Abstract
Background
Atopic dermatitis (AD) is a chronic inflammatory disorder that can affect the physical and psychological health of individuals worldwide. Mangiferin has been previously shown to alleviate allergic diseases.
Objectives
To investigate the effect of mangiferin on AD-like pathologies and the mechanism of action.
Methods
After establishing a mouse model of AD by challenging male BALB/c mice with 1% 2,4-dinitrochlorobenzene (DNCB), 10, 50, and 100 mg/kg mangiferin was administered orally for 21 days. Scratching behaviors, dermatitis score, spleen weight, and serum immunoglobulin E (IgE) levels were assessed. Histopathological changes were determined using toluidine blue and hematoxylin–eosin staining. A cellular model of AD was established by co-stimulating HaCaT keratinocytes with tumor necrosis factor (TNF)-α and interferon (IFN)-γ. The mRNA expression of chemokines and Th2-related cytokines was examined using RT-qPCR. Western blot and immunofluorescence were used to detect the protein levels of markers of the AKT/NF-κB/STAT1 and MAPK pathways.
Results
Mangiferin exhibited anti-atopic effects in DNCB-induced AD-like mice as evidenced by decreased scratching behaviors, dermatitis score, spleen weight, IgE levels, skin thickness, and mast cell infiltration in mice. Additionally, mangiferin inhibited expression of chemokines and Th2 type cytokines (IL-4, IL-5, and IL-13) in AD-like mice and TNF-α/IFN-γ-co-stimulated HaCaT cells. Moreover, mangiferin attenuated TNF-α/IFN-γ-induced release of various inflammatory factors (such as TNF-α, IL-1β, and IL-6) in HaCaT cells. Mangiferin repressed the activation of the AKT/NF-κB/STAT1 and MAPK signaling pathways under both in vivo and in vitro conditions.
Conclusion
Overall, mangiferin inhibits the inflammatory and pruritic responses in AD, which might be an effective drug for AD treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atopic dermatitis (AD) is an allergic inflammatory skin disease, which is primarily characterized by recurrent eczematous lesions and severe itching (Weidinger et al. 2018; Karuppagounder et al. 2016). Its pathophysiology can be attributed to both the genetic and environmental factors that can lead to various immunologic and barrier dysfunctions (Hou et al. 2017). It has been established that several factors and cells could be involved in the development of AD. The Th1/Th2 balance is found to play a critical role in the pathological processes of AD (Yang et al. 2015). For instance, maladjustment of Th1 cells can produce interferon (IFN)-γ that leads to chronic inflammatory responses in AD, whereas Th2-type cytokines contribute to immunoglobulin E (IgE) generation as well as Th2 cell differentiation (Choi et al. 2016; Aslam et al. 2018). Immunosuppressive agents and topical corticosteroids are the standard treatment modalities used for the management of AD patients; however, long-term use can cause severe side effects, such as organ toxicity, vasodilation, bleeding, and skin atrophy. Therefore, herb-derived medicines with relatively low toxicity may be preferred to steroids (Jung et al. 2015; Lee et al. 2014). A number of transcription factors have been reported to mediate cellular signal transduction events. As vital transcription factors, nuclear factor-kappa B (NF-κB) and signal transducer and activator of transcription 1 (STAT1) have been linked to the allergic inflammatory response (Choi et al. 2017). The IκB-α protein is subsequently phosphorylated upon stimulation, thereby resulting in the proteasomal degradation and ubiquitination of IκB, which leads to nuclear translocation and activation of NF-κB. STAT1, which can be activated by NF-κB and IFN-γ in the cytoplasm, translocates into the nucleus. It has been implicated in the regulation of expression of various pro-inflammatory mediators. Therefore, targeting these transcription factors pharmacologically may be useful in finding new therapeutic approaches for allergic diseases (Jung et al. 2012; Han et al. 2011).
Mangiferin (2-β-d-glucopyranosyl-1,3,6,7-tetrahydroxy-9H-xanthen-9-one) is a bioactive component derived primarily from the mango tree. Mangiferin has been reported to display multiple pharmacological effects, including anti-oxidant, anti-inflammatory, anti-fatty liver, anti-metabolic syndrome, and anti-diabetic (Imran et al. 2017; Du et al. 2018). In addition, several prior studies have revealed the anti-inflammatory potential of mangiferin, and it is mainly related to the ability of mangiferin in regulating three signaling pathways, namely NF-κB, mitogen-activated protein kinase (MAPK), and Janus kinase-signal transduction and transcription activator (JAK/STAT) cascades (Mei et al. 2021). For instance, mangiferin administration reduced the symptoms of nasal allergy and inflammatory responses in experimental models with acute rhinitis by inhibiting NF-κB signaling pathway (Wang et al. 2020; Piao et al. 2020). Mangiferin ameliorated the imbalance between Th1 and Th2 cytokine in ovalbumin-evoked mouse models of asthma through the suppression of STAT6 signaling (Guo et al. 2014). Mangiferin has also exhibited remarkable potential in the management of skin conditions. Mangiferin showed a promising effect against skin inflammation by inhibiting the NF-κB signaling cascade (Zhao et al. 2017). In addition, mangiferin suppressed the expression of the proinflammatory cytokines as well as NF-κB activation induced by IgE-antigen complex, thereby alleviating the passive cutaneous anaphylaxis reaction (Lee et al. 2009). Thus, controlling inflammation and allergic reactions may serve as a crucial strategy in AD management. These findings prompted us to investigate the anti-inflammatory effects of mangiferin on AD.
