Abstract
Background
The invasion of trophoblast cells into the maternal uterine decidua is crucial for the formation of the placenta and establishment of pregnancy. Stromal cell-derived factor-1 (SDF-1) regulates cellular functions such as migration, adhesion, and proliferation. During pregnancy, both SDF-1 and its receptor CXCR4 are expressed in the placenta. Abnormal expression of the SDF-1 system has been linked to pregnancy disorders such as pre-eclampsia.
Objective
The purpose of this study was to investigate the effect of SDF-1/CXCR4 signaling on trophoblast cells and the related molecular mechanism(s).
Results
The invasiveness of HTR8/SVneo human trophoblast cells was stimulated by extracellular SDF-1. SDF-1 activated the phosphatidylinositol 3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MAPK) signaling pathways. The ability of SDF-1 to stimulate HTR8/SVneo cell invasion was blocked in the presence of a PI3K inhibitor (wortmannin), an ERK1/2 MAPK inhibitor (U0126), or a P38 MAPK inhibitor (SB203580). The expression of the matrix metalloproteinase (MMP)-2 and MMP-9 genes increased in SDF-1-treated HTR8/SVneo cells, and the up-regulation of MMPs expression was inhibited by blocking the SDF-1 receptor. Additionally, SDF-1 treatment increased MMP-2 and MMP-9 protein levels in HTR8/SVneo cells, and the increase of MMP-2 and MMP-9 was reduced by inhibiting the PI3K and/or MAPK intracellular signaling pathway.
Conclusion
These findings provide evidence that appropriate SDF-1-mediated signaling pathways contribute to the regulation of trophoblast invasiveness into the endometrium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Normal placental development is characterized by extravillous cytotrophoblasts (EVTs) displaying unique capabilities of invasion into decidual tissue and migration into decidual spiral arteries so as to alter the vasculature inside the uterus (Timeva et al. 2014; Chen et al. 2012). These activities of trophoblast cells are critical events during pregnancy; however, they stringently controlled for the establishment of a normal placenta (Hunkapiller and Fisher 2008; Knofler and Pollheimer 2012). Inappropriate trophoblast cell invasion has been demonstrated to be associated with many pregnancy-related diseases and miscarriage. For example, insufficient invasion of EVTs is believed to be associated with poor remodeling of the uterine spiral arteries, which is a key feature of preeclampsia (Chaddha et al. 2004; Lim et al. 1997; Kaufmann et al. 2003; Shah 2001).
The process of trophoblast invasion is precisely regulated by a collection of multiple factors including growth factors, cytokines, and chemokines, which are produced locally or distally in the trophoblast microenvironment (Paiva et al. 2011; Oreshkova et al. 2012). Chemokines, a superfamily of structurally-associated chemotactic proteins, play a crucial role in orchestrating the immune response as mediators of cell recruitment (Zlotnik and Yoshie 2012). Several chemokines produced either by trophoblasts themselves or other component cells have been found at the maternal–fetal interface, and increased and/or decreased levels of these chemokines in serum or placental tissue have been demonstrated in association with abnormal placental development (Makrigiannakis et al. 2006; He et al. 2012; Wallace et al. 2013).
A member of the CXC chemokine family, stromal cell-derived factor-1 (SDF-1, systematic name C-X-C motif chemokine 12; CXCL12), functions as a potent chemotactic factor for many kinds of cells (Kantele et al. 2000; D'Apuzzo et al. 1997; Sozzani et al. 1997; Kawabata et al. 1999; Naiyer et al. 1999). It has been shown that first-trimester human placental trophoblasts secrete SDF-1, which not only recruits immune cells into the decidua, but also regulates trophoblast cell survival and the cross-talk between trophoblasts and decidual stromal cells (Wu et al. 2005; Carlino et al. 2008; Jaleel et al. 2004). Levels of SDF-1 in maternal serum and placental tissue were observed to be increased in patients with preeclampsia compared with normal control patients (Boij et al. 2012; Schanz et al. 2011), which suggests that SDF-1 might play a role in the invasive capacity of human trophoblast cells and normal placentation. Therefore, the aims of this study were to (1) investigate the ability of SDF-1 to induce trophoblast invasiveness; and (2) determine the intracellular signaling pathways and molecular mechanisms underlying SDF-1-induced trophoblast invasion.
