 6-well plate overnight and transfected with 100 pmole/mL of siRNA against CypA (siCypA) or scrambled control siRNA (SC) for 6 h. Lipofectamine™ 2000 (Invitrogen) was used as a transfection reagent. Cells were then cultured in a complete medium until harvesting time. Cells collected at 24 h were used for messenger RNA (mRNA) extraction. Cells collected at 24 h after transfection were used for Western blot analyses of p38 MAPK, NF-κB pathway, CD147, and CypA protein expressions, whereas those collected at 48 h were used for STAT3 analysis. The siCypA and the scrambled sequence siRNA were as described in the study of Obchoei et al. [19].
CypA and CD147 mRNA quantitation by real-time PCR
Total RNA extraction and quantification of the CypA and CD147 using real-time reverse transcriptase-PCR were as previously described [19]. The mRNA level of each sample was normalized to that of β-actin mRNA and presented as relative mRNA level of 2[Ct(β-actin)-Ct(target)]. The primer design and sequences for human CypA gene, human CD147 gene, and the housekeeping gene β-actin were previously described [16]. Each sample was run in triplicate.
Cell cycle analysis
Cells were trypsinized and fixed in 70 % ethanol for 1 h on ice. After washing twice with PBS, cells were stained with propidium iodide (40 μg/mL) for 5 min at room temperature and placed on ice until analysis. The distribution of the cell cycle in each phase was analyzed with FlowJo software (Tree Star, Inc., Ashland, OR).
Cell proliferation assay
Cells (2 × 103cells/well) were seeded in a 96-well plate and cultured in serum-free medium (SFM) for 24 h. Cells were cultured in specified medium for 48 h before subjection to the MTS assay using the CellTiter 96® AQueous One Solution Cell Proliferation Assay Kit from Promega (Madison, WI). Cells treated with SFM were used as a non-treated control. The purified recombinant human CypA (rhCypA; Sigma Aldrich) 0, 0.1, and 1 ng/mL in RPMI 1640 supplemented with 1 % FBS or boiled rhCypA (1 ng/mL) for 5 min were used to determine the action of extracellular CypA. To investigate the involvement of CD147, cells were pre-incubated with either anti-CD147 (5 μg/mL) or IgG isotype control antibody (Sigma) prior to rhCypA treatment.
Statistical analysis
All quantitative data were expressed as mean ± SD. The two-tailed Student’s t test was used for comparison between two groups. Statistical significance was established at P < 0.05.
Results
CCA cells secrete CypA based on the inCypA level
The CypA levels in the CM from six human CCA cell lines and a non-tumor human cholangiocyte cell line, MMNK1, were examined. Concentrated CM and the whole cell lysates were subjected to Western blot analysis for CypA. As shown in Fig. 1a, sCypA was detected in all CM with the apparent molecular weight of ∼18 kDa similar to that of inCypA. Semi-quantitative analysis indicated that sCypA levels were positively correlated with the inCypA levels (Fig. 1b). β-actin as an intracellular marker was detected in all cell lysates, but not in any CM tested. In addition, sCypA and inCypA were dramatically suppressed in the stable CypA knockdown cells, KKU-M139-shCypA, compared with those in the vector control cells, KKU-M139-shV (Fig. 1c, d).
inCypA and sCypA in CCA cell lines. a Western blot, inCypA protein levels in total protein lysates and sCypA protein levels in culture mediums of six CCA cell lines and MMNK1 cells. b Quantitative results of a. c Western blot, inCypA levels detected in whole cell lysates and sCypA detected in CM from KKU-M139-shV and KKU-M139-shCypA cells. d Quantitative results of c. Relative band intensity = inCypA/β-actin; sCypA/20 μg protein
sCypA activates cell proliferation
To demonstrate that sCypA is a key factor in the CM which activates cell proliferation, the CM with low sCypA from KKU-M139-shCypA (shCypA-CM) and the CM with high sCypA from KKU-M139-shV (shV-CM) were used to activate cell proliferation of two CCA cell lines; KKU100 (low endogenous CypA), and KKU-M139 (high endogenous CypA). Cells incubated in serum-free medium (SFM) were used as controls. The results showed that cells incubated with shV-CM (high sCypA) had a high cell proliferation rate, whereas the cells cultured with shCypA-CM (low sCypA) had a low proliferation rate similar to that of the SFM controls (Fig. 2a). The proliferation rates of KKU100 and KKU-M139 cells cultured in shCypA-CM were 23 % and 18 % lower than those of shV-CM, respectively, (P < 0.05).
