Abstract
Background
Bladder cancer (BC) originating from the bladder mucosa is a malignant tumor of the genitourinary system. Long non-coding RNAs (lncRNAs) can participate in cell proliferation and differentiation at multiple levels, and lncRNA dysregulation involves in the processes of malignant tumors.
Methods
Focused on lncRNA small nucleolar RNA host gene 12 (SNHG12) and its target miR-143-3p, the specific mechanism related to BC was discovered. SNHG12 and miR-143-3p expression in BC samples was checked, together with the association with the malignant activities of BC cells.
Results
As texted, SNHG12 expression went upward and miR-143-3p expression went downward in BC. SNHG12 depletion or miR-143-3p over-expression was causal for the suppression of BC cell activities. SNHG12 expression was directly associated with miR-143-3p expression. Ablating miR-143-3p abrogated changes in BC cell function induced by SNHG12 depletion.
Conclusions
Generally, SNHG12/miR-143-3p induces the malignant activities of BC cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bladder cancer (BC) is the most common malignancy of the genitourinary system (Torre et al. 2015). In 2020, BC ranked 9th in incidence globally and 13th in cancer-related deaths among all cancers (Sung et al. 2021). About 75% are non-muscle-invasive, the rest are muscle-invasive (MIBC) or metastatic (Antoni et al. 2017). Currently, the clinical treatment of BC patients mainly relies on radical cystectomy, chemotherapy, transurethral cystectomy, or neoadjuvant chemotherapy (DeGeorge et al. 2017; Dai et al. 2021). However, conventional treatment options for tumor distant metastasis are limited, resulting in poor prognosis and low survival rate of patients (Yuan et al. 2021). Therefore, it is particularly emergent to develop reliable biomarkers and effective therapeutic targets for BC.
Long non-coding RNAs (lncRNAs) are non-coding RNA transcripts that modify molecular interactions and cellular pathways during tumor development (Zhang et al. 2021a). Abnormal expression of lncRNAs has been recognized to modify cellular functions in tumors (Liu 2022). As exampled, aberrant high expression of LINC00649 enhances the malignancy of BC cells (Chen and Chen 2021) and abnormal down-regulation of lncRNA ADAMTS9-AS2 blocks the malignant activities of BC cells (Guo et al. 2021a). LncSTYK1-2 regulates ITGA2 expression and AKT/STAT3/NF-kB signaling by targeting miR-146b-5p, and inhibits BC cell proliferation, migration, and tumorigenicity (Dai et al. 2021). It is shown that lncRNA HNF1A-AS1 promotes human BC cell viability and migration (Feng and Wang 2018). LncRNA small nucleolar RNA host gene 12 (SNHG12) is a newly discovered lncRNA that is abnormally expressed in a variety of human cancers (Wang et al. 2021; Cheng et al. 2020). Recent studies have shown that up-regulated SNHG12 could drive tumorigenesis and cancer phenotypes, such as proliferation, metastasis, invasion, and anti-apoptosis (Zhang and Lu 2018; Wang et al. 2017; Chen et al. 2019). High expression of SNHG12 is associated with poor prognosis in patients with BC, and down-regulation of SNHG12 can inhibit the proliferation of BC cells in vitro (Jiang, et al. 2018). SNHG12 is highly expressed in BC tissues and cells, promotes the proliferation, invasion, and migration of BC cells in vitro, and promotes tumor growth in vivo (Zhang et al. 2022a). However, the regulatory mechanism of SNHG12 in BC remains to be fully elucidated.
It is widely known that lncRNAs act as miRNA sponges (Salmena et al. 2011). LncRNAs-mediated miR-143-3p serves a part in tumors, such as renal cell carcinoma (Li et al. 2022) and non-small cell lung cancer (Li et al. 2021). Many studies have proved that miR-143-3p is related to BC progression (Huang, et al. 2022; Zhang et al. 2021b; Dong et al. 2021).
This study aimed to investigate the potential molecular mechanism of SNHG12 in BC and may provide a possible strategy for the treatment and diagnosis of BC. BC tissue and adjacent normal tissue were sampled for clinicopathological analysis and BC cells were obtained for in vitro experiments. The interaction and function of SNHG12/miR-143-3p were probed in BC.