The AD-like mouse model induced by 2,4-dinitrochlorobenzene (DNCB) is a frequently utilized and reliable animal model (Kang et al. 2021). Covalent conjugates formed by a variety of skin proteins combining with DNCB serve as immunogen leading to typical AD responses, causing inflammatory injury and skin allergy. Human HaCaT keratinocytes co-stimulated by TNF-α and IFN-γ are widely utilized for identifying the promising candidate agents or drugs in AD (Choi et al. 2017; An et al. 2018). TNF-α and IFN-γ are typical inflammatory factors in the skin lesions of AD, which can activate keratinocytes from patients to produce overexpressed inflammatory factors (Kim et al. 2016; Leung and Bieber 2003). Therefore, the effects of on AD were investigated in DNCB-induced AD-like mice and TNF-α/IFN-γ-stimulated HaCaT cells, respectively, for exploring whether mangiferin is promising agent for the treatment of AD.
Materials and methods
Drugs and reagents
Mangiferin (purity > 95%) was purchased from Shenzhen Haisi’an biotechnology Co, Ltd. (PC1393, Shenzhen, China). DNCB and Dexamethasone (DEX) were obtained from Shanghai North Connaught biotechnology Co, Ltd. (046-03421, Shanghai, China) and Sinopharm Chemical Reagent Co, Ltd. (XW03279241, Shanghai, China), respectively. Recombinant human TNF-α (300-01A) and recombinant human IFN-γ (300-02) were commercially purchased from Peprotech (NJ, USA). All the reagents were authenticated endotoxin-free.
Animals
Male BALB/c mice (6–8 weeks old) were procured from Vital River Co. Ltd. (Beijing, China). They were housed in the specific pathogen-free room at optimal temperature of 20–26 ℃, under a 12-h light/dark cycle, with 40–70% humidity. They had free access to clean water and food. The experimental procedures were approved by the Ethics Committee of Huangshi Central Hospital, Affiliated Hospital of Hubei Institute of Technology in accordance with the China National Law on animal care and use.
Establishment of AD-like mouse model
After shaving the dorsal skin regions, all the mice were randomly divided into six distinct groups: Control (Vehicle control) (n = 8), DNCB (n = 8), DNCB + mangiferin (10 mg/kg) (n = 12), DNCB + mangiferin (50 mg/kg) (n = 12), DNCB + mangiferin (100 mg/kg) (n = 12), and DNCB + hydrocortisone (1%; H0135, Sigma, MO, USA) (n = 12) (Voss et al. 2021). From day 1 to day 3, the dorsal skins of the control mice were treated with 200 μL of vehicle solution (acetone/olive oil; 4:1, v/v) whereas DNCB group mice were treated with 200 μL of 1% DNCB dissolved in acetone/olive oil (4:1, v/v) once a day. The different groups received no-treatment from day 4 to day 7. Thereafter, mice were treated with 200 μL of vehicle or 0.5% DNCB solution applied daily on dorsal skin and were administered mangiferin daily by gavage for 21 days. The dosage of mangiferin used for in vivo experiments was selected based on a previous report (Lee et al. 2009). Hydrocortisone (1%) was applied topically to the backs of the mice daily for 21 days. Subsequently, on day 29, the spleen was weighed and the blood samples along with dorsal skin were collected. The dorsal skin samples were kept in a – 80 ℃ refrigerator until further use. To obtain the serum, blood samples were subjected to centrifugation (3000 × g, 4 ℃, 15 min) and stored at – 20 ℃. The serum IgE level was determined using a mouse IgE Enzyme-linked immunosorbent assay (ELISA) assay kit (EK-M26877, Shanghai Biological Technology Co., Ltd. enzyme research, Shanghai) with optical density measured at 450 nm wavelength as per the manufacturer's instructions.
Evaluation of dermatitis severity
The severity of clinical dermatitis severity was analyzed using the method described previously by Yamamoto and colleagues (Nutten 2015). The development of erythema/hemorrhage, scarring/dryness, edema, and excoriation/erosion was scored as following: 0, none; 1, mild; 2, moderate; 3, severe. The scores were then determined by consensus among three different observers, and the sum of the individual scores was used as the dermatitis score.
Measurement of scratching behavior
Mice were housed individually in four-chamber acrylic cages measuring 15 cm × 10 cm × 10 cm. There are small holes in each chamber for air circulation. On behavior recording days, mice were allowed to acclimate to the test cage for 15 min before the actual recording. The number of scratching behaviors was counted for the next 10 min. Consecutive scratching movements of the hind paw were defined as one scratching event (Yu et al. 2017). The scorers were blinded to the different treatment groups.
Histopathological analysis
The dorsal skin samples were collected from the different treatment groups of mice after killing them. Thereafter, 10% buffered formalin (SRRG-NBF250M, Shanghai SCR-Biotech Co., Ltd., Shanghai) was used to fix the collected skin samples, which were then embedded in paraffin (LMAI Bio, Shanghai). After cutting into 4-μm-thick sections, the samples were stained with toluidine blue and hematoxylin and eosin (H&E). The number of mast cells was counted in five randomly selected fields of view at 400 × magnification. The dermal thicknesses was measured in H&E-stained sections viewed under a magnification of × 400. A DFC295 (Leica, Wetzlar, Germany) and a DM IL LED microscope (Leica) were employed to observe the pathological changes of different stained sections.
Cell culture
Human HaCaT keratinocytes were commercially purchased from Beijing PUFFE Biotechnology Co., Ltd (Beijing). The cells were maintained in Dulbecco’s Modified Eagle’s Medium (Shanghai Yihui Biological Technology Co., Ltd., Shanghai). The medium was supplemented with 10% fetal bovine serum (FBS; Sigma). Cells were cultured in a 37 ℃ humidified incubator with 5% CO2.