Effect of extracellular CypA on CCA cell proliferation. a Effect of sCypA on CCA cell proliferation. KKU100 and KKU-M139 cells were incubated for 48 h with the fresh CM collected from KKU-M139-shCypA and KKU-M139-shV cell cultures with or without pre-incubating with 1 μg/mL anti-CypA for 1 h prior to use. Bar graphs represent means ± SD; n = 3, *P < 0.05 versus shV-CM-treated groups. b Effect of rhCypA on CCA cell proliferation of KKU100 and KKU-M139 cells. Cells were incubated with rhCypA 0, 0.1, and 1 ng/mL in RPMI 1640 supplemented with 1 % FBS for 48 h before subjected to cell growth determination using the MTS test. 1 ng/mL rhCypA boiled for 5 min was used as a non-active CypA control. Bar graphs represent means ± SD; n = 3, *P < 0.05 versus non-treated, # P < 0.05 versus 1 ng/mL-treated groups
To confirm that the mitogenic effect observed in shV-CM is attributed to sCypA in CM, anti-CypA antibody was added to CM to neutralize the action of sCypA. As shown in Fig. 2a, anti-CypA antibody treatment, but not IgG isotype control treatment, significantly reduced the enhancement of shV-CM on cell proliferation to the basal level of SFM controls (P < 0.05 versus shV-CM). The similar treatment had no effect on cells cultured with shCypA-CM.
To ascertain the action of extracellular CypA on cell proliferation, KKU100 and KKU-M139 cells were cultured in the absence or presence of various concentrations of rhCypA. The results showed that rhCypA significantly increased cell proliferation in a concentration-dependent fashion. The treatment enhanced cell proliferation by 46 and 37 % in KKU100 and KKU-M139, respectively, (P < 0.05 versus control) (Fig. 2b). In addition, the mitogenic effect of rhCypA was abolished when rhCypA was denatured by heat at 100 °C for 5 min (P < 0.05 versus 1 ng/mL-treated group).
sCypA enhances CCA cell proliferation through CD147
To determine whether CD147 is involved in the mitogenic activity of sCypA, KKU100 and KKU-M139 cells were pre-treated with specific antibody against CD147 or IgG isotype control before incubating with the CMs. Cells incubated with shV-CM plus IgG had the highest proliferation rate. Pre-treated cells with anti-CD147 monoclonal antibody, however, significantly abolished shV-CM-induced cell proliferation to the basal level of SFM controls (P < 0.05). A similar treatment had no effect on proliferation of the cells cultured in shCypA-CM (Fig. 3a).
Involvement of transmembrane-CD147 on sCypA-mediated CCA cell proliferation. Cell proliferation was determined using the MTS assay. Bar graphs represent means ± SD from three independent experiments. a Effect of CD147 blocking on sCypA-induced CCA cell proliferation. Cells were pre-treated with either anti-CD147 or IgG isotype control for 1 h before adding CMs. *P < 0.05 versus shV-CM + IgG. b Effect of CD147 blocking on rhCypA-induced CCA cell proliferation. KKU100 and KKU-M139 cells were pre-incubated with either anti-CD147 or IgG isotype control antibody prior to rhCypA treatment. *P < 0.05 versus non-treated, # P < 0.05 versus rhCypA + IgG. c Western blot analysis confirmed the suppression of CD147 expression in M213-shCD147 compared to M213-shV, β-actin was used as an internal control. d Effect of rhCypA on cell proliferation of CD147 knockdown (shCD147) cells. The stable cells were incubated for 48 h with 0, 0.1, and 1 ng/mL rhCypA and boiled rhCypA (1 ng/mL). *P < 0.05 versus non-treated, # P < 0.05 versus shV cells treated with rhCypA 1 ng/mL
To emphasize the significance of CD147 on extracellular CypA action, cells were pre-treated with monoclonal antibody against CD147 prior the treatment of rhCypA. As shown in Fig. 3b, CD147 antibody significantly abolished the activation of rhCypA on cell proliferation to similar levels as two controls: cells treated with IgG or anti-CD147 antibody alone. The important role of CD147 on sCypA action was further shown in the CD147 knockdown cells. Western blotting of CD147 revealed that M213-shCD147 had a much lower expression of CD147 compared with the vector control M213-shV cells (Fig. 3c). Adding rhCypA to the medium did not augment cell proliferation of M213-shCD147 cells compared with that of M213-shV cells (Fig. 3d).
rhCypA mediates G1 to S phase transition possibly through ERK1/2-induced cyclin D1 expression
The effect of extracellular CypA on cell cycle distribution was examined next. As shown in Fig. 4, rhCypA treatment significantly increased the proportion of S phase cells of KKU100 (10 %, Fig. 4a) and KKU-M139 cells (13 %, Fig. 4b); while G1 phase populations of KKU100 and KKU-M139 were significantly decreased (P < 0.05 versus controls).
