Abstract
Background
Endocrine-disrupting compounds (EDCs) disrupt homeostasis via the dysregulation of hormone synthesis and metabolism. Bisphenol A (BPA) is widely used in consumer products, such as thermal receipts, water bottles, and baby bottles. However, BPA is also an EDC that acts as an estrogen agonist, and human exposure to BPA can lead to estrogenic effects. Consequently, manufacturers have started investigating the properties and effects of alternatives to BPA, molecules such as bisphenol F (BPF) and bisphenol S (BPS).
Objective
Although multiple studies have demonstrated the adverse effect of bisphenols, it remains unknown whether bisphenols affect human lung fibroblast cells. In this study, we investigated the cytotoxic effects of BPA, BPF, and BPS on the MRC5 human lung fibroblast cell line.
Results
We examined and compared the effects of BPA and its alternatives on cell proliferation, cell cycle progression, and apoptosis.
Conclusion
Brief exposures to low concentrations of BPA, BPF, and BPS had no effects on cell viability, cell cycle progression, or apoptosis among MRC5 human lung fibroblast cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endocrine-disrupting compounds (EDCs) are environmental agents that inhibit hormone synthesis, secretion, transport, and metabolism, resulting in disrupted endocrine homeostasis (Diamanti-Kandarakis et al. 2009). EDCs can be found in various materials, including pesticides, cosmetics, metals, personal care products, and food additives or contaminants (Caserta et al. 2011). Continuous human exposure to EDCs, such as phenols, pesticides, paraben, and phthalates, causes adverse biological effects. For example, paraben and phenols increase the incidence of breast cancer, obesity, and altered reproductive functions (Darbre 2017; Darbre and Harvey 2008). Moreover, EDCs have been demonstrated to cause lower sperm counts in men (Crews and McLachlan 2006). Human exposure to EDCs occurs through the consumption of tainted foods, contaminated water and soil, and breathing polluted air (Muncke 2011).
Among the various EDCs, bisphenol A (BPA) is a carbon-based chemical with a structure characterized by two hydroxyphenyl groups, typical of diphenylmethane derivatives and bisphenols. BPA is well known as an EDC and is widely used in consumer products, such as epoxy resins, baby bottles, water bottles, and thermal receipts (Im and Loffler 2016; Peretz et al. 2014). Human BPA exposure generally occurs via oral consumption (Le et al. 2008; Vandenberg et al. 2007). Moreover, high temperatures cause the release of higher concentrations of BPA into beverages, foods, and the environment (Sakurai and Mori 2000). Recently, many studies have revealed BPA as a xenoestrogen or an estrogen agonist. BPA interacts with estrogen receptors in the same manner as estradiol, leading to estrogenic effects (Dairkee et al. 2008). For this reason, the European Union restricted or prohibited the use of BPA in many daily-use consumer products, such as baby bottles and foods. This has prompted several global manufacturers to seek alternatives to BPA with two hydroxyphenyl groups, including bisphenol B (BPB), bisphenol F (BPF), and bisphenol S (BPS) (Ullah et al. 2019). However, recent studies have demonstrated that BPA alternatives can be ingested or inhaled by humans and still cause adverse effects on the reproductive system (Ullah et al. 2019).
Bisphenols promote cell proliferation, invasion, and migration, leading to cancer development and progression. For example, BPA treatment affects transforming growth factor beta and the PI3K signaling pathway, which is associated with ovarian cancer progression (Ptak and Gregoraszczuk 2012). BPS induces cell migration in the epithelial–mesenchymal transition of breast cancer (Deng et al. 2018). Additionally, BPF activates GPER1-mediated signaling, which causes DNA damage and cell cycle inhibition in breast cancer cells (Lei et al. 2018). Although several studies have demonstrated bisphenols to have adverse and cancer-causing effects, it remains unknown whether bisphenols cause physiological changes in human lung fibroblast cells.
