Abstract
Background
Chronic renal failure (CRF) is the result of kidney damage. Puerarin is a flavonoid with specific nephroprotective effect, but its effect on CRF needs further research. This study explored the effect of puerarin on CRF and the potential molecular mechanism.
Methods
Adenine was used to establish an in vivo CRF model in rats, and rats were intragastrically administered with puerarin at a dose of 400 mg/kg body weight once a day from day 1 to day 28. Hematoxylin and eosin (HE) and Masson staining were used to observe the morphology and fibrosis of kidney tissue. Lipopolysaccharide (LPS) (400 ng/mL)/H2O2 (200 µM) was applied to human kidney 2 (HK-2) cells to construct an in vitro CRF model. Enzyme-linked immunosorbent assay (ELISA) was performed to validate interleukin (IL)-1β and IL-18 levels. Quantitative reverse transcription polymerase chain reaction (RT-qPCR) was performed to detect microRNA (miR)-342-3p levels. Transforming growth factor beta (TGF-β)1, SMAD2, SMAD3, and pyroptosis marker proteins were detected by Western blot. The interaction between miR-342-3p and TGF-β/SMAD was determined by a dual-luciferase reporter gene assay. Cell Counting Kit-8 (CCK-8) assay was utilized to determine cell viability.
Results
In the CRF model, puerarin alleviated renal injury and fibrosis and reduced creatinine (Cr) and blood urea nitrogen (BUN) levels. At the same time, miR-342-3p was downregulated, while the TGF-β/SMAD axis was activated and levels of IL-1β and IL-18 were increased. After treatment of CRF rats with puerarin, the expression level of miR-342-3p was increased, the TGF-β/SMAD axis was inhibited, and the secretion of IL-1β and IL-18 was decreased. MiR-342-3p directly bound to and negatively regulated the expression of TGF-β1, SMAD2, and SMAD3. In the in vitro CRF model, miR-342-3p inhibited HK-2 cell pyroptosis by inhibiting the TGF-β/SMAD axis.
Conclusion
Puerarin reduced renal injury and pyroptosis in CRF rats by targeting the miR-342-3p/TGF-β/SMAD axis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is characterized by high prevalence, poor prognosis, and high medical costs and is a significant danger to human health (Liu et al. 2015). Chronic renal failure (CRF), the advanced stage of CKD, is a clinical syndrome with many complications that seriously affect the quality of life and longevity of patients (Ali et al. 2020). Therefore, a better understanding of how we can delay the progression of CRF, reduce the harm of complications and occurrence of uremia, and improve the quality of life of patients has important academic value and social significance.
Pyroptosis is a type of programmed cell death that is characterized by proinflammatory properties. It can be divided into a classical pyroptosis pathway that depends on caspase-1 and a nonclassical pathway dependent on caspase-dependent protease-4/5/11 (caspase-4/5/11) (Guo et al. 2019). Studies have shown that pyroptosis is widely involved in the occurrence and development of infectious diseases (Man et al. 2017), nervous system-related diseases (Hu et al. 2022), atherosclerotic diseases (Xu et al. 2018), and kidney diseases (Wang et al. 2022). Current research shows that pyroptosis plays an important role in ischemia-reperfusion renal injury (Zhao et al. 2018), hypoxia-reoxygenation renal injury (Liu et al. 2022), diabetic nephropathy (Al Mamun et al. 2021), lupus nephritis (Cao et al. 2021), and other renal diseases by promoting inflammatory response (Zhang et al. 2022)and regulating renal fibrosis (Qu et al. 2022). Therefore, exploring the mechanism of pyroptosis of renal tubular epithelial cells in CRF is important in the treatment of CRF.
Puerarin is an active ingredient extracted from Pueraria lobata, a plant used in traditional Chinese medicine. Puerarin has antioxidant (Bacanlı et al. 2017), anti-inflammatory (Zeng et al. 2022), antifibrosis (Wang et al. 2021), antitumor (Zhu et al. 2021; , Lang et al. 2022), and vasodilatory properties (Jiang et al. 2022). It has been shown to protect kidneys in rats with diabetic nephropathy through antioxidant and antiapoptotic effects (Shukla et al. 2018). Puerarin has also been reported to alleviate diabetic kidney damage by inhibiting the expression of NADPH oxidase 4 (NOX4) in podocytes (Li et al. 2017). In addition, puerarin alleviated renal fibrosis by reducing oxidative stress-induced epithelial cell apoptosis through the mitogen-activated protein kinase (MAPK) signaling pathwayin vitro and in vivo(Zhong et al. 2014). Therefore, we speculated that puerarin might have the effect of relieving CRF.
The transforming growth factor beta (TGF-β)/SMAD pathway is one of the most important pathways for the inflammatory response and fibrosis that commonly lead to renal function loss and CRF. Activation of the TGF-β/SMAD pathway has been found to promote pyroptosis and be involved in organ fibrosis and loss of function (Yang et al. 2018), suggesting that the TGF-β/SMAD pathway is involved in CRF. MicroRNAs(miRNAs) are a class of endogenous small non-coding Rnas (about 19 to 25 nucleotides) that negatively regulate gene expression at the post-transcriptional level mainly by binding to complementary target sequences in target mRNA (Xiong et al. 2018). Recent studies have shown that some miRNAs are highly expressed in the kidney and are closely related to kidney development and kidney disease (Assmann et al. 2018), suggesting that miRNAs are important regulators in renal physiology and pathology. Studies have shown that in diabetic retinopathy, miR-342 has been reported to be able to inhibit primary human periretinal cell pyroptosis (Yu et al. 2021). Further, A recent study (Shu et al. 2022)showed that microRNA (miR)-342 participated in the process of liver fibrosis by regulating the TGF-β/SMAD pathway. Additionally, miR-342 has been shown to be involved in impaired renal function, and it has been reported to inhibit renal interstitial fibrosis in diabetic nephropathy by targeting SRY-box transcription factor 6 (SOX6) (Shu et al. 2022). At the same time, it has also been reported that puerarin could have an anticancer effect by promoting miR-342 (Huang et al. 2020). Therefore, we speculated that puerarin might alleviate the pyroptosis of renal tubular epithelial cells caused by CRF by regulating the TGF-β/SMAD axis via miR-342.