Cell treatment
HaCaT cells (1 × 105/well) were initially starved in 0.1% FBS media for 24 h, followed by treatment with mangiferin at doses of 5 µM, 10 µM, 25 µM, and 50 µM, respectively, for 1 h at 37 ℃. Thereafter, 10 ng/mL of TNF-α/IFN-γ treatment was used to stimulate the cells at 37 ℃ for 2 h for RT-qPCR analysis and 45 min for western blot analysis. The cells treated with DMSO were used as control cells.
Cell viability assay
The Cell counting Kit-8 (CCK-8) assay was performed to analyze the effects of mangiferin on the viability of HaCaT cells. All the procedures were performed in accordance with the instructions specified in the CCK-8 kit (BB-4202, BestBio, Shanghai). Briefly, HaCaT cells placed in 96-well plates were treated with magniferin (5–50 μM) at 37 ℃ for 24 h. Thereafter, 10 μL of CCK-8 solution was added and incubated with these cells for another 1 h at 37 ℃. Finally, a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) was employed for detecting the absorbance value of each well at 450 nm.
RT-qPCR analysis
RT-qPCR was performed using a thermal cycler Dice TP850 (Takara, Dalian, China) according to the manufacturer's instructions. RNA samples were isolated from mouse skin tissues and HaCaT cells to detect the mRNA expression of chemokines (RANTES, TARC, and TSLP), Th2-type cytokines (interleukin (IL)-13, IL-5, and IL-4), and proinflammatory factors (TNF-α, IL-1β, and IL-6). After the completion of in vivo experiments, dorsal skin tissues of the mice were obtained, and 25% skin tissues were subjected to homogenization using an RNAiso Plus kit (Takara). HaCaT cells were treated as described above and collected. RNAiso Plus kit (Takara) was used to isolate the total RNA. A SYBR Green Master Mix kit (Takara) was employed to reverse transcribe isolated RNAs into cDNA. SYBR Premix Ex Taq (Takara) was utilized to perform qPCR. GAPDH served as internal control for mRNA expression. The 2−ΔΔCt method was used for calculating the gene expression (Livak and Schmittgen 2001). The sequences of various primers used in this study have been listed in Table 1.
Western blot analysis
PRO-PREP™ protein extraction solution (Beijing QiWei YiCheng Tech Co.,Ltd., Beijing) was used to treat the dorsal tissues or HaCaT cells at 4 ℃ for 20 min. To remove cell debris, microcentrifugation (11,000 × g, 4 ℃, 0.5 h) was performed. Thereafter, a BCA protein quantification kit (E112-01/02, Vazyme Biotech Co., Ltd., Nanjing, China) was used to determine the protein concentration. Thereafter, 10–30 µL proteins were resolved by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SY4584, YITA Bio, Beijing) followed by electroblotting onto a polyvinylidene fluoride membrane. The membrane was subsequently incubated with 5% non-fat milk at room temperature and shaken for 60 min to avoid non-specific binding. The membrane was incubated overnight with the various primary antibodies against IκB-α (1:1000, v/v; #4814, Cell Signaling Technology (CST), Inc., MA, USA), p-IκB-α (1:1000, v/v; #5209, CST), STAT1 (1:1000, v/v; #14,994, CST), p-STAT1 (Ser727) (1:1000, v/v; #8826, CST), p-STAT1 (Tyr701) (1:1000, v/v; #9167, CST), AKT (1:1000, v/v; #9272, CST), p-AKT (Ser473) (1:2000, v/v; #4060, CST), p-p38 (1:1000, v/v; #4511, CST), p38 (1:1000, v/v; #9212, CST), p-JNK (1:1000, v/v; #67,096, CST), JNK (1:1000, v/v; #9252, CST), p-ERK (1:1000, v/v; #4370, CST), ERK (1:1000, v/v; #4695, CST), β-actin (1:1000, v/v; #4970, CST) in a refrigerator at 4 ℃. After washing the blots thrice (10 min each time), the membrane was incubated with a secondary antibody (1:1000, v/v; #7074, CST) for 2 h at room temperature. After washing again thrice (10 min each time), the blots were developed by using an enhanced chemiluminescence detection kit (PK-MB902-500–500, Beijing Biocoen Biotechnology CO., LTD, Beijing). Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA) was used to evaluate band intensity. β-actin served as loading control.
Immunofluorescence staining
HaCaT cells were first seeded in a chamber at a density of 1 × 105 cells/mL. They were then fixed with 100% methanol for half an hour at 20 ℃. HaCaT cells were incubated in PBS containing 0.1% Triton-X100 (XiangSheng Biotechnology Co., LTD., Shanghai) for 0.5 h, blocked with 3% bovine serum albumin (YT0230-2, YITA) for 0.5 h, and incubated with p-STAT1 primary antibody overnight. After washing, the samples were incubated with the secondary antibody, and the images were captured using a confocal laser scanning fluorescence microscope (Leica).
Statistical analysis
The data from at least triplicate experiments have been presented as the mean ± standard deviation. The data obtained from multiple groups was compared using one-way analysis of variance (ANOVA) with Tukey’s post hoc test. p value < 0.05 was considered statistically significant. GraphPad Prism (version 5, GraphPad Software, La Jolla, CA, USA) was adopted for performing the statistical analysis.
