Abstract
Background
The Korean Peninsula is a small but unique area showing great endemic Hynobius diversity with H. quelpaertensis, H. yangi, H. unisacculus and three species candidates (HC1, HC3 and HC4). H. quelpaertensis is distributed in the southern part and in Jeju Island, while the remaining species have extremely narrow distributions.
Objectives
To examine the genetic structure of H. quelpaertensis and the phylogenetic placement in Hynobius.
Methods
Three mitochondrial and six microsatellite loci were genotyped for 204 Hynobius quelpaertensis, three H. leechii, three H. yangi, three HC1, two H. unisacculus, three HC3, three HC4 and ten Japanses H. lichenatus.
Results
A high level of mitochondrial diversity was found in H. quelpaertensis. Our mitochondrial data showed evidence of a historical link between inland and Jeju Island despite the signature of founder effect likely experienced by the early island populations. However, our microsatellite analysis showed the fairly clear signature of isolation history between in- and island populations. Upon phylogenetic analysis, H. quelpaertensis, H. unisacculus and HC1 formed a cluster, whereas H. yangi belonged to a separate cluster. HC3 and HC4 were clustered with either H. quelpaertensis or H. yangi depending on the locus used.
Conclusion
Our results show at least partially the historical imprints engraved by dispersal of Korean endemic Hynobius during Pleistocene, potentially providing a fundamental basis in determining the conservation units and finding management strategies for these species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the development and commercialization of molecular genetics tools, many species round the world have been revealed to have a tremendous level of cryptic diversity, and to exist as species complexes (e.g. Fouquet et al. 2007; Oliver et al. 2009; Helms et al. 2015; Greenan et al. 2018). The taxonomic history of a salamander genus, Hynobius, on the Korean Peninsula is a representative model of the birth and growth of a species complex. Hynobius is the largest genus in Hynobiidae and consists of nearly 40 species (https://amphibiaweb.org/). Even a few decades ago, H. leechii, known to be widely distributed throughout East Asia, was thought to be the only Hynobius species on the Korean Peninsula. In the early 2000s, the populations in the southwest of the Korean Peninsula and Jeju Island, which have long been considered as subspecies of H. leechii, were regarded to be a completely different species and named H. quelpaertensis (Jeju salamander; Yang et al. 2001; Kim et al. 2003; Baek et al. 2011a). The H. leechii populations narrowly distributed on the southeastern part of the peninsula were also identified to be a distinct species and named H. yangi (Kori salamander; Yang et al. 2001; Kim et al. 2003; Baek et al. 2011a).
Several phylogenetic studies with Hynobius salamanders proved that H. quelpaertensis and H. yangi were species completely differentiated from H. leechii (Baek et al. 2011a, b; Min et al. 2016). According to these studies: (i) H. leechii and H. yangi are more closely related with each other and (ii) H. quelpaertensis is a phylogenetic sister to the cluster of H. leechii and H. yangi. The growth of H. leechii species complex is still ongoing, given that these studies identified three more new species candidates (i.e., HC1, HC2, HC3; Baek et al. 2011a, b). In an analysis using mitochondrial cytochrome b and 12S rRNA, these species candidates were closely related phylogenetically to H. quelpaertensis rather than the cluster of H. leechii and H. yangi (Baek et al. 2011a). However, in the analysis using cytochrome oxidase c subunit 1 HC3 revealed a stronger phylogenetic affinity to H. leechii and H. yangi (Baek et al. 2011b). HC2 was recently officially named H. unisacculus (Min et al. 2016). Though it has not yet become a formal report, our recent surveys suggested that a fourth new species candidate exists (HC4; personal data). H. unisacculus and the remaining three HCs are distributed in extremely small geographic ranges (personal data).