In this study, we verified through in vivo and in vitro experiments that puerarin alleviated CRF-induced pyroptosis of renal tubular epithelial cells by regulating the miR-342-3p/TGF-β/SMAD axis, providing a potential therapeutic pathway for the treatment of CRF. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-23-64/rc.).
Materials and methods
Establishment of in vivo CRF model
A total of 42 healthy male Sprague-Dawley rats weighing around 300 g and aged 7–8 weeks old were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. The rats were housed in an environment with a controlled temperature of 24–26 ℃ and a cold white light source alternating with darkness (12 hours/12 hours). Rats had free access to food and water throughout the study. Standard granular rat chow containing 0.63% phosphorus, 0.74% calcium, 0.53% potassium, and 0.22% sodium was gradually supplemented with adenine to induce CRF as per Kashioulis’ method (the concentration of adenine was 0.5% for the first 3 weeks, 0.3% for the next 2 weeks, and then 0.15% until the animals were sacrificed), with a CRF modeling period of 10 weeks (Nguy et al. 2012). After successful CRF modeling, the CRF rats were randomly divided into 2 groups: the CRF model group (model group, n = 14) and puerarin treatment group (puerarin group, n = 14). We refer to the experimental method of liu et al., the rats in the puerarin group were administered with puerarin (Meilunbio, Dalian, China) by gavage at a dose of 400 mg/kg once a day. The duration of drug treatment lasted 4 weeks (Liu et al. 2019). In addition, normal rats serving as a control group (control group, n = 14) had free access to standard granular rat chow. After the experiment, the rats were fasted for 4 hours, and blood was collected from the tail vein to measure the levels of blood urea nitrogen (BUN) and creatinine (Cr). The rats were then sacrificed under anesthesia with 3% pentobarbital sodium (30 mg/kg), and kidney tissue was collected for subsequent experiments. Animal experiments were performed under a project license (No. kmmu20221002) granted by ethics board of the First Affiliated Hospital of Kunming Medical University, in compliance with The First Affiliated Hospital of Kunming Medical University guidelines for the care and use of animals. A protocol was prepared before the study without registration.
Hematoxylin and eosin (HE) staining
The formalin-fixed kidney tissues were embedded in paraffin and sectioned (5 µm thick). Subsequently, the tissues sections were deparaffinized with xylene, and 100%, 95%, 80%, and 70% alcohol was used for hydration. The tissues were then stained with HE for 5 minutes and 2 minutes, respectively. Sections were then dehydrated in 90% and 100% ethanol for several minutes, respectively, and then placed in xylene. Finally, the sections were mounted with neutral resin and the histomorphological changes of renal tissue were observed under an optical microscope.
Determination of serum BUN and Cr
Blood was obtained from the tail vein of rats, and serum was collected after centrifugation. Conventional biochemical analysis (biochemical analyzer) was used to determine serum BUN and Cr. The urease method was used for the determination of BUN, and the protein removal method was used for the determination of Cr.
Masson staining
Kidney tissues were fixed with 4% paraformaldehyde, ethanol was used for multistage dehydration, and the tissues were subsequently embedded in paraffin, sectioned, and then deparaffinized. The dewaxed sections were sequentially processed through hematoxylin solution, Masson’s lichun red acid staining solution, 2% glacial acetic acid solution, 1% phosphomolybdic acid solution, aniline blue staining solution, and 0.2% glacial acetic acid solution. Finally, alcohol was used for dehydration, and the slides were mounted after being cleared with xylene.
Cell culture and transfection
Human kidney 2 (HK-2) cells were purchased from The American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Roswell Park Memorial Institute (RPMI)-1640 containing 10% fetal bovine serum (FBS, Gibco, Waltham, MA, USA) in an incubator (Gibco) with 5% CO2 at 37 ℃. MiR-342-3p mimics, miR-342-3p inhibitor, negative control (miR-NC), and negative control inhibitor (inhibitor-NC) were purchased from RiboBio (Guangzhou, China). Cell transfection was performed according to the instructions for Lipofectamine 2000 (Invitrogen, Waltham, MA, USA).
Establishment of in vitro CRF model
As previously reported, HK-2 cells were cultured under normal conditions and treated with lipopolysaccharide (LPS) (400 ng/mL)/H2O2(200 µM) for 12 hours to construct an in vitro model of CRF (Sun et al. 2020). For the puerarin treatment group, We followed the dosage of puerarin used by Wang et al.,puerarin (Meilunbio) at a concentration of 100 µM was used to treat cells for 24 hours (Wang et al. 2019).
Dual-luciferase reporter gene assay
The interaction between TGF-β1, SMAD2/3, and miR-342-3p was confirmed by dual luciferase reporter assay. Briefly, the 3'-UTR sequences of wild-type (WT) TGF-β1 and SMAD2/3 (with a binding site for miR-342-3p) were inserted into the pmirGLO vector (Promega, Madison, WI, USA) to form WT luciferase reporter genes for TGF-β1 and SMAD2/3, respectively. At the same time, after the binding site of miR-342-3p was mutated, a mutant (MUT) reporter gene of TGF-β1 and SMAD2/3 was constructed. HK-2 cells were cotransfected with miR-342-3p mimic or NC to construct a dual-luciferase system. Finally, luciferase activity from cell lysates was measured using a luminometer (Promega GloMax 20/20 Luminometer, Madison, WI, USA).
Cell counting Kit-8 (CCK-8) assay
The cells were inoculated into a 96-well plate, CCK-8 reagent was added to each well 2 hours before the end of the culture, and cells continued to be cultured in an incubator for 2 hours at 37 ℃. The absorbance (A) at 450 nm of each well was measured with a microplate reader (BioTek, Winooski, VT, USA). Three replicate wells were set in each group to take the average value, and a single well was set up with only medium added as a blank control. Cell viability (%) = (A detection time point-A0h)/(A0h − A blank control) ×100%.