Results
Mangiferin alleviated DNCB-induced AD in mouse models
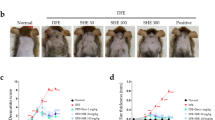
DNCB is a “contact sensitizer” that can cause contact hypersensitivity on mouse skin (Kim et al. 2016). An overview of experimental procedure has been displayed in Fig. 1A. The dorsal skin of DNCB-induced AD mice showed significant lesions on the day of sacrifice. Compared to the model group, the lesions of mice were alleviated in the mangiferin low-dose, mangiferin medium-dose, mangiferin high-dose, and positive control groups, with the significant alleviation in the medium-dose group (Fig. 1B). The scratching frequency and symptom score of the AD models were increased compared to the control group. Compared to the model group, the medium-dose group and HC group exhibited significantly reduced symptom score (p < 0.05; Fig. 1C) and scratching frequency (p < 0.05; Fig. 1D) of mice. Therefore, we selected mangiferin medium-dose in subsequent experiment. The spleen is a pivotal lymphatic organ, which can remove aging blood cells, filter blood, produce hematopoiesis, and be enlarged in inflamed state. When compared to the control group, we observed that the spleen weight of DNCB-treated mice was higher (0.19 vs. 0.12, p < 0.05); however, following treatment with mangiferin (0.14 vs. 0.19) or hydrocortisone (0.13 vs. 0.19), the spleen weight was significantly decreased (p < 0.05; Fig. 1E). An essential characteristic of AD is an elevated level of serum IgE. Serum IgE was measured by ELISA, and the results showed that mangiferin (40.12 vs. 145.54) or hydrocortisone (43.87 vs. 145.54) reduced the DNCB-increased serum IgE levels in comparison to the control group (p < 0.05; Fig. 1F).
Mangiferin alleviated DNCB-induced AD in mouse models. A A schematic diagram representing the experimental procedure used in mouse experiment. B Representative photographs depicting the skin lesions in the control group, DNCB group, DNCB + mangiferin low-dose group, DNCB + mangiferin medium-dose group, DNCB + mangiferin high-dose group, and DNCB + hydrocortisone group. C Scratching times within 10 min in each group. D Dermatitis score in each group. E Spleen weight of the mice in each group was measured. F Serum IgE level in each group was measured by using an ELISA assay. N = 12/group. **p < 0.01, ***p < 0.001; ##p < 0.01, ###p < 0.001
Mangiferin improved the histological changes
The skin lesions were stained with H&E and toluidine blue for histological analysis (Fig. 2A). H&E-stained tissues revealed that the thickness of dermal tissues was markedly higher in DNCB-treated mice in comparison to the control group (478.67 vs. 200.09) due to hyperplasia, hyperkeratosis, and edema. However, the dermal thickening was significantly attenuated after the treatment with mangiferin (249.87 vs. 478.67) or hydrocortisone (231.00 vs. 478.67) (p < 0.05; Fig. 2C). The infiltration of mast cells, a marker of inflammation, was found to be significantly higher in the tissues stained with toluidine blue in the mice treated with DNCB than in the control group (61.56 vs. 11.21). Interestingly, treatment with mangiferin (28.01 vs. 61.56) or hydrocortisone (23.34 vs. 61.56) reduced the number of mast cells (p < 0.05; Fig. 2B, D).
Mangiferin improved histological changes. A Images of skin lesions stained by H&E in the control group, DNCB group, DNCB + mangiferin group, and DNCB + hydrocortisone group (scale bar = 50 μm). B Skin sections observed after staining with toluidine blue (scale bar = 100 μm). C Dermal thickness was analyzed in H&E-stained sections using a microscope. D The number of the mast cells in toluidine blue-stained sections was quantified. N = 12/group. ***p < 0.001
Mangiferin downregulated AD-related cytokine expression in the skin of DNCB-treated mice
Then, we investigated into the suppressive effects of mangiferin on signature cytokines of AD and analyzed the potential mRNA expression of these cytokines in the dorsal skin tissues of AD mice. As important members of the CC chemokine subfamily, thymus, and activation-regulated chemokine (TARC)/CCL17 have been implicated in the process of Th2 lymphocyte recruitment and maintenance of Th2 immune response (Park et al. 2007). TSLP, which is usually known to initiate the Th2 response mediated by dendritic cells and produce allergic inflammation, exhibits a high expression level in the skin lesions and the activated mast cells. Thus, TARC and TSLP are primarily regarded as mediators of inflammatory skin disorders, including AD (Kang et al. 2021). According to RT-qPCR data, repeated DNCB stimulation markedly increased the mRNA expression of TSLP (2.89 vs. 1.00) and TARC (3.10 vs. 1.00), but mangiferin or hydrocortisone markedly reduced the expression of these cytokines (p < 0.05; Fig. 3A). Consistently, DNCB induced upregulation of mRNA levels of Th2-type cytokines including IL-13, IL-5, and IL-4 was markedly inhibited by mangiferin or hydrocortisone treatment (p < 0.05; Fig. 3B).
Mangiferin inhibited NF-κB and STAT1 pathways in the skin tissues of DNCB-induced AD models
The degradation and phosphorylation of IκB and STAT1 activation in AD models were investigated in order to explore the potential signaling pathways involved in the inhibitory effects of mangiferin on the production of cytokines. DNCB-induced upregulation of p-IκB-α protein expression was markedly reversed by mangiferin (1.50 vs. 4.20) or hydrocortisone (1.45 vs. 4.20) treatment (p < 0.05; Fig. 4A). mangiferin (0.88 vs. 0.35) or hydrocortisone (0.85 vs. 0.35) treatment also reversed the DNCB-induced downregulation of IκB-α protein level (p < 0.05; Fig. 4A). This suggests that the treatment of mangiferin significantly suppresses the phosphorylation and degradation of DNCB-generated IκB. Moreover, STAT1 phosphorylation induced at residues Ser727 and Tyr701 by DNCB was partially attenuated upon mangiferin treatment (1.50 vs. 3.89 at Ser727; 1.56 vs. 2.90 at Tyr701) (p < 0.05; Fig. 4B).