Methods
Clinical tissue collection
BC tissues were surgical specimens from BC patients diagnosed by pathological examination. Inclusion criteria: (1) no history of tumor, and (2) no history of chemotherapy, radiotherapy, or anti-tumor therapy. Forty patients underwent surgery in Tongde Hospital of Zhejiang Province from October 2018 to October 2020. Adjacent noncancerous bladder tissues were taken as controls. Each patient provided written consent. The study plan was approved by the Medical Ethics Committee of Tongde Hospital of Zhejiang Province.
Cell culture
BC cell line T24 (#CC1001, CellCook, Guangzhou, China) and human embryonic kidney cell HEK293T (#CC4003, CellCook, Guangzhou, China) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Thermo, Waltham, MA, USA) supplemented with 10% FBS and penicillin/streptomycin.
Cell transfection
Short hairpin RNA (shRNA) targeting SNHG12 (sh-SNHG12), SNHG12 over-expression vector (oe-SNHG12), miR-143-3p mimic/inhibitor, and corresponding negative controls were produced by GenePharma (Shanghai, China). According to the protocol, T24 cells were conditioned to transfection based on Lipofectamine 3000 reagent (Thermo, Waltham, MA, USA).
Cell proliferation
CCK-8 (Yeasen, Shanghai, China) was used to detect cell proliferation according to the manufacturer’s instructions. In short, T24 cells (5 × 103 cells/well in 96-well plates) were allowed to hatch with 10 μl of CCK-8 reaction solution for 3 h and the optical density450 nm was measured on a microplate reader (Thermo, Waltham, MA, USA).
Cell colony-forming ability
T24 cells (600 cells/well in 24-well plates) were cultured at 37 °C, 5% CO2 and saturated humidity for 14 days. After that, the colonies were stained with Giemsa staining solution (Sinochrome, Shanghai, China) for 25 min after fixation with methanol, and those with at least ten cells were counted under a light microscope.
Cell migration and invasion
Transwell (Corning, NY, USA) was used to detect cell migration and invasion according to the manufacturer's instructions. In short, T24 cells (1 × 105 cells/ml) were inoculated in Transwell upper chamber containing serum-free DMEM, while the lower chamber was filled with DMEM containing 10% FBS. Cells that migrated to the lower chamber after 48 h were stained with 1% crystal violet solution for 12 min and photographed under a microscope to evaluate migration. To assess invasion capacity, T24 cells were inoculated in Transwell upper chamber pre-coated with Matrigel (Corning, NY, USA) following the same experimental procedure.
Cell apoptosis
Apoptosis was detected using a commercial apoptosis assay kit (Invitrogen, USA) according to the manufacturer’s instructions. In short, T24 cells were incubated with Annexin V-FITC and PI solutions and examined on a FACSCalibur flow cytometer (Becton Dickinson, USA).
qPCR gene expression study
Total RNA from BC tissues and cells was extracted using a Trizol kit (Invitrogen) and its concentration was determined on a NanoDrop 2000 (Thermo, Waltham, MA, USA). cDNA was synthesized from approximately 1.5 μg RNA using the M-MLV RT kit (#M1701; Promega, Madison, WI, USA). Then, according to the manufacturer's instructions, SNHG12 expression was determined using the Platinum II Hot-Start Green PCR Master Kit (#14001013, Thermo, Waltham, MA, USA). miScript II RT Kit (#218161, Qiagen, Dusseldorf, Germany) was used to detect miR-143-3p. GAPDH and U6 were internal parameters of SNHG12 and miR-143-3p, respectively. The primer sequences used for the expression assay are shown in Table 1.
Dual-luciferase reporter gene assay
According to the protocol, the binding of SNHG12 to miR-143-3p was verified using the dual-luciferase reporter gene detection kit (#E1910, Promega, Madison, WI, USA). Shortly, the SNHG12 wild-type (WT) or mutant (MUT) sequence ligated to the pmirGLO vector was co-transfected with miR-143-3p mimic or mimic NC into HEK-293 T cells. Then, luciferase activity in cell lysates was measured using a GloMax-20/20 luminometer (Promega, Madison, WI, USA).