In this study, we investigated the cytotoxic effects of BPA, BPF, and BPS on the MRC5 human lung fibroblast cell line. We examined and compared the effects of BPA and its alternatives on cell proliferation, cell cycle progression, and apoptosis.
Material and methods
Chemicals and reagents
BPA (cat. no. 47889), BPF (cat. no. 51453), and BPS (cat. no. 43034) were purchased from Sigma-Aldrich (Oakville, Ontario, Canada). A 1 M stock solution of BPA, BPF and BPS were prepared and diluted in 100% dimethyl sulfoxide (DMSO) or ethanol (EtOH). We used 20, 40, 80, and 100 μM of BPA, BPF and BPS in DMEM containing 10% fetal bovine serum (FBS). MTS assay kit (Cell Titer 96® AQueous One Solution cell proliferation assay kit) was purchased from Promega (cat. no. G3581, Madison, USA), and the LIVE/DEAD kit (cat. no. L3224, CA, USA) was purchased from Invitrogen. Apoptosis Detection kit (Cat. No. 556547, CA, USA) was purchased from BD Pharmingen™. The Ki-67 antibody (Cat. No. ab15580), cyclins B1 (Cat. No. ab32053, CA, USA) and cyclin D1 (Cat. No. 2978S, Beverly, MA, USA) were obtained from Abcam, Santa Cruz Biotechnology, Cell Signaling, respectively. The secondary goat anti-rabbit antibody was purchased from Jackson ImmunoResearch (Cat. No. 111-095-144, West Grove, PA, USA).
Cell culture
All reagents for cell culture were obtained from Welgene (Seoul, South Korea). The human lung fibroblast cell line, MRC5, was purchased from the Korean Cell Line Bank (KCLB) (Seoul, South Korea). MRC5 cells were grown in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in an incubator in an atmosphere of 5% CO2. At first, 0.1 mg/ml of poly-D lysine was placed on coverslips for 6 h at 23 °C, then MRC5 cells were seeded in 100 mm culture dishes at a density of 2.5 × 106 cells/dish. After 24 h, various concentrations of 20, 40, 80, and 100 μM BPA, BPF, and BPS was treated for 24 h and 48 h. The cells on coverslips were used for the live/dead cell assay. The cells in the 10-cm culture dishes were washed with Dulbecco’s phosphate-buffered saline (DPBS) without calcium and magnesium and processed for further experimental analyses. For each assay, 0.1% DMSO or 0.6% EtOH was used as the negative control.
MTS assay
MRC5 cells were seeded at a density of 2 × 104 cells/well in 48-well plates and were subsequently treated with 20, 40, 80, and 100 μM BPA, BPF, and BPS in 100 μl DMEM supplemented with 10% FBS for 24 or 48 h. The BeWo cells were treated with 10% MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) and incubated for 1 h at 37 °C. Cell viability was determined by measuring the absorbance at 490 nm using a Multiskan GO microplate reader (Waltham, MA).
Morphological change and live/dead cell assay
MRC5 cells were seeded on coated coverslips and treated with 100 μM BPA, BPF, and BPS at different concentrations for 24 and 48 h. The morphological changes were observed using an inverted microscope. For live/dead cell assay, MRC5 cells were double-stained with 3 μM ethidium homodimer-1 (EthD-1) and 0.3 μM calcein-AM mixture (Life Technologies, CA, USA) for 30 min in the dark condition; the morphology of the unwashed cells was observed using Nikon Eclipse TE300 inverted fluorescence microscope (Nikon Corp., Tokyo, Japan).
Cell cycle analysis
MRC5 cells were collected and were fixed with 70% ethanol for 1 h at 4 °C. Cell pellets were resuspended in 1X PBS containing 0.25 μg/μl RNase A and incubated for 1 h at 37 °C. The cells were treated with 10 μg/ml PI and incubated for 15 min in the dark at 23 °C. PI-stained cells were added 300 μl 1X PBS and subsequently subjected to analysis using a BD Accuri™ C6 Plus flow cytometer (BD FACS, CA, USA). A minimum of 10,000 cells were considered per sample and the results are represented as histograms for analyzing cell distribution in the different phases of the cell cycle. The cell cycle profile was analyzed using BD Accuri™ C6 Plus software.