Materials and methods
Cell culture
Human trophoblast HTR8/SVneo cell line was maintained in RPMI 1640 (Cat No: A10491, Thermo Fisher Scientific Inc., Waltham, MA) containing 5% fetal bovine serum (FBS) at 37 °C. For assays, monolayer cultures of HTR8/SVneo cells were serum-starved for 24 h prior to treatment and then incubated in the presence of various treatments.
Transwell cell invasion assay
The serum-starved HTR8/SVneo cells (1 × 105 cells per 100 μl serum-free RPMI 1640) were seeded on Matrigel-coated transwell inserts (Cat No: 354480, Corning, Inc., Corning, NY) and treatments were added to each bottom well (n = 3 wells per treatment). After 20 h of incubation, the insert membranes and non-invading cells were removed from the chamber. Transwell membranes were fixed in methanol for 10 min and placed on a glass slide. The number of invading cells was counted in five non-overlapping locations of triplicate membranes under a DM3000 (Leica) microscope. Data are expressed as the percent invasion through matrigel matrix-coated membrane relative to the migration through the non-coated membrane.
BrdU cell proliferation assay
Proliferation assays were conducted using a Cell Proliferation ELISA BrdU Kit (Cat No. 11647229001, Roche Molecular Systems, Inc., Basel, Switzerland), according to the manufacturer’s recommendations. Briefly, after 48 h of cell incubation with recombinant SDF-1, 10 μM BrdU was added to the cell culture and the cells were incubated for an additional 2 h at 37 °C. The relative level of the reaction product was quantified by measuring the absorbance at 370 and 492 nm (reference wavelength).
Western blot analyses
Proteins were denatured, separated using SDS-PAGE, and transferred to nitrocellulose membranes. Blots were developed using enhanced chemiluminescence detection (SuperSignal West Pico, Pierce, Rockford, IL, USA) and quantified by measuring the intensity of light emitted from correctly sized bands under ultraviolet light using a ChemiDoc EQ system and Quantity One software (Bio-Rad, Hercules, CA, USA). As a loading control, total proteins or α-tubulin (TUBA) were used to normalize the results from the detection of target proteins. Multiple exposures of each western blot were performed to ensure linearity of the chemiluminescent signals.
Reverse transcriptase PCR and quantitative RT-PCR analysis
Complementary DNA was synthesized from HTR8/SVneo cellular RNA using AccuPower® RT PreMix (Bioneer, Daejeon, Republic of Korea), random hexamer (Invitrogen, Carlsbad, CA) and oligo (dT) primers. All primers were synthesized by Bioneer Inc. (Daejeon, Korea). After RT-PCR using SDF-1 and CXCR4 primers, equal amounts of reaction products were analyzed using a 1% agarose gel, and PCR products were visualized using a Gel Doc system (Bio-Rad, Hercules, CA, USA). Specific primers for individual human MMP-2 (Forward: 5′- TTCCCCTTCATCTTCCTTGG-3′; Reverse: 5′- ATCTGGACAAAAGCCCCACT-3′) and MMP-9 (Forward: 5′-CACTGTCCACCCCTCAGAGC-3′; Reverse: 5′-GCCACTTGTCGGCGATAAGG-3′) were designed using the on-line Primer 3 software. The expression levels of MMP-2 and MMP-9 genes were measured using SYBR® Green (Sigma, St. Louis, MO) and a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA). Sequence-specific products were identified by generating a melting curve, and relative gene expression was quantified using the 2–ΔΔCT method. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin genes were used as endogenous controls to standardize the amount of RNA in each reaction.
Reagents
Recombinant human SDF-1 (catalog number: 350-NS) was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The following were purchased from Cell Signaling Technology (Beverly, MA, USA): antibodies against phospho-AKT (catalog number: 4060), phospho-ERK1/2 (catalog number: 9101), phospho-P70S6K (catalog number: 9204), phospho-S6 (catalog number: 2211), total AKT (catalog number: 9272), ERK1/2 (catalog number: 4695), P70S6K (catalog number: 9202), S6 (catalog number: 2217), and inhibitor for PI3K/AKT (Wortmannin, catalog number: 9951). Enzo Life Sciences, Inc. (Farmingdale, NY) supplied inhibitors for ERK1/2 (U0126, catalog number: EI282) and P38 MAPK (SB203580, catalog number: BML-EI286). Antibodies against MMP-2 (catalog number: sc-13594) and MMP-9 (catalog number: sc-27133) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). AMD3100 (catalog number: 239820-5MGCN) was purchased from Merck Millipore (Darmstadt, Germany).