Enzyme-linked immunosorbent assay (ELISA)
The level of interleukin (IL)-1β and IL-18 in the homogenate supernatant of kidney tissue was determined by ELISA. About 100 mg of kidney tissues were homogenized in 1 mL of 0.05 M precooled phosphate buffered saline (pH 7.4) and centrifuged at 12,000 r/min at 4 ℃. After 10 minutes, the supernatant was collected, and the assay was carried out according to the instructions of the ELISA kit (Abcam, Cambridge, MA, USA).
Next, the level of IL-1β and IL-18 in HK-2 cell culture medium was determined. Differently treated cells were grown into 12-well plates at a density of 2×105. The instructions of the ELISA kit (R&D Systems, Minneapolis, MN, USA) were strictly followed to measure IL-1β and IL-18 levels, and the optical density (OD) value at 450 nm was measured by an ELISA reader (Spectramax 250, Molecular Devices, Sunnyvale, CA, USA).
Quantitative reverse transcription polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent (Invitrogen). The complementary DNA (cDNA) of miR-342-3p was synthesized by All-in-One First-Strand cDNA Synthesis Kit (FulenGen Co., Ltd., Guangzhou, China). Next, RT-qPCR was performed using SYBR green (Applied Biosystems, Waltham, MA, USA). U6 was used as an internal control for miR-342-3p. The following were the primer sequences: miR-342-3p sense (5'-3'): GGGTCTCACACAGAAATCGC and antisense (5'-3'), CAGTGCGTGTCGTGGAGT; and U6 sense (5'-3'): CGCGCTTCGGCAGCACATATACT and antisense (5'-3'): ACGCTTCACGAATTTGCGTGTC.
Western blot
Radioimmunoprecipitation assay (RIPA) lysis buffer was used to extract total protein. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to a nitrocellulose membrane. The membrane were blocked with 5% skim milk at 25 ℃ for 1 hour, after which primary antibody was added, and it was then incubated overnight. After washing the membrane, horseradish peroxidase-labeled secondary antibody was added and incubated at 25 ℃ for 2 hours. The enhanced chemiluminescence (ECL) reagent was developed, the gel imaging system was exposed and photographed, and ImageJ software was used to analyze the gray value of protein bands. Primary antibodies was as follows: TGF-β1 (1:1,000, ab215715, Abcam); SMAD2/3 (1:1,000, ab202445, Abcam), pro-caspase-1 (1:1,000, ab32150, Abcam), caspase-1 (1:1,000, ab207802, Abcam), GAPDH (1:2,000, ab8245, Abcam), and β-actin (1:2,000, ab8226, Abcam).
Statistical analysis
Statistical analysis was performed by SPSS software (IBM, Armonk, NY, USA) and GraphPad Prism 7 (GraphPad Inc., San Diego, CA, USA), the Student's test was used to analyze the differences between the two groups and the results are expressed as mean ± standard deviation (SD). Statistical significance was P < 0.05.
Results
Puerarin protects renal function and alleviates fibrosis in CRF rats
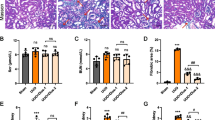
First, we investigated whether puerarin had a protective effect on renal injury in CRF rats. The results of histological analysis of the kidneys by HE staining showed that the rats in the control group showed normal renal tissue morphology without obvious inflammatory cells. However, in the model group, the renal tubular lumen was enlarged, the renal tubular epithelial cells were vacuolated, the interstitium was expanded, and a large number of inflammatory cells were infiltrated around the glomerulus. These negative changes were effectively mitigated in the puerarin treatment group (Fig. 1A). Meanwhile, compared with the control group, the levels of Cr and BUN in the model group were significantly increased, while compared with the model group, the levels of Cr and BUN in the puerarin group were significantly decreased (P < 0.01, P < 0.001, Fig. 1B, C). Masson staining of the kidney tissues revealed that the model group had focal fibrosis compared with the control group, and puerarin significantly inhibited the occurrence of renal lesions in CRF rats (Fig. 1D). The above results suggested that puerarin could effectively protect renal function and alleviate fibrosis in CRF rats.
Puerarin protects renal function and alleviates fibrosis in a rat model of CRF. A HE staining was used to observe the morphology of rat kidney tissue. The magnification is 200 times, scale bar = 100 µm. B, C Kidney function indicators BUN and Cr were detected by an automatic biochemical analyzer. D Masson staining was used to detect the expression of collagen in rat kidney tissue. The magnification is 200 times, scale bar = 100 µm. ***, P < 0.001 vs. Control group; ##, P < 0.01, ###, P < 0.001 vs. Model group. CRF, chronic renal failure; HE, hematoxylin and eosin; BUN, blood urea nitrogen; Cr, creatinine
Puerarin upregulates miR-342-3p, inhibits TGF-β/SMAD signaling activation, and alleviates pyroptosis in CRF rats
To further explore whether puerarin-induced protection of renal function was associated with the miR-342-3p/TGF-β/SMAD axis, we detected the expression of miR-342-3p in kidney tissues of CRF rats. The results showed that miR-342-3p was significantly decreased in the CRF group compared with the control group, while after treatment with puerarin, the level of miR-342-3p was markedly increased (P < 0.05, P < 0.01, P < 0.001, Fig. 2A). In addition, we found that the TGF-β/SMAD signaling-related proteins TGF-β1, SMAD2, and SMAD3 in the model group were much higher than that in the control group, while puerarin administration significantly reduced these increases (P < 0.05, P < 0.01, P < 0.001, Fig. 2B, C). At the same time, we explored the effect of puerarin on pyroptosis, with the results showing that puerarin essentially inhibited the expression of pyroptosis marker caspase-1 (P < 0.05, P < 0.01, P < 0.001, Fig. 2D, E) and inflammatory factors, including IL-1β and IL-18 (P < 0.05, P < 0.01, P < 0.001, Fig. 2F) in CRF rats. Taken together, these results indicated that puerarin increased miR-342-3p expression, inhibited TGF/SMAD signaling, and alleviated pyroptosis in CRF rats.