Statistical analysis
Statistical significance was assessed for quantitative results expressed as mean ± standard deviation using SPSS 20.0. Student’s t test or analysis of variance was dedicated to comparative assessment. P < 0.05 defined significant differences.
Results
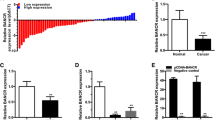
SNHG12 is up-regulated in BC
A qPCR gene expression study measured the increase in SNHG12 expression in BC tissue (Fig. 1A) and further discovered that SNHG12 expression was induced with the advancement of the clinical stage (Fig. 1B). According to that, the SNHG12 high expression group and low-expression group were formulated and followed up. Table 2 presents that SNHG12 expression was not correlated with age, tumor size, or histopathological type, but with tumor stage, lymph-node metastasis, and distant metastasis. Especially, SNHG12 expression was higher in BC patients with metastasis than in those without metastasis (Fig. 1C).
Ablating SNHG12 inhibits BC cell activities
To analyze the functional influence of SNHG12 during cancer development, the T24 cell line was transfected with sh-SNHG12 and oe-SNHG12 to downregulate and upregulate SNHG12, respectively (Fig. 2A). As examined by functional assays, it was observable that SNHG12-depleted T24 cells had decreased proliferative, migratory, and invasive abilities and increased apoptotic ability. SNHG12-overexpressed T24 cells presented the contrast malignant phenotypes (Fig. 2B–F). In general, ablating SNHG12 inhibits BC cell activities, while upregulating SNHG12 has the opposite effect.
Ablating SNHG12 inhibits BC cell activities. A SNHG12 expression in BC cells interfered with sh-SNHG12 or oe-SNHG12; B–F proliferation, migration, invasion, and apoptosis after intervening SNHG12 expression; the values were expressed as mean ± standard deviation. *vs. sh-NC, P < 0.05; #vs. oe-NC, P < 0.05. NC negative control
SNHG12 is a functional modifier of miR-143-3p expression
On the bioinformatics website starBase (http://starbase.sysu.edu.cn/), a targeted binding site existed between SNHG12 and miR-143-3p (Fig. 3A). Considering the potential binding relation, luciferase activity analysis was implemented to discover that miR-143-3p mimic conferred a repressive impact on the luciferase activity of SNHG12-WT (Fig. 3B). Moreover, miR-143-3p expression was maintained at a low level in BC tissues and negatively correlated with SNHG12 expression (Fig. 3C, D). Specifically, after transfecting SNHG12 low-expression and over-expression vector into T24 cells, miR-143-3p expression was recovered or further suppressed in T24 cells, respectively (Fig. 3E). In conclusion, SNHG12 is a functional modifier of miR-143-3p expression.
SNHG12 is a functional modifier of miR-143-3p expression. A, B The targeted binding site of SNHG12 and miR-143-3p; C miR-143-3p expression in BC tissues; D correlation analysis of the relationship between SNHG12 and miR-143-3p expression in BC; E miR-143-3p expression after intervening SNHG12; the values were expressed as mean ± standard deviation. *vs. sh-NC, P < 0.05; #vs. oe-NC, P < 0.05
Restoring miR-143-3p hampers the malignant activities of BC cells
Pivoted on the potential function of miR-143-3p in BC, miR-143-3p mimic/inhibitor was transfected in T24 cells to elevate or suppress miR-143-3p expression, respectively (Fig. 4A). The cellular experiments suggested that miR-143-3p over-expression had similar effects to SNHG12 depletion on BC cells, while miR-143-3p down-regulation exerted as SNHG12 over-expression (Fig. 4B–F). In summary, restoring miR-143-3p hampers the malignant activities of BC cells, while inhibiting miR-143-3p has the opposite effect.