Statistical analyses
All quantitative data were subjected to least squares analysis of variance (ANOVA) using the General Linear Model procedures of the Statistical Analysis System (SAS Institute Inc., Cary, NC, USA). Pooled data from three independent experiments performed in triplicate are presented as mean (LSMs) ± standard errors (SEs).
Results
SDF-1 shows dose-dependent stimulating effect on HTR8/SVneo cell invasion
To investigate the functional role of SDF-1 in trophoblastic cells, we first performed invasion assays using the HTR8/SVneo cell line established from first trimester human cytotrophoblasts (Graham et al. 1993). As illustrated in Fig. 1A, SDF-1-induced cell invasion gradually increased as the dose of SDF-1 treatment increased, and reached 213% increase (P < 0.05) in the HTR8/SVneo cells treated with 50 ng/ml of SDF-1, compared to non-treated (0 ng/ml) cells. To rule out the possibility that the observed invasion changes were due to the effects of SDF-1 on cell proliferation, we further investigated the effects of SDF-1 on trophoblast cell proliferation. The results showed that SDF-1 had no significant effect (P > 0.05) on cell proliferation compared with the control group (Fig. 1B). Also, the cultured HTR8/SVneo cells expressed SDF-1 and CXCR4 genes (Fig. 1C).
Pro-invasive effect of SDF-1 on HTR8/SVneo cells. A Dose-dependent (0, 1, 10, 50, 100, and 200 ng/ml) effects of SDF-1 on HTR8/SVneo cell invasion was determined by using in vitro transwell cell invasion assay. B Dose-dependent (0, 1, 10, 50, 100, and 200 ng/ml) effects of SDF-1 on HTR8/SVneo cell proliferation was determined by using BrdU cell proliferation assay. SDF-1-induced cell invasion and proliferation rates are presented as relative percentage changes relative to control (0 ng/ml) HTR8/SVneo cells (100%). The asterisks indicate significant effect of treatment (*P < 0.05). C Expression of SDF-1 and CXCR4 genes in HTR8/SVneo cells. RT-PCR analysis was performed to detect SDF-1 and CXCR4 transcripts in HTR8/SVneo cells. The GAPDH gene was used as the endogenous control to standardize the amount of RNA in each reaction
SDF-1 induces activation of PI3K/AKT and MAPK signaling pathways in HTR8/SVneo cells
Because treatment in vitro cultured HTR8/SVneo cells with SDF-1 induced cell invasion, we hypothesized that SDF-1 transduces its pro-invasiveness signal into HTR8/SVneo cells through activation of intracellular signaling cascades. We revealed that the abundance of phosphorylated (p)-status signaling molecules (AKT, ERK1/2, P38, P70S6K, and S6) increased as the SDF-1-treatment dose increases (0 to 100 ng/ml). In the HTR8/SVneo cells treated with 50 ng/ml SDF-1, the levels of phospho-AKT (Fig. 2A), phospho-ERK1/2 (Fig. 2B), phospho-P38 (Fig. 2C), phospho-P70S6K (Fig. 2D) and phospho-S6 (Fig. 2E) reached 2.9- (P < 0.01), 4.9- (P < 0.001), 4.5- (P < 0.01), 2.1- (P < 0.05), and 5.0- (P < 0.01) fold increases, respectively, compared to those in non-treated cells.
Inducible effect of SDF-1 on activation of PI3K and MAPK signaling pathways in HTR8/SVneo cells. Dose-dependent (0, 10, 25, 50, and 100 ng/ml) effects of SDF-1 on phosphorylation of A AKT, B ERK1/2, C P38, D P70S6K and E S6 were determined by using western blot analyses. Serum-starved HTR8/SVneo cells were treated with SDF-1 for 30 min. Bar graphs show the abundance of phospho-proteins normalized with abundance of total-proteins, which values are presented as fold changes relative to non-treated (0 ng/ml) HTR8/SVneo cells. The asterisks indicate significant differences compared to non-treated cells (***P < 0.001, **P < 0.01, and *P < 0.05)
SDF-1-induced PI3K/AKT and MAPK signaling proteins cross-talk each other and activate their co-downstream targets
To clarify the activation sequences of SDF-1-induced signaling molecules and interrelations among them, HTR8/SVneo cells were pre-incubated with 1 μM wortmannin (PI3K inhibitor), 20 μM U0126 (MEK1/2 inhibitor) or 20 μM SB203580 (P38 inhibitor) prior to SDF-1 treatment. The results show that SDF-1-mediated AKT phosphorylation was completely blocked by PI3K inhibition and partially reduced by ERK1/2 inhibition, while SDF-1-induced AKT activation was maintained in the presence of the P38 inhibitor (Fig. 3A). SDF-1-induced ERK1/2 activation was significantly inhibited in cells pre-treated with wortmannin or SB203580, or U0126 (Fig. 3B). Only the P38 inhibitor completely inhibited SDF-induced P38 phosphorylation, and the presence or absence of PI3K or ERK1/2 inhibitor did not significantly affect it (Fig. 3C). The increase in phospho-P70S6K and phospho-S6 proteins in response to SDF-1 was returned to near-basal levels by blocking the PI3K, ERK1/2, or P38 pathway (Fig. 3D, E).