Effect of rhCypA on cell cycle distribution of KKU100 and KKU-M139 cells. KKU100 and KKU-M139 cells were treated with 1 ng/mL rhCypA for 48 h. The cells were stained with propidium iodide and then were subjected to flow cytometry analysis. a The cells in the G0/G1 phase, S phase, and G2/M phase were analyzed with FlowJo software. b Bar graphs show percentage of cell cycle distribution, data represent means ± SD; n = 3, *P < 0.05 versus non-treated control
Since sCypA and rhCypA activated proliferation of CCA cell lines via its receptor, CD147, the intracellular signaling downstream of CypA-CD147 interaction was explored. KKU100 and KKU-M139 cells were treated with rhCypA. Phosphorylation of ERK1/2 (pERK1/2) and its down-stream signals cyclin D1, retinoblastoma protein (RB) and phosphorylated RB (p-RB) proteins were examined by Western blot at different time points up to 120 min. As shown in Fig. 5a (KKU100) and Fig. 5b (KKU-M139), pERK1/2 was increased rapidly within 5–10 min, whereas cyclin D1 and p-RB were upregulated thereafter.
rhCypA enhances G1 to S phase transition via CD147 and activation of ERK1/2 pathway. a Effects of rhCypA on ERK1/2 activation and on the expression of cell cycle mediators in KKU100. b Effects of rhCypA on ERK1/2 activation and on the expression of cell cycle mediators in KKU-M139. Cells were treated with 1 ng/mL rhCypA for 0, 5, 10, 30, 60, and 120 min. Expression levels of p-ERK1/2, total ERK1/2 proteins, cyclin D1, p-RB, and RB proteins were analyzed by Western blot. Anti-CD147 blocked rhCypA-mediated ERK1/2 activation in c KKU100 and d KKU-M139. The cells were pre-treated with anti-CD147 or IgG isotype control for 1 h and then the cells were incubated with 1 ng/mL rhCypA for 10 min, and the samples were then collected for ERK1/2 and pERK1/2 detection. The data shown are the ratios of band intensities between pERK1/2 and ERK1/2
To confirm that the activation of ERK1/2 by CypA was mediated via CD147, CCA cells were pre-incubated with either anti-CD147 or IgG isotype control antibodies for 1 h. Cells then were challenged with rhCypA for 10 min prior to Western blot analysis. Compared with the cells treated with IgG isotype control antibodies, the pre-treated cells with anti-CD147 antibodies effectively reduced ERK1/2 phosphorylation activated by rhCypA in KKU100 (Fig. 5c) and KKU-M139 cells (Fig. 5d).
As CypA action involves many signal transduction pathways, i.e., p38 MAPK [24], signal transducers and activators of transcription 3 (STAT3) [25], and NF-κB pathways [26–28], we further investigated whether silencing of CypA affected these pathways in CCA cell lines. Total STAT3 and phosphorylated STAT3 (pSTAT3), p38 and phosphorylated p38 (p-p38), and NF-κB in nuclear and cytosolic fractions were determined in CypA knockdown and scrambled control cells. STAT3, a transcription factor, is aberrantly activated in many cancer cells, including CCA [29–31]. Constitutively activated STAT3 is oncogenic, presumably as a consequence of the genes that it differentially regulates. Activated STAT3 can upregulate the mRNA levels of many genes, including cyclin D1 [32, 33]. Therefore, it was tested whether STAT3 pathway also contributes to cyclin D1 induction and G1 to S progression upon sCypA activation. Total STAT3 and pSTAT3 were examined in siCypA and SC of KKU100 and KKU-M139 cell lines. As shown in Fig. 6a, unlike the ERK pathway, STAT3 protein, both total and phosphorylated forms, remained unchanged in siCypA-transfected KKU-M139 cell line as compared to the scrambled control. As the constitutive phosphorylation of STAT3 in KKU100 cell line was very low and barely detectable by Western blot and to assure that knockdown CypA did not involve STAT3 protein expression or activation, a similar experiment in another CCA cell line, KKU-M214 was performed. As observed in the KKU-M139, total STAT3, and pSTAT3 proteins in siCypA-treated KKU-M214 were not altered as compared with the control cells.