Annexin V-FITC/propidium iodide (PI) apoptosis assay
An FITC Annexin-V Apoptosis Detection Kit I was used for determining cellular apoptosis according to the manufacturer’s instructions. Briefly, cells were collected and washed with 1X PBS and resuspended in 1X Annexin-V binding buffer [140 mM NaCl, 2.5 mM CaCl2, and 10 mM HEPES/NaOH (pH 7.4)]. Next, they were stained with 5 μl PI and/or 5 μl Annexin-V Alexa Fluor 488 and incubated for 15 min in the dark and were washed with 1X Annexin-V binding buffer. At least 10,000 cells were considered per sample and apoptosis was measured using the BD Accuri™ C6 Plus flow cytometer (BD FACS, San Jose, CA).
Immunostaining for fluorescence-activated cell sorting (FACS) analysis
BPA, BPF, and BPS-treated MRC5 cells were fixed in 1% paraformaldehyde for 5 h on a rotator at 4 °C and centrifuged at 3000 rpm for 3 min. Each sample was resuspended with solution A (75 mM sodium acetate, 0.1% saponin, 0.1% BSA, and 25 mM HEPES, pH 7.2) and was incubated in the diluted indicated antibodies against cyclin B1 (1: 200), cyclin D1 (1:200), and Ki-67 (1:400) for 1 h at 23 °C. After washing with 1X PBS, cells were incubated with FITC-labeled goat anti-rabbit secondary antibody (1:200) for 30 min in the dark at 23 °C. The expression of the target proteins was measured using a BD Accuri™ C6 Plus flow cytometer (BD FACS, CA, USA). A minimum of 10,000 cells were considered per sample and the results are represented as histograms for analyzing cell distribution in the different phases of the cell cycle. The cell cycle profile was analyzed using BD Accuri™ C6 Plus software.
Statistical analyses
Data are presented as the mean ± standard error of the mean (SEM); experiments were performed in triplicate. Data were analyzed using two-way ANOVA followed by Tukey’s multiple comparison test using GraphPad Prism5 software (California, USA). Differences between the groups were considered to be significant at p < 0.05.
Results
Bisphenols did not affect the viability of MRC5 human lung fibroblast cells
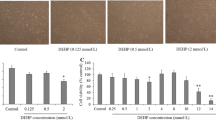
We first investigated the viability of MRC5 cells in association with exposure to BPA, BPF, and BPS at various concentrations (Fig. 1). MRC5 cells were treated with 20–100 μM of BPA and its alternatives, and the MTS assay was performed after treatment for 24 and 48 h. BPA had no effect on human lung fibroblast cell viability (Fig. 1a). Similarly to BPA, BPF and BPS also did not affect the proliferation of MRC5 cells (Fig. 1b and c). Based on the MTS results, the MRC5 cells were treated with 100 μM of bisphenol for subsequent experiments. Next, we examined the morphological changes in the MRC5 cells using phase-contrast microscopy (Fig. 2a). Consistent with the MTS assay findings, treatment with BPA and its alternatives did not induce morphological changes. We further examined the MRC5 cell viability in association with the administration of BPA, BPF, and BPS using the live/dead assay (Fig. 2b). Unlike with the untreated group, dead cells were not observed among bisphenol-treated MRC5 cells (Fig. 2b), suggesting that, below 100 μM, BPA had no cytotoxic effects on MRC5 human lung fibroblast cells.