Cross-talk between intracellular signaling pathways induced by SDF-1 in HTR/SVneo cells. The abundance of phospho-status of A AKT, B ERK1/2, C P38, D P70S6K and E S6 were determined in HTR8/SVneo cells treated with SDF-1 alone or SDF-1 plus each pharmacological inhibitor by using western blot analyses. Serum-starved HTR8/SVneo cells were pre-treated with inhibitors for 30 min before SDF-1 treatment and then incubated with SDF-1 for an additional 30 min. The abundance of phospho-proteins normalized with abundance of total-proteins are presented as fold changes relative to control (non-SDF-1 and -inhibitors) cells. The asterisks indicate significant differences compared to cells treated with only SDF-1 (***P < 0.001, **P < 0.01, and *P < 0.05)
SDF-1 receptor-conjugated intracellular signaling pathways are associated with up-regulation of MMP-2 and MMP-9
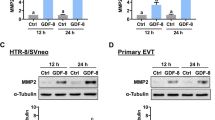
Next, we investigated whether there is a close correlation between SDF-1-induced pathways and the ability of SDF-1 to stimulate HTR8/SVneo cell invasion (Fig. 4). Consistent with the findings shown in Fig. 1, 50 ng/ml SDF-1 alone increased HTR8/SVneo cell invasion by 237% (P < 0.01); however, the stimulatory effect of SDF-1 on HTR8/SVneo cell invasion was partially or completely blocked in the presence of wortmannin, U0126, or SB203580 (P < 0.01) despite the existence of SDF-1 stimulation, when compared against cells treated with SDF-1 alone. In addition, SDF-1 alone significantly increased the abundance of MMP-2 and MMP-9 transcripts by approximately 6.2-fold (P < 0.01) and 5.2- (P < 0.01), respectively, compared to the control cells (Fig. 5A). These increased levels of MMP-2 and MMP-9 transcripts by SDF-1 stimulation were reduced by an SDF-1 antagonist (AMD3100), despite the existence of SDF-1 stimulation. SDF-1 stimulation was positively correlated with the abundance of MMP-2 and MMP-9 proteins in HTR8/SVneo cells. The effect of SDF-1 in up-regulating MMP-2 protein expression was reduced by PI3K-, ERK1/2, or P38 pathway blockage (Fig. 5B), and the increase in MMP-9 protein in response to SDF-1 stimulation was inhibited in the presence of ERK1/2 or P38 inhibitor, but not PI3K inhibitor (Fig. 5C).