CypA silencing (siCypA) suppresses p38 protein expression and phosphorylation. Three possible signal pathways related to CypA: a STAT3, b NF-κB, and c p38 MAPK were examined. Key molecular proteins of each pathway were determined using Western blotting. β-actin and histone H1 protein were used as internal controls for cytosolic proteins and nuclear proteins, respectively. Cells treated with siCypA or scrambled control siRNA for 24 h were used for p38 MAPK and NF-κB pathways, and those treated for 48 h were used for STAT3 detection
To determine the association of CypA and NF-κB, all NF-κB subunits (p50, p52, p65, and p100) were examined in the siCypA-treated and the SC cells. As shown in Fig. 6b, the NF-κB subunits detected in the cytosolic and nuclear fractions were not significantly different between siCypA-treated and the SC cells. In contrast to STAT3 and NF-κB, p38 MAPK expression and phosphorylation were reduced significantly upon CypA silencing in all three CCA cell lines tested (Fig. 6c).
CypA regulates CD147 expression
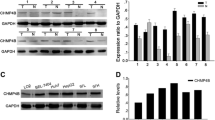
Since CypA is shown to regulate the expression of CD147 [34–36], we further elucidated whether alteration of CypA expression affects CD147 expression level. Real-time PCR and Western blot of CD147 were determined in siCypA-treated and the SC cells of three CCA cell lines. The results showed that silencing of CypA reduced CD147 expressions at both mRNAs (Fig. 7a) and proteins (Fig. 7b) of KKU-M139 and KKU-M214 but not of KKU-100.
siCypA suppresses expression of CD147. Cells were treated with siCypA or scrambled control siRNA for 24 h. The expression levels of CD147 were determined at mRNA level using real-time PCR (a) and at protein level using Western blot (b). Two CCA cell lines with high expression of CD147 (KKU-M139 and KKU-M214) and one with low expression of CD147 (KKU-100) were used in this study. β-actin was used as an internal control
Discussion
We have recently shown that CypA is over-expressed in CCA patients’ tissues and the endogenous levels of CypA are positively correlated with cell growth potential of CCA cell lines [19]. In general, CypA is found predominantly as a cytoplasmic protein, but it can be detected as a secretory protein in some pathological conditions. In the present study, it is further demonstrated that CCA cells do secrete CypA based on inCypA levels and sCypA promotes cell growth via the CD147/ERK1/2 pathway.
Our results clearly show that CCA cell lines are able to secrete CypA. Firstly, CypA was detected in the CM of all CCA cell lines studied. Secondly, the levels of sCypA were positively correlated with inCypA levels; and thirdly, suppression of CypA expression lowered the level of inCypA and consequently reduced the sCypA level. Concomitantly, CypA has also been identified as a protein secreted by human cancer cells, e.g., head and neck cancer [37], irradiated breast cancer [38], and prostate cancer [39].
The role of sCypA in proliferation of CCA cell lines was investigated in the current study. High sCypA-CM from shV-harbored cells, but not low sCypA-CM from shCypA-transfected cells, significantly enhanced cell proliferation compared with SFM controls. To ensure that the growth enhancing effect observed in shV-CM was attributed to the sCypA present in the CM, anti-CypA antibodies were used to neutralize sCypA activity in the CM prior to the treatment. sCypA was shown to be a key molecule in activating CCA cell proliferation because the proliferation-enhancement observed with the shV-CM was mostly abolished to the basal level of cells cultured in SFM when sCypA was neutralized by anti-CypA antibody. The action of sCypA on activation of cell proliferation was confirmed by the fact that addition of rhCypA, to the cell culture media, mediated cell proliferation in a dose-dependent fashion. CypA-mediated cell proliferation has been reported in small cell lung cancer [15] and pancreatic cancer [16] in which synthetic rhCypA also activated cancer cell proliferation. In addition to the published information, direct evidence was provided here that sCypA originated from inCypA of cancer cells enhanced cell growth in an autocrine/paracrine manner.