Puerarin promotes the expression of miR-342-3p and inhibits the activation of the TGF-β/SMAD signaling pathway. A RT-qPCR was used to detect miR-342-3p levels. B, C Western blot was performed to detect TGF-β/SMAD pathway-related proteins. D, E Western blot was used to detect the levels of pro-caspase-1 and caspase-1. F ELISA was used to detect the levels of IL-1β and IL-18 in kidney tissue homogenates. *, P < 0.05, **, P < 0.01, ***, P < 0.001 vs. Control group; #, P < 0.05, ##, P < 0.01, ###, P < 0.001 vs. Model group. TGF, transforming growth factor; RT-qPCR, quantitative reverse transcription polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay; IL, interleukin
MiR-342-3p targets inhibition of the TGF-β/SMAD signaling pathway in HK2 cells
To explore the potential relationship between miR-342-3p and the TGF-β/SMAD axis, we predicted potential binding sites for miR-342-3p using TargetScan (http://www.targetscan.org). the results showed that miR-342-3p fully matched the 3'-UTR sequences of TGF-β1(Fig. 3A), Meanwhile, a dual-luciferase reporter assay further confirmed that TGF-β1 was the direct target genes of miR-342-3p (P < 0.001, Fig. 3B). Moreover, SMAD2 was also shown to be a target of miR-342-3p (P < 0.001,Fig. 3C, D). Not surprisingly, SMAD3 was also found to be regulated by miR-342-3p as a target of miR-342-3p (P < 0.001,Fig. 3E, F). Finally, we transfected miR-342-3p mimic into HK-2 cells. The successful transfection of HK-2 cells with miR-342-3p mimic was verified by RT-qPCR (P < 0.001, Fig. 3G), and protein levels of TGF-β1 and SMAD2/3 were significantly reduced in HK-2 cells transfected with miR-342-3p mimic (P < 0.05, P < 0.01, P < 0.001, Fig. 3H, I). Taken together, these results suggested that TGF-β1 and SMAD2/3 were direct targets of miR-342-3p.
Targets of miR-342-3p, TGF-β1, and SMAD2/3. A, C, E Starbase tool showing schematic representation of putative binding sites for miR-342-3p in 3'-UTRs of TGF-β1, SMAD2, and SMAD3. B, D, F The dual-luciferase reporter assay was used to verify the targeting relationship between miR-342-3p and TGF-β1, SMAD2, and SMAD3 respectively. ***, P < 0.001 vs. NC (Wt-Smad2/3) group, ###, vs. Mimic(Mut-Smad 2/3) group. G RT-qPCR was used to verify the transfection efficiency of miR-342-3p. P < 0.05 vs. NC group. H, I The protein level of TGF-β1, SMAD2, and SMAD3 was detected by Western blot., P < 0.05; **, P < 0.01; ***, P < 0.001 vs. NC group. Wt, wild-type; Mut, mutant; TGF, transforming growth factor; RT-qPCR, quantitative reverse transcription polymerase chain reaction; NC, negative control
Puerarin alleviates pyroptosis in CRF model cells in vitro by inducing miR-342-3p
Finally, we explored the effect of miR-342-3p on pyroptosis in an in vitro model of CRF. We first incubated cells with different concentrations of puerarin to find the appropriate concentration for treating the cells before the establishment of the in vitro CRF model. The results showed that higher concentrations (150 and 200 µM) of puerarin markedly reduced cell viability compared with the control, while the lower concentration (0–100 µM) of puerarin had no significant effect on cell viability. Thus, a concentration of 100 µM puerarin was selected for the subsequent experiments to treat renal tubular epithelial cells (P < 0.05, Fig. 4A). We also verified the transfection efficiency of HK-2 cells transfected with NC inhibitor and mR-342-3p inhibitor and found miR-342-3p inhibitor significantly inhibited the expression of miR-342-3p compared with the control group (P < 0.01, Fig. 4B). Next, we treated HK-2 cells with LPS (400 ng/mL)/H2O2 (200 µM) for 12 hours to construct a CRF model in vitro. Cell viability in the model group was much lower than that in the control group, and puerarin (100 µM) could ameliorate the decreasing trend to a certain extent, while mR-342-3p inhibitor blocked the rescue effect of puerarin (P < 0.01, P < 0.01, Fig. 4C). The TGF-β/SMAD signaling pathway was activated in the model group, and puerarin inhibited the TGF-β/SMAD pathway induced by CRF mode. In addition, mR-342-3p inhibitor reduced the blocking effect of puerarin on the TGF-β/SMAD pathway (P < 0.05, P < 0.01, P < 0.001, Fig. 4D, E). Puerarin also blocked the level of pyroptosis and inflammation in the model group, and consistent with the previous results, mR-342-3p inhibitor alleviated the blocking effect of puerarin on pyroptosis and inflammation (P < 0.05, P < 0.01, P < 0.001, Fig. 4F−H). Taken together, the results indicated that puerarin alleviated the pyroptosis of CRF model cells in vitro by inducing miR-342-3p.