Effect of SDF-1-mediated signaling pathways on HTR8/SVneo cell invasiveness. Effects of SDF-1 alone (50 ng/ml) or SDF-1 plus each pharmacological inhibitor on HTR8/SVneo cell invasion were analyzed by using in vitro transwell cell invasion assay. Cell invasion rates are presented as relative percentage changes relative to control (non-SDF-1 and -inhibitors) cells (100%). The asterisks indicate significant differences compared to control cells (**P < 0.01)
Effect of SDF-1 receptor-conjugated signaling pathways on the expression of pro-invasive genes. A The abundance of MMP-2 and MMP-9 transcripts in HTR8/SVneo cells treated with SDF-1 (0, 25, or 50 ng/ml) alone or SDF-1 (50 ng/ml) plus AMD3100 (SDF-1 antagonist; 10 or 30 ng/ml) were analyzed by quantitative RT-PCR analyses. Data are presented as fold changes relative to control (non-SDF-1 and AMD3100) cells. The asterisks indicate significant differences compared to control cells (*P < 0.05) and the sharps indicate significant differences compared to cells treated with only 50 ng/ml SDF-1 (###P < 0.001, ##P < 0.01, and #P < 0.05). The abundance of B MMP-2 and C MMP-9 proteins were determined in HTR8/SVneo cells treated with SDF-1 alone or SDF-1 plus each pharmacological inhibitor by using western blot analyses. The asterisks indicate significant differences compare to SDF-1 only treated cells (***P < 0.001, **P < 0.01, and *P < 0.05)
Discussion
A better understanding of the molecular mechanisms underlying the association between chemoattractants and trophoblast invasion will help to explain in detail the establishment of pregnancy and the progression of many pregnancy-related diseases, such as preeclampsia. Several cytokines and chemokines have been implicated in trophoblast migration and/or invasion; for example, epidermal growth factor (EGF), tumor necrosis factor-alpha (TNFα), interleukin-1-beta (IL-1β), transforming growth factor-beta 1 (TGF-β1), and chemokine CCL24 (Han et al. 2010; Huber et al. 2006; Karmakar and Das 2002; Li et al. 2015; Qiu et al. 2004; Gleeson et al. 2001; McKinnon et al. 2001; Cartwright et al. 2002). SDF-1 (also known as CCL12) has been identified as a potential contributor to controlling the migration and/or invasion of a variety of immune and non-immune cells including T cells (Kantele et al. 2000), B cells (D’Apuzzo et al. 1997), hematopoietic stem cells (Kawabata et al. 1999; Naiyer et al. 1999), endothelial cells (Feil and Augustin 1998) and neuronal cells (Bajetto et al. 1999; Ji et al. 2004). Elevated levels of SDF-1 have been observed in the maternal serum and placenta in women with preeclampsia (Boij et al. 2012; Schanz et al. 2011).
We report here that extracellular SDF-1 acts as a chemoattractant to increase the invasiveness of HTR-8/SVneo cells. Inappropriate placentation and several pregnancy-related diseases are associated with deficiencies in trophoblast invasion and increased trophoblast apoptosis (Hunkapiller and Fisher 2008; Knofler and Pollheimer 2012; Lim et al. 1997; Kaufmann et al. 2003; Norwitz 2006). Although a previous study by Jaleel et al. described that SDF-1 suppressed apoptosis and enhanced trophoblast cell survival via the MAPK pathway (Jaleel et al. 2004), we did not observe a significant increase in trophoblast cell proliferation by SDF-1 stimulation. Several molecules are known to stimulate migration and/or invasion of cells through MAPK activation and PI3K activation (Li et al. 2015; Qiu et al. 2004; Gleeson et al. 2001; Cartwright et al. 2002; Cheng et al. 2021). Recently, Li et al. reported that chemokine CCL24 promotes the invasiveness of trophoblasts through PI3K and ERK1/2 signaling pathways (Li et al. 2015). Our findings indicate that SDF-1-induced HTR8/SVneo cell invasion depends on the activation of PI3K/AKT, ERK1/2 and P38 MAPK signaling pathways, and that these pathways appear to cross-talk with each other and transduce signals to their co-downstream targets, P70S6K and S6.
SDF-1-induced invasiveness and activation of intracellular signaling cascades are suggested to be dependent on SDF-1 binding to its receptor at the cell surface (Koch and Engele 2020). Here, both SDF-1 and CXCR4 genes were observed to be expressed in HTR8/SVneo cells. Although most chemokines activate multiple receptors and CXCR7 was identified as a further receptor for SDF-1 (Balabanian et al. 2005; Liu et al. 2015), evidence suggests that SDF-1 binds exclusively to CXCR4, a G protein-coupled receptor in the heptahelical family (Loetscher et al. 1994; Zlotnik and Yoshie 2000). We determined that treatment with SDF-1 up-regulated MMP-2 and MMP-9 expression in HTR-8/SVneo cells and that blockage of CXCR4 by its antagonist AMD3100 inhibited the SDF-1-mediated MMP increase. Proteolysis plays a crucial role in the regulation of cell motility. MMP-2 and MMP-9 have been implicated in remodeling of the extracellular matrix during trophoblast invasion by acting as key enzymes that degrade components of the basement membrane, such as collagen (Staun-Ram et al. 2004). Upregulation and secretion of MMPs by trophoblast cells may lead to increased capacity to degrade the extracellular matrix and invade the base membrane (Singh et al. 2011; Zhu et al. 2012). The current results also show reducible effects of inhibitors of PI3K/AKT and MAPKs on SDF-1-mediated MMP increase. These findings indicate that SDF-1/CXCR4-conjugated PI3K/AKT and MAPK activation is required for the upregulation of MMP expression and trophoblast invasion in response to SDF-1 (Fig. 6). However, further research is needed to determine whether SDF-1 secreted by trophoblasts actually stimulates trophoblasts to increase invasiveness in an autocrine manner and whether activation of the SDF-1/CXCR4 axis is central to trophoblast invasion.