CD147 is the only known signaling receptor for sCypA. Blocking sCypA/CD147 interaction by monoclonal antibody against CD147 or reduced CD147 expression significantly suppressed the effect of sCypA or rhCypA on cell proliferation and ERK1/2 activation in KKU100 and KKU-M139 cells. The requirement of CD147 for CypA-induced cancer cell proliferation through ERK1/2 pathway is also reported for pancreatic cancer cells [16]. The mechanisms by which CypA-CD147 activates the signaling pathway are not fully elucidated. In the present study, the ERK1/2 pathway was shown to be a critical signaling pathway that regulates cell proliferation upon sCypA action. This observation is supported by the previous report that CypA had a positive correlation with the phosphorylation of ERK1/2 [19] and transient/stable knockdown of inCypA inhibited the pERK1/2 in KKU-M139 cells. In this study, a rapidly increase of ERK1/2 phosphorylation was observed within 5–10 min after stimulation by rhCypA. The rapid activation of ERK1/2 seems to be a common phenomenon of ERK1/2 in response to virtually all mitogenic factors [40, 41]. Increasing pERK1/2 has been detected in a variety of human malignant cells including CCA. Almost 50 % (29/59) of CCA patients exhibited ERK1/2 activation, whereas none was found in the normal bile duct epithelium [42].
In this study, we demonstrate, for the first time, that rhCypA drives cells from G1 to S phase, upregulates cyclin D1 levels, and concomitantly increases the amount of p-RB protein. This activation may be mediated by ERK1/2 signaling pathway as it is known that ERK1/2 promotes cell proliferation through the G1 phase by driving the expression of the D-type cyclins such as cyclin D1 [43]. Apart from ERK pathway, STAT3 was shown to be an important pathway for CCA cell growth and survival by both constitutively activated [30] or induction by autocrine/paracrine growth factors such as interleukin-6 [44, 45]. Activated STAT3 can upregulate cyclin D1 and drive cells from G1 to S phase [32, 33]. The observed cyclin D1 upregulation and G1 to S phase progression and increased cell proliferation upon sCypA action in this study, however, may not link to STAT3 activation because depletion of inCypA and presumably sCypA by siRNA had no effect on either total STAT3 or pSTAT3 protein levels in three different CCA cell lines studied.
The link between CypA and MAPK/NF-κB pathway activation has been reported in many cancers [26–28]. Currently, overexpression of NF-κB in human CCA tissues was reported [46]. Specific NF-κB inhibitors could significantly suppress growth of CCA cell lines in vitro and in CCA-xenografted mice model [46, 47]. In the present study, we did not observe the link between CypA and NF-κB pathway, however, the link between CypA and p38 MAPK pathway was noted. p38 MAPK kinase signaling is a pathway activated in response to a wide range of cellular stress stimuli and cytokines. In CCA, p38 MAPK signaling was shown to mediate the anchorage-independent growth and tumor transformation of CCA cell line (KMCH-1) [48] and exhibit the pro-tumorigenic activity via inhibiting degradation of c-Met [49]. As activation of p38 was shown to decrease CDK inhibitor p21 expression and promote proliferation in CCA cells [48], therefore, activation of p38 may supplement the ERK1/2-mediated G1 to S phase transition observed in this study.
It should be noted here that although all cellular responses reported in the present study are facilitated through sCypA-CD147 interaction, inCypA may also play a role in activation of cell proliferation. inCypA may activate CXCR4-mediated cell proliferation via regulation of ERK1/2 nuclear translocation [50], which is critical for the activation of several transcription factors that regulate cell proliferation and migration.
In the present study, we also showed that CD147 expression was partly regulated by CypA. This observation was in agreement with previous reports [35, 36]. Since cellular chemotactic and signaling responses to sCypA are correlated with CD147 expression levels, CD147 is considered as a rate-limiting factor in the receptor complex responding to sCypA [34]. Anti-CD147 monoclonal antibody has already been used for preventing invasion and metastasis of human cancer, e.g., head and neck carcinoma [51–53]. Metuximab (Licartin), a murine HAb18 F(ab′)2 fragment specific for CD147, was developed in the 131I-labeled form and approved as a new drug for clinical therapy of primary hepatocellular carcinoma by the China State Food and Drug Administration in April 2005 [54]. It may thus be worthwhile to explore the function of CD147 in CypA-dependent and CypA-independent manner in CCA. Thus, it is possible that targeting CypA using cyclosporin A (CsA) as a chemosensitizer, CsA derivatives without immunosuppressant activity, novel inhibitors of CypA, and monoclonal antibody against CD147 or combinational therapy by targeting both CypA/CD147 interaction and its downstream pathway molecules may likely improve the treatment of CCA.