Puerarin alleviates pyroptosis in CRF model cells in vitro by inducing miR-342-3p. A CCK-8 assay was used to detect the viability of HK-2 cells treated with different concentrations of puerarin. B RT-qPCR was used to detect the transfection effect of miR-342-3p. C The viability of HK-2 cells in different treatment groups was detected by CCK-8 assay. D, E Western blot was performed to detect of the level of TGF-β1, SMAD2, and SMAD3. F, G The protein levels of pro-caspase-1 and caspase-1 were detected by Western blot. H ELISA was used to detect the levels of IL-1β and IL-18 in the culture medium. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. Control group. #, P < 0.05; ##, P < 0.01; ###, P < 0.001 vs. Model group. ^, P < 0.05; ^^, P < 0.01 vs. Puerarin group. CRF, chronic renal failure; CCK-8, Cell Counting Kit-8; HK-2, human kidney 2; RT-qPCR, quantitative reverse transcription polymerase chain reaction; TGF, transforming growth factor; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; NC, negative control
Discussion
CRF is a clinical syndrome in the late stage of a variety of CKDs (Su et al. 2018). Due to advancements in dialysis and kidney transplantation technology, the efficacy of CRF treatment has improved significantly. However, the high cost of treatment is unmanageable for most patients and their families. Therefore, the search for effective non-dialysis methods and drugs is a current focus of research. Traditional Chinese medicine has been shown to have a significant effect on relieving symptoms, protecting residual renal function, and delaying the disease (Ye et al. 2018), and thus it is expected to be effective in the treatment of CRF. Puerarin is an isoflavone compound extracted fromPuerariaand used in traditional Chinese medicine. In recent years, puerarin has been widely used in the clinical treatment of cardiovascular diseases, cerebrovascular diseases (Qin et al. 2022), and diabetic nephropathy (Zhu et al. 2022). Puerarin has also been reported to play an important role in the treatment of kidney-related diseases (Keskin Alkaç et al. 2022). In this study, we found that puerarin could effectively alleviate the renal injury and fibrosis caused by CRF in rats. Our results were consistent with previous studies (Zhu et al. 2022). In addition, in CRF rats, due to renal excretion dysfunction and increased protein decompositionin vivo, serum BUN and Cr levels were significantly increased, causing the body’s own toxic symptoms. In the puerarin treatment group, the renal tissue damage caused by CRF was effectively reduced and the content of BUN and Cr inhibited. The mechanism may have been related to the vasodilator effect of puerarin, which can improve hemorheology, increase renal tissue perfusion, improve hypoxia, increase Na+/-K+-ATPase activity, decrease intracellular Na content, and reduce Na+/-Ca+exchange protein, reducing the intracellular calcium overload and cell edema (Zhao et al. 2005). Pyroptosis is a novel proinflammatory programmed cell death mediated by Caspase-1-dependent, accompanied by the release of a large number of proinflammatory factors and inducing a cascade of amplified inflammatory responses(Shi et al. 2015)At the same tiome, Puerarin ameliorates acute lung injury by modulating NLRP3 inflammasome-induced pyroptosis (Cai et al. 2022). Our study also showed that puerarine treatment effectively reduced the levels of inflammatory cytokines (IL-1β and IL-18) and pyrogenic markers (pro-caspase-1 and caspase-1) in renal tissue homogenate.. The results of the above studies suggested that puerarin may have alleviated CRF in rats through its vasodilator effect and pyroptosis alleviationmechanism.
Renal interstitial fibrosis is an asymptotic pathological process that occurs after kidney injury caused by various etiologies and is an important part of CRF (Peng et al. 2022). Active prevention and treatment of renal fibrosis is of great significance to the early treatment and prevention of CRF. TGF-β1 is a key factor in renal fibrosis, inducing hypertrophy of glomerular and tubular cells through autocrine and paracrine pathways and promoting extracellular matrix (ECM) accumulation (Peng et al. 2022). SMAD protein, the only TGF-β receptor intracellular kinase substrate discovered in recent years, mediates the intracellular conduction process of TGF-β1, and the TGF-β/SMAD signaling pathway plays an important role in the occurrence and development of renal fibrosis (Zou et al. 2022). Our results showed that TGF-β1, SMAD2, and SMAD3 were upregulated in the kidney tissue of CRF rats, indicating that the TGF-β/SMAD signaling pathway was activated, while puerarin could downregulate TGF-β1, SMAD2 and SMAD3, indicating that the activation of TGF/SMAD signaling was inhibited. The above results suggested that puerarin may have inhibited pyroptosis by blocking the activation of TGF-β/SMAD signaling.
MiRNA is a non-coding RNA involved in the occurrence of various diseases (Jin et al. 2018), and there is growing evidence that miRNAs are key regulators of renal pathophysiology. Studies have shown that miRNAs were associated with nephrotic syndrome (Li et al. 2022), immunoglobulin A (IgA) nephropathy (Szeto et al. 2022), diabetic nephropathy (Asgari et al. 2022), lupus nephritis (Liao et al. 2022), and other kidney injuries. In addition, miRNAs can play a role in kidney disease by regulating pyroptosis. For example, miRNA-92a-3p has been recognized as a vital controller of pyroptosis in renal ischemia-reperfusion damage (Wang et al. 2020). Previous studies have shown that the miR-223-3p/NLRP3 pathway was involved in LPS-induced intense kidney damage and inhibited HK-2 cell pyroptosis (Tan et al. 2020), and that the discharge of miRNA-135b-5p might have hindered LPS-induced pyroptosis and inflammation in HK-2 cells (Huang and Xu 2021). Our experimental results found that miR-342-3p inhibitor could interfere with the pyroptosis level of HK-2 cells, which was consistent with previous findings. In addition, this study revealed the relationship between miRNA-342-3p and pyroptosis for the first time.
In addition, some studies based on gene knockout technology have confirmed that tumor cells can disrupt the miRNA homeostasis mechanism (Balatti and Croce 2022), thereby hindering the antitumor effect of TGF-β, indicating that miRNAs could regulate the TGF-β/SMAD signaling pathway (Hu et al. 2021). It has been reported that miR-342-3p was abnormally expressed in diabetic nephropathy (Clegg et al. 1987), and we found through prediction software that miR-342-3p has binding sites for TGF-β1, SMAD2, and SMAD3. MiR-342-3p negatively regulated the expression of TGF-β1, SMAD2, and SMAD3, and therefore, miR-342-3p inhibited the activation of the TGF-β1/SMAD signaling pathway by targeting the 3'-UTR of TGF-β1, SMAD2, and SMAD3, while puerarin treatment could significantly promote the expression of miR-342-3p. The above results showed that puerarin inhibited the occurrence of pyroptosis through the miR-342-3p/TGF-β/SMAD axis and ultimately alleviated CRF.