Effects of mangiferin on TNF-α/IFN-γ-stimulated HaCaT keratinocytes
It has been found that upon exposure to various stimuli, the immortalized human keratinocyte line HaCaT can release a range of proinflammatory mediators linked to AD (Sung et al. 2012). The results of CCK-8 assay revealed that mangiferin under dose of 50 μM was non-toxic to HaCaT keratinocytes (Fig. 5A). TNF-α/IFN-γ may synergistically promote the production of TARC, TSLP, and RANTES, three key mediators in AD pathogenesis (Choi et al. 2014). In addition, the production of cytokines and chemokines in keratinocytes have been associated with STAT1 and NF-κB activation (Park et al. 2015a). Thus, the effects of mangiferin on the production of chemokines and cytokines in HaCaT keratinocytes stimulated by TNF-α/IFN-γ were investigated. Unsurprisingly, HaCaT cells stimulated with TNF-α/IFN-γ showed markedly elevated mRNA expression of Th2 cytokines IL-4, IL-5, and IL-13, as well as TARC, TSLP, and RANTES. Mangiferin treatment markedly reduced the elevated mRNA expression of IL-4 (1.20 vs. 2.25), IL-5 (1.22 vs. 2.35), IL-13 (1.26 vs. 2.30), TARC (1.27 vs. 2.89), TSLP (1.30 vs. 2.47), and RANTES (1.29 vs. 2.30) in a dose-dependent manner (p < 0.05; Fig. 5B-5C). High levels of inflammatory factors have been reported in HaCaT cells treated with TNF-α/IFN-γ (Sung et al. 2012). Interestingly, mangiferin reduced TNF-α/IFN-γ-induced mRNA expression of pro-inflammatory cytokines TNF-α (1.28 vs. 2.76), IL-1β (1.22 vs. 2.39), and IL-6 (1.31 vs. 2.55) (p < 0.05; Fig. 5D). Subsequently, the influence of mangiferin on activation of Akt and NF-κB pathways was investigated. These pathways regulate the expression of various inflammatory factors in human keratinocytes (Lee et al. 2007). The results of western blot analysis showed that treatment of TNF-α/IFN-γ-stimulated HaCaT cells with 25 μM (3.22 vs. 5.50) or 50 µM (1.13 vs. 5.50) mangiferin significantly suppressed the phosphorylation of Akt. Akt can phosphorylate IκB kinase directly in response to stimuli, which in turn contributes to NF-κB activation. Moreover, both the phosphorylation and degradation of IκB in HaCaT cells stimulated by TNF-α/IFN-γ were reduced by treatment with mangiferin (1.05 vs. 4.39 for p-IκB; 0.75 vs. 0.31 for IκB) (p < 0.05; Fig. 5E), suggesting that mangiferin inhibits Akt and NF-κB activation in keratinocytes.
Expression profile of cytokines, chemokines, and NF-κB and Akt activation in TNF-α/IFN-γ-stimulated HaCaT keratinocytes. A Cell viability was determined by using CCK-8 assay. B–D The mRNA expression levels of Th2-type cytokines (IL-4, IL-5, and IL-13), chemokines (TARC, TSLP, and RANTES), and proinflammatory factors (TNF-α, IL-1β, and IL-6) were analyzed by RT-qPCR. (E) Total proteins were extracted and subjected to western blot analysis for analyzing the expression of AKT and IκB-α proteins. N = 5. **p < 0.01, ***p < 0.001
Suppressive effects of mangiferin on the activation of STAT1 in TNF-α/IFN-γ-treated HaCaT cells
To explore the potential molecular mechanism responsible for the suppressive impact of mangiferin on AD in parallel with the suppression of NF-κB, we primarily focused on the effects of mangiferin on STAT1 activation induced by TNF-α/IFN-γ. In contrast to the control group, TNF-α/IFN-γ stimulation led to the activation of STAT1, and mangiferin greatly reduced the phosphorylation of STAT1 at Tyr701 site (0.90 vs. 4.56) (p < 0.05; Fig. 6A). Furthermore, compared to TNF-α/IFN-γ-stimulated HaCaT keratinocytes, treatment with mangiferin decreased the mRNA levels of IL-2 (1.31 vs. 2.55) and IL-12 (1.31 vs. 2.55), which are important cytokines implicated in STAT1 phosphorylation (p < 0.05; Fig. 6B). Moreover, representative images of immunofluorescence assay revealed that exposure to 50 µM mangiferin repressed STAT1 nuclear translocation in TNF-α/IFN-γ-treated HaCaT cells (Fig. 6C).
Mangiferin inhibited the activation of STAT1 in TNF-α/IFN-γ-treated HaCaT cells. A Total proteins were extracted and then subjected to western blot analysis for examining the protein levels of STAT1. B IL-2 and IL-12 mRNA expression in TNF-α/IFN-γ-stimulated HaCaT cells was determined by RT-qPCR. C STAT1 expression (green) in HaCaT keratinocytes treated with TNF-α/IFN-γ was analyzed by immunofluorescence staining. DAPI was used for the nucleus counterstaining (scale bar = 50 μm). N = 5. **p < 0.01, ***p < 0.001
Mangiferin inhibited MAPK activation in TNF-α/IFN-γ-stimulated HaCaT cells
We further performed a deeper investigation into other potential molecular mechanisms. The MAPK signaling pathway is closely related to the activation of TNFα/IFNγ, and it regulates various reactions downstream of the entire keratinocyte (Kwon et al. 2012). Western blot was used to analyze the protein phosphorylation of p38, JNK, and ERK. The experimental results show that TNF-α/IFN-γ significantly induced the phosphorylation of p38, JNK, and ERK. In the group of HaCaT cells pretreated with mangiferin, we found that the phosphorylation of the proteins decreased (1.15 vs. 2.11 for p-p38; 1.74 vs. 3.36 for p-JNK; 1.65 vs. 3.32 for p-ERK) (p < 0.05; Fig. 7A–C).