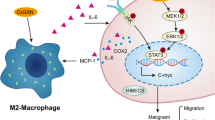
In conclusion, it was demonstrated here that sCypA augments cell proliferation of CCA. CypA is secreted from CCA cells in a reasonable level and corresponded to the inCypA level. sCypA stimulates cell proliferation via an autocrine/paracrine fashion, at least in part via sCypA/CD147 interaction, which then triggers cell signaling cascades (Fig. 8). The possible mechanisms are ERK1/2 signaling pathway that induces G1/S transition by upregulation of cyclin D1 and activation of p38 signaling that decreases CDK inhibitor, p21. Several known drugs can inhibit the sCypA/CD147 signal and suppress growth of cancer cells. A better understanding of the molecular mechanisms underlying CypA function may help to achieve a novel-targeted therapy and improve outcome of CCA treatment in the future.
Proposed cellular and molecular events during sCypA-induced CCA cell proliferation. Unusually high expression levels of CypA gene results in the production of inCypA protein leading to increase of sCypA levels in the extracellular space. sCypA acts as an autocrine/paracrine growth factor through binding with the membrane bound CD147 of CCA cells. The CypA/CD147 interaction initiates growth signals through diverse pathways including ERK1/2 and p38 MAPK signaling pathways that promote G1 to S transition via cyclin D1 and p-RB. CypA also enhances CD147 expression. Specific inhibitors against CypA such as cyclosporin A (CsA) or against CypA/CD147 binding (Licartin) may effectively inhibit CCA cell proliferation and tumor progression. Dotted lines represent molecular events demonstrated in the present study
References
Vatanasapt V, Tangvoraphonkchai V, Titapant V, Pipitgool V, Viriyapap D, Sriamporn S. A high incidence of liver cancer in Khon Kaen Province, Thailand. Southeast Asian J Trop Med Public Health. 1990;21(3):489–94.
Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003;88(3):209–20.
Wiedmann MW, Mossner J. Molecular targeted therapy of biliary tract cancer-results of the first clinical studies. Curr Drug Targets. 2010;11(7):834–50.
Zhu AX, Hezel AF. Development of molecularly targeted therapies in biliary tract cancers: reassessing the challenges and opportunities. Hepatology. 2011;53(2):695–704.
Steinmann B, Bruckner P, Superti-Furga A. Cyclosporin A slows collagen triple-helix formation in vivo: indirect evidence for a physiologic role of peptidyl-prolyl cis-trans-isomerase. J Biol Chem. 1991;266(2):1299–303.
Zhu C, Wang X, Deinum J, Huang Z, Gao J, Modjtahedi N, et al. Cyclophilin A participates in the nuclear translocation of apoptosis-inducing factor in neurons after cerebral hypoxia-ischemia. J Exp Med. 2007;204(8):1741–8.
Krummrei U, Bang R, Schmidtchen R, Brune K, Bang H. Cyclophilin-A is a zinc-dependent DNA binding protein in macrophages. FEBS Lett. 1995;371(1):47–51.
Galat A. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity–targets–functions. Curr Top Med Chem. 2003;3(12):1315–47.
Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J Leukoc Biol. 2007;82(3):613–8.
Gwinn WM, Damsker JM, Falahati R, Okwumabua I, Kelly-Welch A, Keegan AD, et al. Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention. J Immunol. 2006;177(7):4870–9.
Sherry B, Yarlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci U S A. 1992;89(8):3511–5.
Xu Q, Leiva MC, Fischkoff SA, Handschumacher RE, Lyttle CR. Leukocyte chemotactic activity of cyclophilin. J Biol Chem. 1992;267(17):11968–71.
Seizer P, Schonberger T, Schott M, Lang MR, Langer HF, Bigalke B, et al. EMMPRIN and its ligand cyclophilin A regulate MT1-MMP, MMP-9 and M-CSF during foam cell formation. Atherosclerosis. 2010;209(1):51–7.
Yang Y, Lu N, Zhou J, Chen ZN, Zhu P. Cyclophilin A upregulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology. 2008;47(9):1299–310.
Yang H, Chen J, Yang J, Qiao S, Zhao S, Yu L. Cyclophilin A is upregulated in small cell lung cancer and activates ERK1/2 signal. Biochem Biophys Res Commun. 2007;361(3):763–7.
Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE, et al. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006;106(10):2284–94.
Yurchenko V, Constant S, Eisenmesser E, Bukrinsky M. Cyclophilin-CD147 interactions: a new target for anti-inflammatory therapeutics. Clin Exp Immunol. 2010;160(3):305–17.
Obchoei S, Wongkhan S, Wongkham C, Li M, Yao Q, Chen C. Cyclophilin A: potential functions and therapeutic target for human cancer. Med Sci Monit. 2009;15(11):RA221–32.