Conclusions
In conclusion, puerarin played an anti-CRF role by inhibiting cell pyroptosis. Pyroptosis was regulated by miR-342-3p targeting 3'-UTR of TGF-β1, SMAD2, and SMAD3 and then inhibiting the activation of the TGF-β/SMAD signaling pathway. This study provided preliminary evidence of a new mechanism for CRF treatment based on puerarin. In future studies, the dose-response relationship between puerarin and CRF needs to be further explored.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Al Mamun A, Ara Mimi A, Wu Y, Zaeem M, Abdul Aziz M, Aktar Suchi S, Alyafeai E, Munir F, Xiao J (2021) Pyroptosis in diabetic nephropathy. Clin Chim Acta 523:131–143. https://doi.org/10.1016/j.cca.2021.09.003
Ali I, Chinnadurai R, Ibrahim S, Green D, Kalra P (2020) Predictive factors of rapid linear renal progression and mortality in patients with chronic kidney disease. BMC Nephrol 21:345. https://doi.org/10.1186/s12882-020-01982-8
Asgari M, Salehi I, Ranjbar K, Khosravi M, Zarrinkalam E (2022) Interval training and Crataegus persica ameliorate diabetic nephropathy via miR-126/Nrf-2 mediated inhibition of stress oxidative in rats with diabetes after myocardial ischemia-reperfusion injury. Biomed pharmacotherapy = Biomedecine pharmacotherapie 153:113411. https://doi.org/10.1016/j.biopha.2022.113411
Assmann T, Recamonde-Mendoza M, de Souza B, Bauer A, Crispim D (2018) MicroRNAs and diabetic kidney disease: Systematic review and bioinformatic analysis. Mol Cell Endocrinol 477:90–102. https://doi.org/10.1016/j.mce.2018.06.005
Bacanlı M, Başaran A, Başaran N (2017) The antioxidant, cytotoxic, and antigenotoxic effects of galangin, puerarin, and ursolic acid in mammalian cells. Drug Chem Toxicol 40:256–262. https://doi.org/10.1080/01480545.2016.1209680
Balatti V, Croce C (2022) Small Non-Coding RNAs in Leukemia. Cancers. https://doi.org/10.3390/cancers14030509
Cai D, Zhao Y, Yu F (2022) Puerarin ameliorates acute lung injury by modulating NLRP3 inflammasome-induced pyroptosis. Cell death discovery 8:368. https://doi.org/10.1038/s41420-022-01137-8
Cao H, Liang J, Liu J, He Y, Ke Y, Sun Y, Jiang S, Lin J (2021) Novel Effects of Combination Therapy Through Inhibition of Caspase-1/Gasdermin D Induced-Pyroptosis in Lupus Nephritis. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.720877
Clegg M, Ferrell F, Keen C (1987) Hypertension-induced alterations in copper and zinc metabolism in Dahl rats. Hypertens (Dallas Tex : 1979) 9:624–628. https://doi.org/10.1161/01.hyp.9.6.624
Guo H, Xie M, Zhou C, Zheng M (2019) The relevance of pyroptosis in the pathogenesis of liver diseases. Life Sci 223:69–73. https://doi.org/10.1016/j.lfs.2019.02.060
Hu Y, Lou X, Liu B, Sun L, Wan S, Wu L, Zhao X, Zhou Q, Sun M, Tao K et al (2021) E2F3TGF-β1-regulated miR-3691-3p targets and to inhibit prostate cancer progression. Asian J Androl 23:188–196. https://doi.org/10.4103/aja.aja_60_20
Hu Y, Wang B, Li S, Yang S (2022) Pyroptosis, and its Role in Central Nervous System Disease. J Mol Biol 434:167379. https://doi.org/10.1016/j.jmb.2021.167379
Huang S, Jin S, Xu B, Wang R (2020) Puerarin alleviates the progression of non-small cell lung cancer by regulating the miR-342/CCND1 axis. Neoplasma 67:1244–1255. https://doi.org/10.4149/neo_2020_191107N1145
Huang J, Xu C (2021) LncRNA MALAT1-deficiency restrains lipopolysaccharide (LPS)-induced pyroptotic cell death and inflammation in HK-2 cells by releasing microRNA-135b-5p. Ren Fail 43:1288–1297. https://doi.org/10.1080/0886022x.2021.1974037
Jiang Z, Cui X, Qu P, Shang C, Xiang M, Wang J (2022) Roles and mechanisms of puerarin on cardiovascular disease: A review. Biomed pharmacotherapy = Biomedecine pharmacotherapie 147:112655. https://doi.org/10.1016/j.biopha.2022.112655
Jin X, Cai L, Wang C, Deng X, Yi S, Lei Z, Xiao Q, Xu H, Luo H, Sun J (2018) CASC2/miR-24/miR-221 modulates the TRAIL resistance of hepatocellular carcinoma cell through caspase-8/caspase-3. Cell Death Dis. https://doi.org/10.1038/s41419-018-0350-2
Keskin Alkaç Z, Ahmet Korkak F, Dağoğlu G, Akdeniz İncili C, Dağoğlu Hark B, Tanyıldızı S (2022) Puerarin mitigates oxidative injuries, opening of mitochondrial permeability transition pores and pathological damage associated with liver and kidney in Xanthium strumarium-intoxicated rats. Toxicon: official journal of the International Society on Toxinology 213:13–22. https://doi.org/10.1016/j.toxicon.2022.04.004
Lang J, Guo Z, Xing S, Sun J, Qiu B, Shu Y, Wang Z, Liu G (2022) Inhibitory role of puerarin on the A549 lung cancer cell line. Translational cancer research 11:4117–4125. https://doi.org/10.21037/tcr-22-2246
Li X, Cai W, Lee K, Liu B, Deng Y, Chen Y, Zhang X, He J, Zhong Y (2017) Puerarin attenuates diabetic kidney injury through the suppression of NOX4 expression in podocytes. Sci Rep 7:14603. https://doi.org/10.1038/s41598-017-14906-8