Discussion
AD is one of the complicated skin diseases and its relationship with allergy remians controversial. Epidermal keratinocytes are the first line of defense in the skin. In the case of AD patients, allergens, irritants, and pathogen invasion damage the skin barrier, which triggers an immune response in stimulated keratinocytes and intensifies skin inflammation (Kang et al. 2021). The expression of a variety of chemokines and inflammatory cytokines originating from keratinocytes play crucial roles in AD pathogenesis (Kawahara et al. 2017; Zhu et al. 2018). The onset and acute phases of AD are primarily influenced by Th2-based immune responses (Nutten 2015).
As magngiferin can exhibit both anti-inflammatory and anti-allergic functions, an AD mouse model induced by DNCB was employed for evaluating the potential influence of mangiferin on AD in vivo. It was observed that DNCB-induced contact dermatitis accompanied with immunological and skin alterations, sharing similarities with AD patients including clinical characteristics such as erythema, hemorrhage, edema, erosion, skin dryness, and upregulated IgE levels (Hou et al. 2017). The primary features of AD are represented by mast cells and eosinophils infiltrating into inflammatory skin lesions (Jiao et al. 2016; Liu et al. 2011). Our findings revealed that mangiferin attenuated serum IgE levels, blocked mast cell infiltration, decreased scratch reactions, and dermal as well as epidermal thickness, among other AD-like symptoms. Topical corticosteroids, such as hydrocortisone, are widely used in patients with AD (Mohamed et al. 2023). In this study, 1% hydrocortisone was used to act as a therapeutic control to analyze the efficacy of mangiferin. Our results demonstrated that the efficacy of mangiferin was satisfactory in the analyzed parameters, showing similar results to hydrocortisone (Figs. 1, 2, 3, 4). It suggests that mangiferin has a potential as a therapeutic agent for AD. However, the safety of mangiferin needs to be further verified.
In chronic allergic diseases, the involvement of TARC has been found to correlate with the severity of the disease, as AD is regarded as a chronic inflammatory skin disorder dominated by Th2 in the acute phase (Jiao et al. 2016; Liu et al. 2011). As a cytokine originating from epithelial cells, TSLP plays a significant role in the pathophysiology of chronic allergies mediated by Th2 cells. This results in specifically elevated frequency of IL-4-, IL-5-, and IL-13-expressing effector cells, thereby enhancing the production of cytokines (Rochman et al. 2018). Thus, we examined the impact of mangiferin on TARC and TSLP mRNA levels and found that in DNCB-treated AD mice, the mRNA expression of TARC and TSLP was significnatly downregulated upon mangiferin treatment. Moreover, mangiferin also inhibited the mRNA expression of IL-4, IL-5, and IL-13 in DNCB-treated AD mice. Keratinocytes are typical cells for analyzing AD response, which can induce the differentiation of T lymphocytes and cause the production of various inflammatory chemokines and cytokines when exposed to different irritants, such as IFN-γ and TNF-α (Kang et al. 2021). A large group of cytokines, such as TNF-α and IFN-γ, stimulate the proliferation and differentiation of keratinocytes. Not only the keratinocytes are the target cells of these cytokines, but also they secrete many kinds of cytokines by themselves, including some chemokines, which play a key role in the pathogenesis of AD (Si-Si et al. 2011). In this study, we examined the mechanism of action of mangiferin in AD using TNF-α/IFN-γ-stimulated keratinocytes. As expected, co-stimulation with TNF-α and IFN-γ induced the expression of chemokines (RANTES, TARC, and TSLP), Th1 cytokimes (IL-2 and IL-12), Th2 cytokimes (IL-4, IL-5, and IL-13), and proinflammatory cytokines (TNF-α, IL-1β, and IL-6) in HaCaT keratinocytes, consistent with the in vivo results. Interestingly, enhanced mRNA levels of these factors were significnatly reduced upon mangiferin treatment. Our results demonstrated that mangiferin suppressed the expression of chemokines and Th2 cytokimes in AD in vitro and in vivo. These findings indicated that mangiferin exerts a potential suppressive effect on Th2 immune responses in the activation of keratinocytes, which provides insights into the protective effects of mangiferin against AD-like lesions in vivo.
NF-κB and STAT1 are important allergic inflammation-related transcription factors (Choi et al. 2017). A previous report has revealed that the activation of STAT1 and NF-κB can induce the expression of inflammatory genes associated with AD (Lim et al. 2016). Upon activation of STAT1 and NF-κB pathways, these two proteins are translocated into the nucleus, where they can effectively promote the expression of AD-related cytokine genes (Stanley and Lacy 2010). Thus, AD symptoms can be significantly alleviated by inhibiting the activation of NF-κB and STAT1 signaling pathways that might function as important targets in developing novel therapeutics against AD (Stanley and Lacy 2010). It has been established that IFN-γ receptor engagement can activate receptor-associated tyrosine kinases. Once tyrosine kinases are activated, they can subsequently phosphorylate a single tyrosine residue (Tyr701) on STAT1. However, in most cases, Tyr701 phosphorylation has been found to be insufficient to initiate transcription. Thus, STAT1 must also be phosphorylated on its serine site (Ser727) by a serine kinase independent of tyrosine to achieve full transcriptional activity, and thereby to initiate effective STAT1-targeted gene transcription (Zhou et al. 2004; Stephanou et al. 2001). We found in our study, that the DNCB-evoked degradation and phosphorylation of IκB-α as well as the phosphorylation of STAT1 at Tyr701 and Ser727 residues were markedly attenuated in AD mouse models by post treatment of mangiferin. Furthermore, the phosphorylation and degradation of IκB-α as well as the phosphorylation of Akt (Ser473) and STAT1 (Tyr701) were also significnatly inhibited by mangiferin in TNF-α/IFN-γ-stimulated HaCaT cells. Moreover, mangiferin repressed STAT1 nuclear translocation in TNF-α/IFN-γ-stimulated HaCaT cells. These results indicated that mangiferin represses the production of inflammatory cytokines and chemokines through blocking the activation of both STAT1 and NF-κB signaling pathways in AD in vitro and in vivo. Previous studies have shown that the mitogen-activated protein kinase (MAPK) signaling pathway is involved in the regulation of NF-κB and STAT1 in response to cytokines, such as TNF-α and IFN-γ (Darnell et al. 1994; Park et al. 2015b). Interestingly, our results indicated that mangiferin markedly suppressed the TNF-α/IFN-γ-induced phosphorylation of MAPKs, including p38, JNK, and ERK, suggesting that the expression of cytokines and chemokines in TNF-α/IFN-γ-stimulated HaCaT keratinocytes can be reduced via suppression of MAPKs, under the action of mangiferin. However, whether mangiferin can modulate the activation of MAPK pathway in animal models of AD needs further verification.