To date, genetic studies of Hynobius on the Korean Peninsula have primarily focused on revealing precise phylogenetic and micro-distributional differences among species. The fine-scale genetic structure has not yet been studied for the new species members identified since 2000 in H. leechii species complex. In particular, the detailed population structure of H. quelpaertensis, which occupies the widest area among the endemic Hynobius on the peninsula, must be studied to provide insight into the following two important historical and evolutionary aspects. First, the distribution of Korean Hynobius salamanders is the historical signature of southward dispersal caused by Quaternary climate fluctuation (Hewitt 2004; Hoarau et al. 2007; Provan and Bennett 2008). Although the Korean Peninsula has never been completely covered by glaciers, the impact of glaciers in the north likely maintained cold and dry climate in the middle of the peninsula for an extended period of time, eventually leading to the southern migration of many terrestrial animals (d’Alpoim Guedes et al. 2016). Therefore, the genetic and phylogenetic structure among the populations of H. quelpaertensis and other Korean Hynobius species can provide an important clue to reconstruct the isolation process among refugia provided in many areas on this peninsula. Second, the distribution of H. quelpaertensis appears as a continuous pattern from the southwestern part of the peninsula to Jeju Island, which may help precisely trace the biogeographic inland-to-island route. There have been several studies on the genetic differentiation of terrestrial vertebrate species between Jeju Island and the Korean Peninsula inland region (Jo et al. 2012; Choi et al. 2014; Lee et al. 2015), but no study has yet attempted to characterize the historical process shaping the contemporary distribution encompassing inland and island areas.
Here, mitochondrial and microsatellite loci were used to examine the fine-scale genetic structure of H. quelpaertensis populations and the evolutionary relationship with other Korean endemic species. First, mitochondrial haplotypes from all H. quelpaertensis populations were applied to examine the evolutionary relationship among the species or species candidates in the H. leechii species complex. Second, based on the intra- and interpopulation genetic diversity data obtained, insights were gleaned into the pattern of colonization and demographic changes that past populations have undergone. Finally, the biogeodispersal history was estimated to explain how this species was distributed to the southernmost islands of the peninsula, based on the pattern of the population structure. Although this species is not currently designated as a statutory protected species in South Korea, the data in this study can be crucial to help establish conservation strategies when/if the species is in serious ecological crisis perhaps. It should be considered that globally this is a quite rare species living only on the southern part of this tiny peninsula.
Materials and methods
Samples and DNA isolation
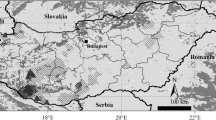
DNA samples used in this study were isolated from the tissue or whole-body (frozen or ethanol-immersed) specimens stored in the Conservation Genome Resource Bank for Korean Wildlife (CGRB). These specimens were the individuals collected or donated from 2001 to 2013. All experimental procedures were conducted under the permission by CGRB and in accordance with the guidelines by Seoul National University Institutional Animal Care and Use Committee (SNUIACUC). The individuals analyzed included 204 Hynobius quelpaertensis, three H. leechii, three H. yangi, three HC1, two H. unisacculus, three HC3, three HC4 and ten Japanses H. lichenatus (Fig. 1; Table 1). The sequence data of H. nebulosus, H. chinesis, H. formasanus and H. tokyoensis were retrieved from NCBI GenBank and used as outgroup species for the phylogenetic analysis (Table 1). Genomic DNA was isolated using DNeasy Blood and Tissue Kit (Qiagen, Dusseldorf, Germany) according to the manufacturer’s instructions.

http://widemaphst.cafe24.com/wmap/html/kbma4_down.html and https://rgm-79.tistory.com/703 (both were freely available for non-commercial purposes) (color figure online)
The 34 collection localities of Hynobius species used in this study. Populations comprise 24 Hynobius quelpaertensis populations (blue circle: inland; green circle: island), three H. leechii (red circle) populations, three H. yangi (pink circle) populations, three HC1 (blue triangle) populations, one H. unisacculus (HC2; red triangle) population, one HC3 (marron triangle) population, one HC4 (pink triangle) population and one H. lichenatus population (blue diamond). This picture was generated by modifying the shape files retrieved from
Mitochondrial sequencing
Three mitochondrial loci, cytochrome oxidase subunit 1 (COI), cytochrome b (cyt b) and d-loop, were amplified and sequenced for all 231 Hynobius species samples. Two previously reported primer sets were used for the amplification of COI and cyt b (Oh et al. 2007). d-loop was amplified using the primer set specifically designed for Hynobius species in the present study (Table S1). The PCR reaction mixture (30 μl) consisted of 10-50 ng genomic DNA, 0.2 mM dNTPs, 1.5 mM MgCl2, 1 μM of each primer, 1 unit of taq™ (Takara Bio, Shiga prefecture, Japan), and 1 × PCR buffer. Thermal cycling was composed of a denaturation at 94 °C for 2 min, 30 cycles of a denaturation at 94 °C for 45 s, an annealing at 53 (cyt b and d-loop) or 55 °C (COI) for 1 min and an extension at 72 °C for 1 min, and an extra extension at 72 °C for 7 min. PCR products were run on 1.5% agarose gels to examine the quality, purified using a Primeprep PCR Purification Kit (GenetBio, Daejeon, South Korea) and sequenced by Biomedic Inc (Bucheon, South Korea) on an ABI3730XL (Applied Biosystems, Foster City, CA, USA) with BigDye Terminator 3.1 Cycle Sequencing Kit (Applied Biosystems).