Li Q, Yin M, Zhang Z, Yu Y, Liu F (2022) Study on the Mechanism of circRNA Regulating the miRNA Level in Nephrotic Syndrome. Evidence-based complementary and alternative medicine: eCAM. https://doi.org/10.1155/2022/3729995
Liao W, He X, Zhang W, Chen Y, Yang J, Xiang W, Ding Y (2022) MiR-145 participates in the development of lupus nephritis by targeting CSF1 to regulate the JAK/STAT signaling pathway. Cytokine 154:155877. https://doi.org/10.1016/j.cyto.2022.155877
Liu W, Gan Y, Ding Y, Zhang L, Jiao X, Liu L, Cao H, Gu Y, Yan L, Wang Y et al (2022) Autophagy promotes GSDME-mediated pyroptosis via intrinsic and extrinsic apoptotic pathways in cobalt chloride-induced hypoxia reoxygenation-acute kidney injury. Ecotoxicol Environ Saf 242. https://doi.org/10.1016/j.ecoenv.2022.113881
Liu F, Gao X, Inglese G, Chuengsaman P, Pecoits-Filho R, Yu A (2015) A Global Overview of the Impact of Peritoneal Dialysis First or Favored Policies: An Opinion. Perit dialysis international: J Int Soc Perit Dialysis 35:406–420. https://doi.org/10.3747/pdi.2013.00204
Liu H, Zhang X, Zhong X, Li Z, Cai S, Yang P, Ou C, Chen M (2019) Puerarin inhibits vascular calcification of uremic rats. Eur J Pharmacol 855:235–243. https://doi.org/10.1016/j.ejphar.2019.05.023
Man S, Karki R, Kanneganti T (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277:61–75. https://doi.org/10.1111/imr.12534
Nguy L, Nilsson H, Lundgren J, Johansson ME, Teerlink T, Scheffer PG, Guron G (2012) Vascular function in rats with adenine-induced chronic renal failure. Am J Physiol Regul Integr Comp Physiol 302:R1426–1435. https://doi.org/10.1152/ajpregu.00696.2011
Peng Z, Guo H, Li Y, Li J, Yang X, Liu J, Hu Q, Wang H, Wang L (2022) The Smad3-dependent microRNA let-7i-5p promoted renal fibrosis in mice with unilateral ureteral obstruction. Front Physiol. https://doi.org/10.3389/fphys.2022.937878
Qin W, Guo J, Gou W, Wu S, Guo N, Zhao Y, Hou W (2022) Molecular mechanisms of isoflavone puerarin against cardiovascular diseases: What we know and where we go. Chin Herb Med 14:234–243. https://doi.org/10.1016/j.chmed.2021.12.003
Qu X, Zhai B, Liu Y, Chen Y, Xie Z, Wang Q, Wu Y, Liu Z, Chen J, Mei S et al (2022) Pyrroloquinoline quinone ameliorates renal fibrosis in diabetic nephropathy by inhibiting the pyroptosis pathway in C57BL/6 mice and human kidney 2 cells. Biomed pharmacotherapy = Biomedecine pharmacotherapie. https://doi.org/10.1016/j.biopha.2022.112998
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. https://doi.org/10.1038/nature15514
Shu B, Zhang R, Zhou Y, He C, Yang X (2022) METTL3-mediated macrophage exosomal NEAT1 contributes to hepatic fibrosis progression through Sp1/TGF-β1/Smad signaling pathway. Cell death discovery 8:266. https://doi.org/10.1038/s41420-022-01036-y
Shukla R, Banerjee S, Tripathi Y (2018) Antioxidant and Antiapoptotic effect of aqueous extract of Pueraria tuberosa (Roxb. Ex Willd.) DC. On streptozotocin-induced diabetic nephropathy in rats. BMC Complement Altern Med 18:156. https://doi.org/10.1186/s12906-018-2221-x
Su L, Huang G, Yin S, Hua X, Tang X (2018) A clinical analysis of vitrectomy for severe vitreoretinopathy in patients with chronic renal. BMC Ophthalmol. https://doi.org/10.1186/s12886-018-0704-7
Sun L, Xu H, Wang Y, Ma X, Xu Y, Sun F (2020) The mitochondrial-targeted peptide SBT-20 ameliorates inflammation and oxidative stress in chronic renal failure. Aging 12:18238–18250. https://doi.org/10.18632/aging.103681
Szeto C, Ng J, Fung W, Chan G, Luk C, Lai K, Wang G, Chow K, Mac-Moune Lai F (2022) Urinary mi-106a for the diagnosis of IgA nephropathy: Liquid biopsy for kidney disease. Clin Chim Acta 530:81–86. https://doi.org/10.1016/j.cca.2022.03.006
Tan J, Fan J, He J, Zhao L, Tang H (2020) Knockdown of LncRNA DLX6-AS1 inhibits HK-2 cell pyroptosis via regulating miR-223-3p/NLRP3 pathway in lipopolysaccharide-induced acute kidney injury. J Bioenerg Biomembr 52:367–376. https://doi.org/10.1007/s10863-020-09845-5
Wang J, Ge S, Wang Y, Liu Y, Qiu L, Li J, Huang X, Sun L (2021) Puerarin Alleviates UUO-Induced Inflammation and Fibrosis by Regulating the NF-κB P65/STAT3 and TGFβ1/Smads Signaling Pathways. Drug Des Devel Ther 15:3697–3708. https://doi.org/10.2147/dddt.s321879
Wang Y, Li Y, Xu Y (2022) Pyroptosis in Kidney Disease. J Mol Biol 434:167290. https://doi.org/10.1016/j.jmb.2021.167290
Wang B, Ma W, Yang H (2019) Puerarin attenuates hypoxia-resulted damages in neural stem cells by up-regulating microRNA-214. Artif cells Nanomed Biotechnol 47:2746–2753. https://doi.org/10.1080/21691401.2019.1628040
Wang R, Zhao H, Zhang Y, Zhu H, Su Q, Qi H, Deng J, Xiao C (2020) Identification of MicroRNA-92a-3p as an Essential Regulator of Tubular Epithelial Cell Pyroptosis by Targeting Nrf1 via HO-1. Front Genet. https://doi.org/10.3389/fgene.2020.616947