There are several limitations in our study. First, it is necessary to elucidate the various downstream events involved in the identified mechanisms of action of mangiferin and the effects of mangiferin on different markers associated with skin barrier. Second, it is imperative to understand the anti-allergic inflammatory impact of mangiferin on immune responses, such as T cell responses, innate lymphoid cells, as well as toll-like receptors and thereby develop a novel therapeutic approach to treat allergic disorders. AD is a chronic relapsing and remitting inflammatory skin disease. We did not investigate the long-term effects of mangiferin treatment, which should be addressed in the future work to validate its safety and potential as a therapeutic agent in AD.
In conclusion, we demonstrated that mangiferin can inhibit both the activation of mast cells and keratinocytes in the DNCB-induced AD model, as well as the development of atopic skin inflammation. We also observed that mangiferin inhibits the activation of STAT1, AKT/NF-κB, and MAPK signaling pathways (Fig. 8). These results suggested that mangiferin could be used as a potential therapeutic agent for suppressing the inflammatory and pruritic responses in AD. Our results provide novel insights into the potential pharmacological effects of mangiferin in treating AD.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
An HJ et al (2018) Therapeutic effects of bee venom and its major component, melittin, on atopic dermatitis in vivo and in vitro. Br J Pharmacol 175(23):4310–4324
Aslam H et al (2018) Immunomodulatory effect of thymoquinone on atopic dermatitis. Mol Immunol 101:276–283
Choi HJ, Lee JH, Jung YS (2014) (+)-Nootkatone inhibits tumor necrosis factor α/interferon γ-induced production of chemokines in HaCaT cells. Biochem Biophys Res Commun 447(2):278–284
Choi EJ et al (2016) Topical application of Moringa oleifera leaf extract ameliorates experimentally induced atopic dermatitis by the regulation of Th1/Th2/Th17 balance. Biomed Pharmacother 84:870–877
Choi JK et al (2017) Chrysin attenuates atopic dermatitis by suppressing inflammation of keratinocytes. Food Chem Toxicol 110:142–150
Darnell Jr. JE, Kerr IM, Stark GR (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264(5164):1415–1421
Du S et al (2018) Mangiferin: an effective therapeutic agent against several disorders (Review). Mol Med Rep 18(6):4775–4786
Guo HW et al (2014) Mangiferin attenuates TH1/TH2 cytokine imbalance in an ovalbumin-induced asthmatic mouse model. PLoS ONE 9(6):e100394
Han EH et al (2011) Psidium guajava extract inhibits thymus and activation-regulated chemokine (TARC/CCL17) production in human keratinocytes by inducing heme oxygenase-1 and blocking NF-κB and STAT1 activation. Environ Toxicol Pharmacol 32(2):136–145
Hou DD et al (2017) Sea buckthorn (Hippophaë rhamnoides L.) oil improves atopic dermatitis-like skin lesions via inhibition of NF-κB and STAT1 activation. Skin Pharmacol Physiol 30(5):268–276
Imran M et al (2017) Mangiferin: a natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis 16(1):84
Jiao D et al (2016) NOD2 and TLR2 ligands trigger the activation of basophils and eosinophils by interacting with dermal fibroblasts in atopic dermatitis-like skin inflammation. Cell Mol Immunol 13(4):535–550
Jung MR et al (2012) Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 3-O-β-D-glucopyanosylspinasterol via blocking NF-κB and STAT1 signaling pathways in TNF-α and IFN-γ-induced HaCaT keratinocytes. Biochem Biophys Res Commun 427(2):236–241
Jung M et al (2015) Inhibitory effect of 5,6-dihydroergosteol-glucoside on atopic dermatitis-like skin lesions via suppression of NF-κB and STAT activation. J Dermatol Sci 79(3):252–261
Kang YM et al (2021) Oleanolic acid alleviates atopic dermatitis-like responses in vivo and in vitro. Int J Mol Sci 22(21):12000
Karuppagounder V et al (2016) Molecular targets of quercetin with anti-inflammatory properties in atopic dermatitis. Drug Discov Today 21(4):632–639
Kawahara T et al (2017) Effect of the topical application of an ethanol extract of quince seeds on the development of atopic dermatitis-like symptoms in NC/Nga mice. BMC Complement Altern Med 17(1):80
Kim JE et al (2016) Molecular mechanisms of cutaneous inflammatory disorder: atopic dermatitis. Int J Mol Sci 17(8):1234
Kwon DJ et al (2012) Casuarinin suppresses TARC/CCL17 and MDC/CCL22 production via blockade of NF-κB and STAT1 activation in HaCaT cells. Biochem Biophys Res Commun 417(4):1254–1259
Lee BS et al (2007) Wogonin suppresses TARC expression induced by mite antigen via heme oxygenase 1 in human keratinocytes. Suppressive effect of wogonin on mite antigen-induced TARC expression. J Dermatol Sci 46(1):31–40
Lee B et al (2009) Mangiferin inhibits passive cutaneous anaphylaxis reaction and pruritus in mice. Planta Med 75(13):1415–1417
Lee H et al (2014) The leaves of Broussonetia kazinoki siebold inhibit atopic dermatitis-like response on mite allergen-treated Nc/Nga mice. Biomol Ther (seoul) 22(5):438–444