Restoring miR-143-3p hampers the malignant activities of BC cells. A miR-143-3p expression in BC cells interfered with miR-143-3p mimic/inhibitor; B–F proliferation, migration, invasion, and apoptosis after intervening SNHG12 expression; the values were expressed as mean ± standard deviation. *vs. mimic NC, P < 0.05; #vs. inhibitor NC, P < 0.05
miR-143-3p knockdown promotes the development of SNHG12-depleted BC cells
A co-transfection design was implemented with sh-SNHG12 + miR-143-3p inhibitor to further verify SNHG12/miR-143-3p-mediated progression of BC. miR-143-3p inhibitor suppressed miR-143-3p expression mediated by sh-SNHG12 (Fig. 5A), thereby enhancing the malignant activities of T24 cells (Fig. 5B–F).
miR-143-3p knockdown promotes the development of SNHG12-depleted BC cells. A miR-143-3p expression in BC cells interfered with sh-SNHG12 + miR-143-3p inhibitor; B–F Proliferation, migration, invasion, and apoptosis after intervening SNHG12 expression; the values were expressed as mean ± standard deviation. *vs. sh-SNHG12 + inhibitor NC, P < 0.05
Discussion
Over the past decades, epigenetic regulation of functional genes, such as lncRNAs and miRNAs, are important cancer regulatory mechanisms (Dai et al. 2021; Hosseinahli et al. 2018). In BC cells, the functions of lncRNAs and miRNAs have been also revealed previously (Wieczorek and Reszka 2018). However, their pathogenic effects and underlying mechanisms remain unstudied. The study found that SNHG12 expression was up-regulated in BC tissues. In addition, suppressing SNHG12 depressed proliferation, migration, and invasion of BC cells, and promoted cell apoptosis. In terms of molecular mechanisms, SNHG12 could directly bind and inhibit miR-143-3p expression in BC cells. Finally, suppressing miR-143-3p reversed the regulatory effects of down-regulation of SNHG12 on proliferation, migration, invasion, and apoptosis of BC cells. These studies reveal new molecular mechanisms involved in the regulation of lncRNAs and miRNAs gene expression in BC pathogenesis.
As mentioned above, lncRNAs regulated gene expression has been established as a pathogenic mechanism in BC (Martens-Uzunova et al. 2014; Robertson, et al. 2017; Lv et al. 2017). SNHG12 has recently attracted attention for its important role in human malignancies. SNHG12 is highly expressed in gastric cancer (Zhang et al. 2022b), non-small cell lung cancer (Tan et al. 2022), colorectal cancer (Guo et al. 2021b), renal cell carcinoma (Yu et al. 2021), and prostate cancer (Chen et al. 2020). As expected, the report suggested that SNHG12 was up-regulated in BC tissues. LncRNAs are significantly correlated with the clinicopathological features of BC patients, especially tumor metastasis, and clinical stage (Zhang et al. 2021a). Similarly, by analyzing the relationship of SNHG12 expression with clinicopathological parameters of BC, it was noticed that SNHG12 was correlated with tumor stage, lymph node, and distant metastasis. In addition, it was also found that SNHG12 expression in BC tissues of patients with metastasis was significantly higher than that of patients without metastasis. Finally, to better verify the cancer-promoting effect of SNHG12, functional experiments were conducted. The results showed that SNHG12 acted as an oncogene in BC, and knocking down SNHG12 could effectively inhibit the proliferation, migration, and invasion of BC cells, and promote cell apoptosis.
LncRNAs act as molecular sponges of miRNAs (Mao et al. 2022). Subsequently, SNHG12 showed high specific enrichment of miR-143-3p through the bioinformatics website and luciferin reporter gene analysis. To further verify the relationship between SNHG12 and miR-143-3p, a clinical correlation analysis was conducted. The results showed that miR-143-3p expression in BC tissues was down-regulated and negatively correlated with SNHG12. Consistently, SNHG12 can negatively regulate miR-143-3p expression in BC cells, that is, after transfection of sh-SNHG12, miR-143-3p expression was increased, while after transfection of oe-SNHG12, miR-143-3p expression was decreased. These results all confirmed that SNHG12 acted as an RNA sponge to inhibit miR-143-3p in BC cells. miR-143-3p has been considered a tumor suppressor in cancers. For instance, miR-143-3p suppresses brain metastasis in lung cancer (Wang et al. 2019) and disrupts cellular activities in breast cancer (Pinweha et al. 2019) and BC (Huang, et al. 2022). Therefore, the effects of miR-143-3p on the proliferation, invasion, and apoptosis of BC cells were studied. The results suggested that upregulation of miR-143-3p expression can inhibit the proliferation, migration, and invasion of BC cells, and promote cell apoptosis. The down-regulation of miR-143-3p can reverse the effect of down-regulation of SNHG12 on the biological function of BC cells.