Effect of bisphenols on the viability of human lung fibroblast MRC5 cells. MRC5 cell viability was quantified using the MTS assay. Cells were treated with 20–100 μM of BPA (a), BPF (b), and BPS (c) for 24 or 48 h. Data are presented as the percentage of the values obtained for the solvent-treated cells used as the control. DMSO was used as BPA solvent control, and EtOH was used as BPF and BPS solvent control
Effect of bisphenols on the morphology and viability of MRC5 cells. a The morphology of MRC5 cells was observed by phase-contrast microscopy after treatment with 100 μM of BPA, BPF, and BPS for 24 or 48 h. b MRC5 cells were stained with calcein-AM (representing live cells, green) and ethidium homodimer (representing dead cells, red) in the live/dead assay. DMSO or EtOH were used as negative controls. Images are representative of three independent experiments. Scale bars represent 200 μm
Bisphenols did not alter the proliferation of MRC5 cells
Ki-67 is a well-known marker of cell proliferation, and it can be used to determine the proportion of dividing cells in a population of cells (Sun and Kaufman 2018). To investigate the cytotoxicity of MRC5 cells, we measured Ki-67 protein expression using flow cytometry in association with the administration of BPA and its alternatives for 24 or 48 h. Relative to the control group, the BPA and BPS groups did not exhibit any significant change in the number of Ki-67-positive cells following 24 or 48 h of exposure, respectively. BPF treatment was associated with slightly decreased Ki-67 expression levels after 24 h treatment, but this difference ceased to exist after longer exposure (Fig. 3).
Effect of bisphenol treatment on MRC5 cell proliferation. a The 100 μM of BPA-, BPF-, and BPS-treated MRC5 cells were immunostained with anti-Ki-67 antibodies. The cells were quantified by flow cytometry. b The percentages of Ki-67-positive cells are represented as the mean ± SEM of three independent experiments (n = 6)
Effect of bisphenols on cell cycle progression
Recent studies have shown that BPA increases cell cycle arrest in various cell types (Can et al. 2005; Chen et al. 2002). To demonstrate whether BPA, BPF, and BPS affected cell cycle progression, human lung fibroblast MRC5 cells were treated with each for 24 or 48 h. Relative to the control group (0.4%), the proportion of cells in the sub-G1 phase slightly increased in association with BPA (2.6%), BPF (3.4%), and BPS (5.2%) treatment, but this difference was not significant (Fig. 4). Moreover, the number of cells in the G0/G1 and G2/M phases did not change after bisphenol treatment of MRC5 cells. These results suggested that BPA and its alternatives did not affect cell cycle progression.
Cell cycle progression among MRC5 cells upon bisphenol treatment. a MRC5 cells were treated with 100 μM of BPA, BPF, and BPS for 24 or 48 h. The cell cycle was quantified by flow cytometry with PI staining. b The percentages of cells in the sub-G1, G0/G1, S, and G2/M-phases are represented as the mean ± SEM of three independent experiments (n = 6)
The expression of cyclin B1 and cyclin D1 in bisphenol-treated MRC5 cells
To further determine the molecular mechanisms of the cell cycle, the expression of cell cycle markers, cyclin B1 and cyclin D1, was analyzed by flow cytometry. MRC5 cells were treated with BPA and its alternatives for 24 or 48 h. The cells were immunostained with anti-cyclin B1 and cyclin D1, which are well-known markers of the G0/G1 and G2/M phase, respectively, then quantified using FACS. Consistent with the cell cycle analysis, cyclin B1 and cyclin D1 levels did not change upon BPA and BPS treatment (Figs. 5 and 6). Although the expression of cyclin B1 and cyclin D1 was slightly increased in MRC5 cells treated with 100 μM BPF for 24 h, this change was not statistically significant (Figs. 5 and 6). These results indicate that BPA and its alternatives were not involved in cell cycle regulation.