Leung DY, Bieber T (2003) Atopic dermatitis. Lancet 361(9352):151–160
Lim HS et al (2016) Ma Huang Tang suppresses the production and expression of inflammatory chemokines via downregulating STAT1 phosphorylation in HaCaT keratinocytes. Evid Based Complement Alternat Med 2016:7831291
Liu FT, Goodarzi H, Chen HY (2011) IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol 41(3):298–310
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Mei S, Ma H, Chen X (2021) Anticancer and anti-inflammatory properties of mangiferin: a review of its molecular mechanisms. Food Chem Toxicol 149:111997
Mohamed AA et al (2023) A comparative randomized clinical trial evaluating the efficacy and safety of tacrolimus versus hydrocortisone as a topical treatment of atopic dermatitis in children. Front Pharmacol 14:1202325
Nutten S (2015) Atopic dermatitis: global epidemiology and risk factors. Ann Nutr Metab 66(Suppl 1):8–16
Park EJ et al (2007) Suppression of spontaneous dermatitis in NC/Nga murine model by PG102 isolated from Actinidia arguta. J Invest Dermatol 127(5):1154–1160
Park JH et al (2015a) Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines production via blockade of NF-κB, STAT1 and p38-MAPK activation in human epidermal keratinocytes. J Ethnopharmacol 171:85–93
Park JH et al (2015b) Suppression of Th2 chemokines by crocin via blocking of ERK-MAPK/NF-κB/STAT1 signalling pathways in TNF-α/IFN-γ-stimulated human epidermal keratinocytes. Exp Dermatol 24(8):634–636
Piao CH et al (2020) Mangiferin alleviates ovalbumin-induced allergic rhinitis via Nrf2/HO-1/NF-κB signaling pathways. Int J Mol Sci 21(10):3415
Rochman Y et al (2018) TSLP signaling in CD4(+) T cells programs a pathogenic T helper 2 cell state. Sci Signal 11(521)
Si-Si W et al (2011) Inhibition of TNF-α/IFN-γ induced RANTES expression in HaCaT cell by naringin. Pharm Biol 49(8):810–814
Stanley AC, Lacy P (2010) Pathways for cytokine secretion. Physiology (bethesda) 25(4):218–229
Stephanou A et al (2001) Induction of apoptosis and Fas receptor/Fas ligand expression by ischemia/reperfusion in cardiac myocytes requires serine 727 of the STAT-1 transcription factor but not tyrosine 701. J Biol Chem 276(30):28340–28347
Sung YY, Kim YS, Kim HK (2012) Illicium verum extract inhibits TNF-α- and IFN-γ-induced expression of chemokines and cytokines in human keratinocytes. J Ethnopharmacol 144(1):182–189
Voss GT et al (2021) Suppressive effect of 1,4-anhydro-4-seleno-D-talitol (SeTal) on atopic dermatitis-like skin lesions in mice through regulation of inflammatory mediators. J Trace Elem Med Biol 67:126795
Wang Y, Cui C, Sun H (2020) Anti-inflammatory effect of mangiferin on an experimental model of allergic rhinitis through the inhibition of NF-κB signaling pathways. J Environ Pathol Toxicol Oncol 39(4):357–364
Weidinger S et al (2018) Atopic dermatitis. Nat Rev Dis Primers 4(1):1
Yang G et al (2015) Solanum tuberosum L, cv Jayoung epidermis extract inhibits mite antigen-induced atopic dermatitis in NC/Nga mice by regulating the Th1/Th2 balance and expression of filaggrin. J Med Food 18(9):1013–1021
Yu H et al (2017) Effect of isoliquiritigenin for the treatment of atopic dermatitis-like skin lesions in mice. Arch Dermatol Res 309(10):805–813
Zhao Y et al (2017) Mangiferin antagonizes TNF-α-mediated inflammatory reaction and protects against dermatitis in a mice model. Int Immunopharmacol 45:174–179
Zhou L et al (2004) Trapidil inhibits monocyte CD40 expression by preventing IFN-gamma-induced STAT1 S727 phosphorylation. Int Immunopharmacol 4(7):863–871
Zhu TH et al (2018) Epithelial barrier dysfunctions in atopic dermatitis: a skin-gut-lung model linking microbiome alteration and immune dysregulation. Br J Dermatol 179(3):570–581
Acknowledgements
The authors appreciate all the participants providing supports for this study.
Funding
None.
Author information
Authors and Affiliations
Contributions
Cuilin Xie and MengYao Hu conceived and designed the experiments. Cuilin Xie, MengYao Hu and Bin Niu carried out the experiments. Cuilin Xie, MengYao Hu and Bin Niu analyzed the data. Cuilin Xie, MengYao Hu and Bin Niu drafted the manuscript. All authors agreed to be accountable for all aspects of the work. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Author Cuilin Xie declares that she has no conflict of interest. Author MengYao Hu declares that she has no conflict of interest. Author Bin Niu declares that he has no conflict of interest.
Ethical approval
The experimental procedures were conducted after obtaining the approval from the Ethics Committee of Huangshi Central Hospital, Affiliated Hospital of Hubei Institute of Technology in accordance to the China National Law on animal care and use.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, C., Hu, M. & Niu, B. Mangiferin can alleviate atopic dermatitis-like responses in mice and HaCaT cells. Mol. Cell. Toxicol. (2024). https://doi.org/10.1007/s13273-024-00476-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s13273-024-00476-0