Considering the interaction of SNHG12 and miR-143-3p in BC cells revealed here, its potential for the diagnosis or treatment of cancer patients needs to be further evaluated by large-scale preclinical experiments. In addition, future experiments need to further mine the downstream target genes of miR-143-3p. Finally, future animal experiments are needed to elucidate the molecular mechanism of SNHG12 in BC.
Conclusion
Our study found that SNHG12 expression was inversely associated with poor prognosis in BC. SNHG12, as a novel BC oncogenic factor, promoted the malignant behaviors of BC cells by sponge-absorbing miR-143-3p. These findings provide a new perspective on the pathogenesis and development of BC and can serve as a new target for BC prevention, diagnosis, and targeted therapy.
Availability of data and materials
Data are available from the corresponding author on request.
References
Antoni S et al (2017) Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol 71(1):96–108
Chen X, Chen S (2021) LINC00649 promotes bladder cancer malignant progression by regulating the miR-15a-5p/HMGA1 axis. Oncol Rep. https://doi.org/10.3892/or.2021.7959
Chen Q et al (2019) Overexpression of SNHG12 regulates the viability and invasion of renal cell carcinoma cells through modulation of HIF1alpha. Cancer Cell Int 19:128
Chen Z et al (2020) Long noncoding RNA SNHG12 promotes prostate tumor occurrence and progression via AKT regulation. Biomed Res Int 2020:8812923
Cheng G et al (2020) Long noncoding RNA SNHG12 indicates the prognosis of prostate cancer and accelerates tumorigenesis via sponging miR-133b. J Cell Physiol 235(2):1235–1246
Dai R et al (2021) Lnc-STYK1-2 regulates bladder cancer cell proliferation, migration, and invasion by targeting miR-146b-5p expression and AKT/STAT3/NF-kB signaling. Cancer Cell Int 21(1):408
DeGeorge K, Holt H, Hodges S (2017) Bladder cancer: diagnosis and treatment. Am Fam Physician 96(8):507–514
Dong L et al (2021) LINC00511/miRNA-143-3p modulates apoptosis and malignant phenotype of bladder carcinoma cells via PCMT1. Front Cell Dev Biol 9:650999
Feng Z, Wang B (2018) Long non-coding RNA HNF1A-AS1 promotes cell viability and migration in human bladder cancer. Oncol Lett 15(4):4535–4540
Guo Q et al (2021a) Long-chain noncoding RNA ADAMTS9-AS2 regulates proliferation, migration, and apoptosis in bladder cancer cells through regulating miR-182-5p. J Interferon Cytokine Res 41(2):60–71
Guo K et al (2021b) LncRNA SNHG12 promotes the development and progression of colon cancer by regulating the miR-15a/PDK4 axis. Am J Transl Res 13(9):10233–10247
Hosseinahli N et al (2018) Treating cancer with microRNA replacement therapy: a literature review. J Cell Physiol 233(8):5574–5588
Huang C et al (2022) Long noncoding RNA LINC02470 sponges MicroRNA-143-3p and enhances SMAD3-mediated epithelial-to-mesenchymal transition to promote the aggressive properties of bladder cancer. Cancers 14(4):968
Jiang B et al (2018) Identification of oncogenic long noncoding RNA SNHG12 and DUXAP8 in human bladder cancer through a comprehensive profiling analysis. Biomed Pharmacotherapy = Biomedecine & Pharmacotherapie 108:500–507
Li Y et al (2021) Downregulation of LINC01296 suppresses non-small-cell lung cancer via targeting miR-143-3p/ATG2B. Acta Biochim Biophys Sin 53(12):1681–1690
Li Y et al (2022) Silencing lncRNA SLC16A1-AS1 induced ferroptosis in renal cell carcinoma through miR-143-3p/SLC7A11 signaling. Technol Cancer Res Treat 21:15330338221077804
Liu Q (2022) The emerging roles of exosomal long non-coding RNAs in bladder cancer. J Cell Mol Med 26(4):966–976
Lv M et al (2017) lncRNA H19 regulates epithelial-mesenchymal transition and metastasis of bladder cancer by miR-29b-3p as competing endogenous RNA. Biochim Biophys Acta Mol Cell Res 1864(10):1887–1899