Cyclin B1 expression in bisphenol-treated MRC5 cells. a Cells were treated with BPA, BPF, and BPS for 24 or 48 h. Cells were fixed with 1% PFA and stained with anti-cyclin B1 antibody. Expression levels of cyclin B1 were analyzed by flow cytometry. b The percentages of cyclin B1-positive cells are represented as the mean ± SEM of three independent experiments (n = 6). DMSO or EtOH was used as the negative control
Cyclin D1 expression in bisphenol-treated MRC5 cells. a Cells were treated with BPA, BPF, and BPS for 24 or 48 h. Cells were fixed with 1% PFA and stained with anti-cyclin D1 antibody. Expression levels of cyclin D1 were analyzed by flow cytometry. b The percentages of cyclin D1-positive cells are represented as the mean ± SEM of three independent experiments (n = 6). DMSO or EtOH was used as the negative control
Effect of bisphenols on apoptotic cell death in human lung fibroblast cells
Cellular growth is modulated by two key events: cell cycle progression and apoptosis (Vermeulen et al. 2003). Since we observed that cell cycle progression did not change upon bisphenols treatment (Fig. 4), we next investigated whether BPA and its alternatives could induce apoptosis among MRC5 cells. We performed an apoptosis assay using Annexin V/propidium iodide (PI) staining, and then we analyzed the apoptotic population by flow cytometry. BPA, BPF, and BPS treatment did not affect early apoptotic cells (double-positive for Annexin V and PI) or late apoptotic cells (Annexin V-positive and PI-negative) (Fig. 7). These results showed that, among MRC5 cells, brief exposures to low concentrations of BPA, BPF, and BPS had no effect on apoptosis.
Effect of bisphenol treatment on apoptosis among MRC5 cells. a Cells were treated with 100 μM of BPA, BPF, and BPS for 24 h or 48 h and double-stained with Annexin V-FITC and PI. The proportion of apoptotic cells was assessed by flow cytometry. The scatter plots represent PI (y-axis) and Annexin V-FITC (x-axis). b The percentages of cells in the live, early apoptotic, late apoptotic, and necrotic stages are expressed as the mean ± SEM of three independent experiments (n = 6)
Discussion
In this study, we investigated the adverse effects of BPA, BPF, and BPS on human lung fibroblast MRC5 cells using cell viability assays. Although BPA has cytotoxic effects on various cancer cells, it is unclear whether BPA and its alternatives have cytotoxic effects on lung fibroblast cells. Surprisingly, we observed that treatment with BPA, BPF, and BPS in MRC5 cells did not affect cell proliferation, cell cycle progression, or apoptosis. We used 20–100 μM of BPA and its alternatives, and we observed no significant differences between the bisphenol groups and the control. These results indicated that low concentrations of BPA, BPF, and BPS had no adverse effects on human lung fibroblast cells.
BPA is widely used in products, such as infant bottles, plastic storage containers, food, and beverages (Aljadeff et al. 2018). BPA has been shown to have estrogenic and androgenic properties, and it is associated with human reproductive diseases (Dairkee et al. 2008). Consequently, some countries have restricted the use of BPA in food and beverage packages. Due to regulations on BPA usage, manufacturers have tried to develop interchangeable BPA alternatives, such as the structurally similar bisphenols, BPB, BPF, BPS (Ullah et al. 2019). Although BPF and BPS are commonly used in thermal paper receipts, canned food, and electroplating solvents, recent studies have shown that these BPA substitutes still have adverse effects on the endocrine system (Liao et al. 2012b; Liao and Kannan 2013, 2014).
It has been shown that the atmosphere has been exposed to over 100 tons of bisphenols (Vandenberg et al. 2012). BPA, BPF, and BPS are detectable at concentrations of about 1.33, 0.054, and 0.34 μg/g, respectively, in indoor house dust (Liao et al. 2012a). Even though lung exposure to BPA and its alternatives occurs through inhalation, the effects of BPA on the lungs remain poorly understood. BPA has been shown to induce rapid upregulation of ERK1/2 via the estrogenic receptor GPER, resulting in the activation and migration of A549 lung cancer cells (Zhang et al. 2014). Moreover, BPS also can stimulate the migration of non-small-cell lung cancer cells through the activation of the TGF-β/Smad-2/3 pathway (Song et al. 2019). However, there is little evidence regarding the adverse effects of bisphenols in lung fibroblast or lung cancer cells, compared to the numerous studies linking bisphenols to various other cancer types. Therefore, we demonstrated the mode of action of BPA, BPF, and BPS in human lung fibroblast MRC5 cells. Based on our MTS results, we decided to use 100 mM of each of the bisphenols, and this had no effect on cell proliferation. We found that low concentrations of BPA, BPF, and BPS had no significant effects on MRC5 cell viability, cell cycle progression, or apoptosis.