Obchoei S, Weakley SM, Wongkham S, Wongkham C, Sawanyawisuth K, Yao Q, et al. Cyclophilin A enhances cell proliferation and tumor growth of liver fluke-associated cholangiocarcinoma. Mol Cancer. 2011;10:102.
Sawanyawisuth K, Wongkham C, Araki N, Zhao Q, Riggins GJ, Wongkham S. Serial analysis of gene expression reveals promising therapeutic targets for liver fluke-associated cholangiocarcinoma. Asian Pac J Cancer Prev. 2012;13(Suppl):89–93.
Sripa B, Leungwattanawanit S, Nitta T, Wongkham C, Bhudhisawasdi V, Puapairoj A, et al. Establishment and characterization of an opisthorchiasis-associated cholangiocarcinoma cell line (KKU-100). World J Gastroenterol. 2005;11(22):3392–7.
Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, et al. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation. 2004;77(3):446–51.
Chiampanichayakul S, Peng-in P, Khunkaewla P, Stockinger H, Kasinrerk W. CD147 contains different bioactive epitopes involving the regulation of cell adhesion and lymphocyte activation. Immunobiology. 2006;211(3):167–78.
Li Z, Min W, Gou J. Knockdown of cyclophilin A reverses paclitaxel resistance in human endometrial cancer cells via suppression of MAPK kinase pathways. Cancer Chemother Pharmacol. 2013;72(5):1001–11.
Bauer K, Kretzschmar AK, Cvijic H, Blumert C, Loffler D, Brocke-Heidrich K, et al. Cyclophilins contribute to STAT3 signaling and survival of multiple myeloma cells. Oncogene. 2009;28(31):2784–95.
Sun S, Guo M, Zhang JB, Ha A, Yokoyama KK, Chiu RH. Cyclophilin A (CypA) interacts with NF-kappaB subunit, p65/RelA, and contributes to NF-kappaB activation signaling. PLoS One. 2014;9(8):e96211.
Sun S, Wang Q, Giang A, Cheng C, Soo C, Wang CY, et al. Knockdown of CypA inhibits interleukin-8 (IL-8) and IL-8-mediated proliferation and tumor growth of glioblastoma cells through down-regulated NF-kappaB. J Neurooncol. 2010;101(1):1–14.
Yu G, Wan R, Hu Y, Ni J, Yin G, Xing M, et al. Pancreatic acinar cells-derived cyclophilin A promotes pancreatic damage by activating NF-kappaB pathway in experimental pancreatitis. Biochem Biophys Res Commun. 2014;444(1):75–80.
Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19(21):2474–88.
Dokduang H, Juntana S, Techasen A, Namwat N, Yongvanit P, Khuntikeo N, et al. Survey of activated kinase proteins reveals potential targets for cholangiocarcinoma treatment. Tumour Biol. 2013;34(6):3519–28.
Isomoto H, Kobayashi S, Werneburg NW, Bronk SF, Guicciardi ME, Frank DA, et al. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology. 2005;42(6):1329–38.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. STAT3 as an oncogene. Cell. 1999;98(3):295–303.
Leslie K, Lang C, Devgan G, Azare J, Berishaj M, Gerald W, et al. Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res. 2006;66(5):2544–52.
Trachtenberg A, Pushkarsky T, Heine S, Constant S, Brichacek B, Bukrinsky M. The level of CD147 expression correlates with cyclophilin-induced signalling and chemotaxis. BMC Res Notes. 2011;4:396.
Yang H, Li M, Chai H, Yan S, Lin P, Lumsden AB, et al. Effects of cyclophilin A on cell proliferation and gene expressions in human vascular smooth muscle cells and endothelial cells. J Surg Res. 2005;123(2):312–9.
Yurchenko V, Pushkarsky T, Li JH, Dai WW, Sherry B, Bukrinsky M. Regulation of CD147 cell surface expression: involvement of the proline residue in the CD147 transmembrane domain. J Biol Chem. 2005;280(17):17013–9.
Ralhan R, Masui O, Desouza LV, Matta A, Macha M, Siu KW. Identification of proteins secreted by head and neck cancer cell lines using LC-MS/MS: strategy for discovery of candidate serological biomarkers. Proteomics. 2011;11(12):2363–76.
Chevalier F, Depagne J, Hem S, Chevillard S, Bensimon J, Bertrand P, et al. Accumulation of cyclophilin A isoforms in conditioned medium of irradiated breast cancer cells. Proteomics. 2012;12(11):1756–66.