Xiong H, Yan T, Zhang W, Shi F, Jiang X, Wang X, Li S, Chen Y, Chen C, Zhu Y (2018) miR-613 inhibits cell migration and invasion by downregulating Daam1 in triple-negative breast cancer. Cell Signal 44:33–42. https://doi.org/10.1016/j.cellsig.2018.01.013
Xu Y, Zheng L, Hu Y, Wang Q (2018) Pyroptosis and its relationship to atherosclerosis. Clin Chim Acta 476:28–37. https://doi.org/10.1016/j.cca.2017.11.005
Yang F, Qin Y, Lv J, Wang Y, Che H, Chen X, Jiang Y, Li A, Sun X, Yue E et al (2018) Silencing long non-coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis. https://doi.org/10.1038/s41419-018-1029-4
Ye M, Hu K, Jin J, Wu D, Hu P, He Q (2018) The association between time-mean serum uric acid levels and the incidence of chronic kidney disease in the general population: a retrospective study. BMC Nephrol 19:190. https://doi.org/10.1186/s12882-018-0982-6
Yu X, Ma X, Lin W, Xu Q, Zhou H, Kuang H (2021) Long noncoding RNA MIAT regulates primary human retinal pericyte pyroptosis by modulating miR-342-3p targeting of CASP1 in diabetic retinopathy. Exp Eye Res 202:108300. https://doi.org/10.1016/j.exer.2020.108300
Zeng J, Zhao N, Yang J, Kuang W, Xia X, Chen X, Liu Z, Huang R (2022) Puerarin Induces Molecular Details of Ferroptosis-Associated Anti-Inflammatory on RAW264.7 Macrophages. Metabolites. https://doi.org/10.3390/metabo12070653
Zhang M, Zhi D, Lin J, Liu P, Wang Y, Duan M (2022) miR-181a-5p Inhibits Pyroptosis in Sepsis-Induced Acute Kidney Injury through Downregulation of NEK7. J Immunol Res. https://doi.org/10.1155/2022/1825490
Zhao Y, Du G, Cui H, Cao C, Wang X, Zhang C (2005) [Experimental study of protective effect of pueraria compound on the cerebral ischemic injury]. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica 30:548–551
Zhao H, Huang H, Alam A, Chen Q, Suen K, Cui J, Sun Q, Ologunde R, Zhang W, Lian Q et al (2018) VEGF mitigates histone-induced pyroptosis in the remote liver injury associated with renal allograft ischemia-reperfusion injury in rats. Am J transplantation: official J Am Soc Transplantation Am Soc Transpl Surg 18:1890–1903. https://doi.org/10.1111/ajt.14699
Zhong Y, Zhang X, Cai X, Wang K, Chen Y, Deng Y (2014) Puerarin attenuated early diabetic kidney injury through down-regulation of matrix metalloproteinase 9 in streptozotocin-induced diabetic rats. PLoS ONE 9:e85690. https://doi.org/10.1371/journal.pone.0085690
Zhu H, Xiao Y, Guo H, Guo Y, Huang Y, Shan Y, Bai Y, Lin X, Lu H (2021) The isoflavone puerarin exerts anti-tumor activity in pancreatic ductal adenocarcinoma by suppressing mTOR-mediated glucose metabolism. Aging 13:25089–25105. https://doi.org/10.18632/aging.203725
Zhu Q, Yang S, Wei C, Lu G, Lee K, He J, Liu R, Zhong Y (2022) Puerarin attenuates diabetic kidney injury through interaction with Guanidine nucleotide-binding protein Gi subunit alpha-1 (Gnai1) subunit. J Cell Mol Med 26:3816–3827. https://doi.org/10.1111/jcmm.17414
Zou J, Zhou X, Ma Y, Yu R (2022) Losartan ameliorates renal interstitial fibrosis through metabolic pathway and Smurfs-TGF-β/Smad. Biomed pharmacotherapy = Biomedecine pharmacotherapie 149:112931. https://doi.org/10.1016/j.biopha.2022.112931
Funding
This study was supported by Medical Discipline Leader Training Program of Health Commission of Yunnan Province in 2019 (Grant Number D-2019015); 2023 Yunnan Provincial Department of Science and Technology-Kunming Medical University Applied Basic Research Joint Special Fund General Project (Project number: 202301AY070001-154); Regional Project of National Natural Science Foundation of China (Grant Number 82160142) in 2022; Outstanding Youth Cultivation Project of Applied Basic Research Joint Special Fund of Yunnan Provincial Department of Science and Technology and Kunming Medical University in 2022 (Grant Number 202201AY070001-044).
Author information
Authors and Affiliations
Contributions
Contributions: (I) Conception and design: JY and WF; (II) Administrative support: WF; (III) Provision of study materials or patients: BL; (IV) Collection and assembly of data: JW; (V) Data analysis and interpretation: JY and BL; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Corresponding author
Ethics declarations
Conflict of interest
We all declare that we have no conflict of interest.
Ethical approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (No. kmmu20221002) granted by ethics board of the First Affiliated Hospital of Kunming Medical University, in compliance with The First Affiliated Hospital of Kunming Medical University guidelines for the care and use of animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Li, B., Wang, J. et al. Puerarin alleviates chronic renal failure-induced pyroptosis in renal tubular epithelial cells by targeting miR-342-3p/TGF-β/SMAD axis. Genes Genom 45, 1563–1573 (2023). https://doi.org/10.1007/s13258-023-01448-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-023-01448-9