Conclusion
To our knowledge, this was the first study to suggest possible effects of BPA, BPF, and BPS on the human lung fibroblast cells and demonstrate that BPA and its alternatives have no cytotoxic effects on human lung fibroblast cells. Even though we did not observe any changes in MRC5 cell viability in association with the administration of BPA and BPA alternatives, further experiments are warranted, including transcriptome profiling by mRNA-seq. This will allow for the prediction of adverse bisphenol effects via the regulation of gene sets.
References
Aljadeff G, Longhi E, Shoenfeld Y (2018) Bisphenol A: a notorious player in the mosaic of autoimmunity. Autoimmunity 51:370–377. https://doi.org/10.1080/08916934.2018.1551374
Can A, Semiz O, Cinar O (2005) Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol Hum Reprod 11:389–396. https://doi.org/10.1093/molehr/gah179
Caserta D, Mantovani A, Marci R, Fazi A, Ciardo F, La Rocca C et al (2011) Environment and women’s reproductive health. Hum Reprod Update 17:418–433. https://doi.org/10.1093/humupd/dmq061
Chen MY, Ike M, Fujita M (2002) Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol 17:80–86. https://doi.org/10.1002/tox.10035
Crews D, McLachlan JA (2006) Epigenetics, evolution, endocrine disruption, health, and disease. Endocrinology 147(6 Suppl):S4-10. https://doi.org/10.1210/en.2005-1122
Dairkee SH, Seok J, Champion S, Sayeed A, Mindrinos M, Xiao W et al (2008) Bisphenol A induces a profile of tumor aggressiveness in high-risk cells from breast cancer patients. Cancer Res 68:2076–2080. https://doi.org/10.1158/0008-5472.CAN-07-6526
Darbre PD (2017) Endocrine disruptors and obesity. Curr Obes Rep 6:18–27. https://doi.org/10.1007/s13679-017-0240-4
Darbre PD, Harvey PW (2008) Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol 28:561–578. https://doi.org/10.1002/jat.1358
Deng Q, Jiang G, Wu Y, Li J, Liang W, Chen L et al (2018) GPER/Hippo-YAP signal is involved in bisphenol S induced migration of triple negative breast cancer (TNBC) cells. J Hazard Mater 355:1–9. https://doi.org/10.1016/j.jhazmat.2018.05.013
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM et al (2009) Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30:293–342. https://doi.org/10.1210/er.2009-0002
Im J, Loffler FE (2016) Fate of bisphenol A in terrestrial and aquatic environments. Environ Sci Technol 50:8403–8416. https://doi.org/10.1021/acs.est.6b00877
Le HH, Carlson EM, Chua JP, Belcher SM (2008) Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett 176:149–156. https://doi.org/10.1016/j.toxlet.2007.11.001
Lei B, Huang Y, Liu Y, Xu J, Sun S, Zhang X et al (2018) Low-concentration BPF induced cell biological responses by the ERalpha and GPER1-mediated signaling pathways in MCF-7 breast cancer cells. Ecotoxicol Environ Saf 165:144–152. https://doi.org/10.1016/j.ecoenv.2018.08.102
Liao C, Kannan K (2013) Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem 61:4655–4662. https://doi.org/10.1021/jf400445n
Liao C, Kannan K (2014) A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch Environ Contam Toxicol 67:50–59. https://doi.org/10.1007/s00244-014-0016-8
Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q et al (2012a) Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol 46:9138–9145. https://doi.org/10.1021/es302004w
Liao C, Liu F, Kannan K (2012b) Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol 46:6515–6522. https://doi.org/10.1021/es300876n