In conclusion, the present study demonstrates that SDF-1 regulates the invasiveness of trophoblasts in human first-trimester pregnancy by activating the PI3K/AKT and MAPKs pathways and by altering the expression of MMPs, which suggests that the SDF-1/CXCR4 axis regulates the appropriate invasion of trophoblasts and abnormal SDF-1 expression may be associated with the abnormal placentation and pathogenesis of pregnancy-related diseases.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bajetto A et al (1999) Expression of chemokine receptors in the rat brain. Ann N Y Acad Sci 876:201–209
Balabanian K et al (2005) The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem 280:35760–35766
Boij R et al (2012) Biomarkers of coagulation, inflammation, and angiogenesis are independently associated with preeclampsia. Am J Reprod Immunol 68:258–270
Carlino C et al (2008) Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood 111:3108–3115
Cartwright JE, Tse WK, Whitley GS (2002) Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Exp Cell Res 279:219–226
Chaddha V, Viero S, Huppertz B, Kingdom J (2004) Developmental biology of the placenta and the origins of placental insufficiency. Semin Fetal Neonatal Med 9:357–369
Chen JZ, Sheehan PM, Brennecke SP, Keogh RJ (2012) Vessel remodelling, pregnancy hormones and extravillous trophoblast function. Mol Cell Endocrinol 349:138–144
Cheng G, Liu X, Li P, Li Y (2021) Down-regulation of PTTG1 suppresses PDGF-BB-induced proliferation, migration and extracellular matrix production of airway smooth muscle cells (ASMCs) by regulating PI3K/AKT/mTOR signaling pathway. Mol Cell Toxicol 17:485–492
D’Apuzzo M et al (1997) The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4. Eur J Immunol 27:1788–1793
Feil C, Augustin HG (1998) Endothelial cells differentially express functional CXC-chemokine receptor-4 (CXCR-4/fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun 247:38–45
Gleeson LM, Chakraborty C, McKinnon T, Lala PK (2001) Insulin-like growth factor-binding protein 1 stimulates human trophoblast migration by signaling through alpha 5 beta 1 integrin via mitogen-activated protein Kinase pathway. J Clin Endocrinol Metab 86:2484–2493
Graham CH et al (1993) Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res 206:204–211
Han J et al (2010) Epidermal growth factor stimulates human trophoblast cell migration through Rho A and Rho C activation. Endocrinology 151:1732–1742
He YY et al (2012) The decidual stromal cells-secreted CCL2 induces and maintains decidual leukocytes into Th2 bias in human early pregnancy. Clin Immunol 145:161–173
Huber AV, Saleh L, Bauer S, Husslein P, Knofler M (2006) TNFalpha-mediated induction of PAI-1 restricts invasion of HTR-8/SVneo trophoblast cells. Placenta 27:127–136
Hunkapiller NM, Fisher SJ (2008) Chapter 12. Placental remodeling of the uterine vasculature. Method Enzymol 445:281–302
Jaleel MA, Tsai AC, Sarkar S, Freedman PV, Rubin LP (2004) Stromal cell-derived factor-1 (SDF-1) signalling regulates human placental trophoblast cell survival. Mol Hum Reprod 10:901–909
Ji JF, He BP, Dheen ST, Tay SS (2004) Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neurosci Lett 355:236–240
Kantele JM, Kurk S, Jutila MA (2000) Effects of continuous exposure to stromal cell-derived factor-1 alpha on T cell rolling and tight adhesion to monolayers of activated endothelial cells. J Immunol 164:5035–5040
Karmakar S, Das C (2002) Regulation of trophoblast invasion by IL-1beta and TGF-beta1. Am J Reprod Immunol 48:210–219
Kaufmann P, Black S, Huppertz B (2003) Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 69:1–7
Kawabata K et al (1999) A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc Natl Acad Sci USA 96:5663–5667
Knofler M, Pollheimer J (2012) IFPA award in placentology lecture: molecular regulation of human trophoblast invasion. Placenta 33(Suppl):S55-62
Koch C, Engele J (2020) Functions of the CXCL12 receptor ACKR3/CXCR7-what has been perceived and what has been overlooked. Mol Pharmacol 98:577–585