Andersen H, Jensen ON, Eriksen EF. A proteome study of secreted prostatic factors affecting osteoblastic activity: identification and characterisation of cyclophilin A. Eur J Cancer. 2003;39(7):989–95.
Alonso L, Okada H, Pasolli HA, Wakeham A, You-Ten AI, Mak TW, et al. Sgk3 links growth factor signaling to maintenance of progenitor cells in the hair follicle. J Cell Biol. 2005;170(4):559–70.
Auciello G, Cunningham DL, Tatar T, Heath JK, Rappoport JZ. Regulation of fibroblast growth factor receptor signalling and trafficking by Src and Eps8. J Cell Sci. 2013;126(Pt 2):613–24.
Jinawath A, Akiyama Y, Yuasa Y, Pairojkul C. Expression of phosphorylated ERK1/2 and homeodomain protein CDX2 in cholangiocarcinoma. J Cancer Res Clin Oncol. 2006;132(12):805–10.
Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26(22):3227–39.
Johnson C, Han Y, Hughart N, McCarra J, Alpini G, Meng F. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer. 2012;1(1):58–70.
Senggunprai L, Kukongviriyapan V, Prawan A, Kukongviriyapan U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother Res. 2013;28(6):841–8.
Seubwai W, Wongkham C, Puapairoj A, Khuntikeo N, Pugkhem A, Hahnvajanawong C, et al. Aberrant expression of NF-kappaB in liver fluke associated cholangiocarcinoma: implications for targeted therapy. PLoS One. 2014;9(8):e106056.
Seubwai W, Vaeteewoottacharn K, Hiyoshi M, Suzu S, Puapairoj A, Wongkham C, et al. Cepharanthine exerts antitumor activity on cholangiocarcinoma by inhibiting NF-kappaB. Cancer Sci. 2010;101(7):1590–5.
Tadlock L, Patel T. Involvement of p38 mitogen-activated protein kinase signaling in transformed growth of a cholangiocarcinoma cell line. Hepatology. 2001;33(1):43–51.
Dai R, Li J, Fu J, Chen Y, Wang R, Zhao X, et al. The tyrosine kinase c-Met contributes to the pro-tumorigenic function of the p38 kinase in human bile duct cholangiocarcinoma cells. J Biol Chem. 2012;287(47):39812–23.
Pan H, Luo C, Li R, Qiao A, Zhang L, Mines M, et al. Cyclophilin A is required for CXCR4-mediated nuclear export of heterogeneous nuclear ribonucleoprotein A2, activation and nuclear translocation of ERK1/2, and chemotactic cell migration. J Biol Chem. 2008;283(1):623–37.
Dean N, Helman E, Aldridge J, Carroll W, Magnuson S, Rosenthal E. Anti-EMMPRIN treatment of HNSCC in an ex vivo model. Laryngoscope. 2010;120 Suppl 4:S146.
Dean NR, Knowles JA, Helman EE, Aldridge JC, Carroll WR, Magnuson JS, et al. Anti-EMMPRIN antibody treatment of head and neck squamous cell carcinoma in an ex-vivo model. Anticancer Drugs. 2010;21(9):861–7.
Dean NR, Newman JR, Helman EE, Zhang W, Safavy S, Weeks DM, et al. Anti-EMMPRIN monoclonal antibody as a novel agent for therapy of head and neck cancer. Clin Cancer Res. 2009;15(12):4058–65.
Chen ZN, Mi L, Xu J, Song F, Zhang Q, Zhang Z, et al. Targeting radioimmunotherapy of hepatocellular carcinoma with iodine (131I) metuximab injection: clinical phase I/II trials. Int J Radiat Oncol Biol Phys. 2006;65(2):435–44.
Acknowledgments
This study was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), and Khon Kaen University to SW, and a Globalization Demonstration Project grant to CC from the Baylor College of Medicine’s Center for Globalization, Houston, Texas, USA, and the postdoctoral fellowship to S. Obchoei (PD 54210). We would like to thank Prof. James A. Will for the English presentation of this manuscript and the Research Instrument Center of KKU, Faculty of Medicine for technical support of the FACS analysis.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obchoei, S., Sawanyawisuth, K., Wongkham, C. et al. Secreted cyclophilin A mediates G1/S phase transition of cholangiocarcinoma cells via CD147/ERK1/2 pathway. Tumor Biol. 36, 849–859 (2015). https://doi.org/10.1007/s13277-014-2691-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2691-5