Muncke J (2011) Endocrine disrupting chemicals and other substances of concern in food contact materials: an updated review of exposure, effect and risk assessment. J Steroid Biochem Mol Biol 127:118–127. https://doi.org/10.1016/j.jsbmb.2010.10.004
Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R et al (2014) Bisphenol a and reproductive health: update of experimental and human evidence, 2007–2013. Environ Health Perspect 122:775–786. https://doi.org/10.1289/ehp.1307728
Ptak A, Gregoraszczuk EL (2012) Bisphenol A induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/Stat, MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol Lett 210:332–337. https://doi.org/10.1016/j.toxlet.2012.02.003
Sakurai K, Mori C (2000) Fetal exposure to endocrine disruptors. Nihon Rinsho 58:2508–2513
Song P, Fan K, Tian X, Wen J (2019) Bisphenol S (BPS) triggers the migration of human non-small cell lung cancer cells via upregulation of TGF-beta. Toxicol In Vitro 54:224–231. https://doi.org/10.1016/j.tiv.2018.10.005
Sun X, Kaufman PD (2018) Ki-67: more than a proliferation marker. Chromosoma 127:175–186. https://doi.org/10.1007/s00412-018-0659-8
Ullah A, Pirzada M, Jahan S, Ullah H, Razak S, Rauf N et al (2019) Prenatal BPA and its analogs BPB, BPF, and BPS exposure and reproductive axis function in the male offspring of Sprague Dawley rats. Hum Exp Toxicol 38:1344–1465. https://doi.org/10.1177/0960327119862335
Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV (2007) Human exposure to bisphenol A (BPA). Reprod Toxicol 24:139–177. https://doi.org/10.1016/j.reprotox.2007.07.010
Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G (2012) Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet 17:407–434. https://doi.org/10.1590/s1413-81232012000200015
Vermeulen K, Van Bockstaele DR, Berneman ZN (2003) The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36:131–149. https://doi.org/10.1046/j.1365-2184.2003.00266.x
Zhang KS, Chen HQ, Chen YS, Qiu KF, Zheng XB, Li GC et al (2014) Bisphenol A stimulates human lung cancer cell migration via upregulation of matrix metalloproteinases by GPER/EGFR/ERK1/2 signal pathway. Biomed Pharmacother 68:1037–1043. https://doi.org/10.1016/j.biopha.2014.09.003
Acknowledgements
This work supported by the Korea Environment Industry & Technology Institute (KEITI) through “The Environmental Health Action Program” funded by Korea Ministry of Environment (MOE), Grant & Award Number: 2017001360007. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (NRF-2018R1D1A1B07050755). This research was also supported by the Chung-Ang University Graduate Research Scholarship in 2019.
Author information
Authors and Affiliations
Contributions
Overall conceptualization, J-YK and J-WK; methodology and investigation, J-YK, MJK, C-HK, M-JA, G-SS, H-ML, and J-WK; data analysis, J-YK and J-WK; statistical analysis, J-YK, MJK, and C-HK writing-original draft, J-YK and J-WK; writing-review and editing, J-YK and J-WK; funding acquisition, J-WK; supervision and project administration, J-WK.
Corresponding author
Ethics declarations
Conflict of interest
Ji-Young Kim, Mi Jin Kim, Mi-Jin An, Geun-Seup Shin, Hyun-Min Lee, Chul-Hong Kim, and Jung-Woong Kim declare that they have no conflicts of interest.
Human and animal rights
The article does not contain any studies with human and animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, JY., Shin, GS., Kim, CH. et al. The cytotoxic effects of bisphenol A alternatives in human lung fibroblast MRC5 cells. Mol. Cell. Toxicol. 17, 267–276 (2021). https://doi.org/10.1007/s13273-021-00133-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-021-00133-w