Li H et al (2015) Chemokine CCL24 promotes the growth and invasiveness of trophoblasts through ERK1/2 and PI3K signaling pathways in human early pregnancy. Reproduction 150:417–427
Lim KH et al (1997) Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am J Pathol 151:1809–1818
Liu X, Dai LI, Zhou R (2015) Association between preeclampsia and the CXC chemokine family (Review). Exp Ther Med 9:1572–1576
Loetscher M et al (1994) Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem 269:232–237
Makrigiannakis A, Minas V, Kalantaridou SN, Nikas G, Chrousos GP (2006) Hormonal and cytokine regulation of early implantation. Trends Endocrinol Metab 17:178–185
McKinnon T, Chakraborty C, Gleeson LM, Chidiac P, Lala PK (2001) Stimulation of human extravillous trophoblast migration by IGF-II is mediated by IGF type 2 receptor involving inhibitory G protein(s) and phosphorylation of MAPK. J Clin Endocrinol Metab 86:3665–3674
Naiyer AJ et al (1999) Stromal derived factor-1-induced chemokinesis of cord blood CD34(+) cells (long-term culture-initiating cells) through endothelial cells is mediated by E-selectin. Blood 94:4011–4019
Norwitz ER (2006) Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod Biomed Online 13:591–599
Oreshkova T, Dimitrov R, Mourdjeva M (2012) A cross-talk of decidual stromal cells, trophoblast, and immune cells: a prerequisite for the success of pregnancy. Am J Reprod Immunol 68:366–373
Paiva P et al (2011) Human chorionic gonadotrophin regulates FGF2 and other cytokines produced by human endometrial epithelial cells, providing a mechanism for enhancing endometrial receptivity. Hum Reprod 26:1153–1162
Qiu Q, Yang M, Tsang BK, Gruslin A (2004) Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol Hum Reprod 10:677–684
Schanz A et al (2011) Pre-eclampsia is associated with elevated CXCL12 levels in placental syncytiotrophoblasts and maternal blood. Eur J Obstet Gynecol Reprod Biol 157:32–37
Shah DM (2001) Perinatal implications of maternal hypertension. Semin Pediatr Neurol 8:108–119
Singh M et al (2011) Matrix metalloproteinases and their inhibitors and inducer in gestational trophoblastic diseases and normal placenta. Gynecol Oncol 122:178–182
Sozzani S et al (1997) Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol 159:1993–2000
Staun-Ram E, Goldman S, Gabarin D, Shalev E (2004) Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod Biol Endocrinol 2:59
Timeva T, Shterev A, Kyurkchiev S (2014) Recurrent implantation failure: the role of the endometrium. J Reprod Infertil 15:173–183
Wallace AE et al (2013) Trophoblast-induced changes in C-x-C motif chemokine 10 expression contribute to vascular smooth muscle cell dedifferentiation during spiral artery remodeling. Arterioscler Thromb Vasc Biol 33:e93–e101
Wu X et al (2005) Human first-trimester trophoblast cells recruit CD56brightCD16- NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol 175:61–68
Zhu JY, Pang ZJ, Yu YH (2012) Regulation of trophoblast invasion: the role of matrix metalloproteinases. Rev Obstet Gynecol 5:e137–e143
Zlotnik A, Yoshie O (2000) Chemokines: a new classification system and their role in immunity. Immunity 12:121–127
Zlotnik A, Yoshie O (2012) The chemokine superfamily revisited. Immunity 36:705–716
Acknowledgements
This research was supported by Regional Innovation Strategy (RIS) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2022RIS-005) and by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1I1A3060225).
Author information
Authors and Affiliations
Contributions
YB performed the experiments and analyzed the data. JK analyzed and reviewed the data. WJ performed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Yeongju Bae, Jiho Jang, Han-Soo Kim, Wooyoung Jeong declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bae, Y., Jang, J., Kim, HS. et al. Blockade of stromal cell-derived factor-1 signaling disturbs the invasiveness of human extravillous trophoblast cells. Mol. Cell. Toxicol. 20, 307–314 (2024). https://doi.org/10.1007/s13273-023-00344-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-023-00344-3