Mao Y, Wen C, Yang Z (2022) Construction of a co-expression network for lncRNAs and mRNAs related to urothelial carcinoma of the bladder progression. Front Oncol 12:835074
Martens-Uzunova ES et al (2014) Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urol 65(6):1140–1151
Pinweha P et al (2019) MicroRNA-143-3p targets pyruvate carboxylase expression and controls proliferation and migration of MDA-MB-231 cells. Arch Biochem Biophys 677:108169
Robertson AG et al (2017) Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171(3):540-556 e25
Salmena L et al (2011) A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146(3):353–358
Sung H et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Tan D et al (2022) LncRNA SNHG12 in extracellular vesicles derived from carcinoma-associated fibroblasts promotes cisplatin resistance in non-small cell lung cancer cells. Bioengineered 13(1):1838–1857
Torre L et al (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Wang JZ et al (2017) LncRNA SNHG12 promotes cell growth and inhibits cell apoptosis in colorectal cancer cells. Braz J Med Biol Res 50(3):e6079
Wang H et al (2019) N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol Cancer 18(1):181
Wang X et al (2021) Long noncoding RNA SNHG12 is a potential diagnostic and prognostic biomarker in various tumors. Chin Neurosurg J 7(1):37
Wieczorek E, Reszka E (2018) mRNA, microRNA and lncRNA as novel bladder tumor markers. Clin Chim Acta Int J Clin Chem 477:141–153
Yu H et al (2021) SNHG12 promotes carcinogenesis of human renal cell cancer via functioning as a competing endogenous RNA and sponging miR-30a-3p. J Cell Mol Med 25(10):4696–4708
Yuan J et al (2021) Annexin A8 regulated by lncRNA-TUG1/miR-140-3p axis promotes bladder cancer progression and metastasis. Mol Therapy Oncolytics 22:36–51
Zhang H, Lu W (2018) LncRNA SNHG12 regulates gastric cancer progression by acting as a molecular sponge of miR-320. Mol Med Rep 17(2):2743–2749
Zhang Y et al (2021a) Biological functions and clinical significance of long noncoding RNAs in bladder cancer. Cell Death Discovery 7(1):278
Zhang Y, Chen L, Luo G (2021b) Long non-coding RNA PCAT6 regulates bladder cancer progression via the microRNA-143-3p/PDIA6 axis. Exp Ther Med 22(3):947
Zhang B et al (2022a) Identification of stemness index-related long noncoding RNA SNHG12 in human bladder cancer based on WGCNA. Mol Cell Probes 66:101867
Zhang F et al (2022b) Gastric cancer cell-derived extracellular vesicles elevate E2F7 expression and activate the MAPK/ERK signaling to promote peritoneal metastasis through the delivery of SNHG12. Cell Death Discovery 8(1):164
Acknowledgements
Not applicable.
Funding
This work was supported by Health Medicine Science and Technology Plan Project of Zhejiang Province, China (Grant No. 2021KY603).
Author information
Authors and Affiliations
Contributions
LC designed the research study. LC and CF performed the research. WZ and WXY provided help and advice. CF and WZ analyzed the data. LC and WXY wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Li Chen declares that he has no conflict of interest. Chao Feng declares that he has no conflict of interest. Wei Zhou declares that he has no conflict of interest. WenXin Ye declares that he has no conflict of interest.
Ethical approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All subjects were approved by Tongde Hospital of Zhejiang Province (No. (2021) 018). Written informed consent was obtained from each subject.
Consent for publication
All authors approved the final manuscript and agreed to publish.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, L., Feng, C., Zhou, W. et al. Molecular mechanism of long non-coding RNA SNHG12 regulating bladder cancer cell activities. Mol. Cell. Toxicol. (2023). https://doi.org/10.1007/s13273-023-00391-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s13273-023-00391-w