Microsatellite characterization and genotyping
A microsatellite DNA-enriched library was constructed based on a new method devised by modifying the previously known method (Hamilton et al. 1999). Genomic DNA of an individual in population GS was digested with MboI (Promega, Fitchburg, WI, USA) to generate fragments of 400–700 bp. These fragments were ligated to a specific double-stranded MboI adaptor (forward: 5′-GAT CGT CGA CGG TAC CGA ATT CT-3′; reverse: 5′-GTC AAG AAT TCG GTA CCG TCG AC-3′) using T4 DNA ligase (Promega). The fragments ligated with the adaptor were recovered by PCR using the sequences of forward and reverse adaptors as primers. The amplified products were hybridized with biotinylated (CT)12 and (GT)12 probes. Hybridized fragments were recovered using Streptavidin-coated magnetic beads (MagneSphere Series Paramagnetic Particles; Promega) and were amplified using single-stranded forward adaptor (annealing temp: 58 °C). The PCR products were inserted into the pGEM-T Easy Vector (Promega) and transformed into competent DH5α cells. A total of 150 positive clones were sequenced with T7 and SP6 universal primers at Biomedic Inc. Of these, 34 sequences were selected as microsatellite locus candidates that contained more than 15 motif repetitions and could generate amplified fragments of about 100–300 bp with primer sets designed using Primer3 software (Rozen and Skaletsky 2000). In order to be recognized as microsatellite loci, the PCR amplification efficiency should be perfect, and polymorphism should be shown in electrophoresis test using randomly selected eight individuals of H. quelpaertensis. Six sequences that fully met the condition were finally selected (Table S1 in supplementary material). Samples (204 H. quelpaertensis and ten Japanese H. lichenatus) were genotyped at these six novel loci. Each forward primer was fluorescently labeled with either FAM, HEX, NED or PET (Applied Biosystems). The fluorescently labeled PCR products were genotyped at Biomedic Inc using an ABI 3730 XL Genetic Analyzer. Alleles were scored using GeneMapper 3.7 (Applied Biosystems).
Mitochondrial data analysis
Sequence reads of three mitochondrial loci were examined with BLAST searches and aligned using ClustalW (Larkin et al. 2007) implemented in MEGA 6.06 (Tamura et al. 2013). All haplotypes revealed in this study were deposited in NCBI GenBank (MK591728–MK591819; MK593473–MK593536; MK604333–MK604450). Intrapopulation genetic diversity was quantified by estimating the number of haplotypes (h), haplotype diversity (Nei 1987) and nucleotide diversity (Nei 1987) in DnaSP 5.10 (Librado and Rozas 2009). Haplotype network was constructed for each mitochondrial locus, based on median-joining algorithm using PopART 1.7 (Leigh and Bryant 2015), to analyze the distributional pattern of haplotype diversity. In order to estimate the historical demographic changes of this species, three different methods, the neutrality tests based on Tajima’s D (Tajima 1989) and Fu’s Fs (Fu 1997) implemented in DnaSP, mismatch distribution analysis (Slatkin and Hudson 1991) implemented in Arlequin 3.5 and Bayesian skyline plot analysis implemented in BEAST 1.8.0 (Drummond et al. 2012). The maximum likelihood (ML) analysis was performed for each mitochondrial locus using IQ-Tree 1.3.10 with 10,000 ultrafast bootstrap replicates (Nguyen et al. 2015). The best-fit substitution model was chosen to be TPM3μ + F + I + G4 for COI tree, TPM2μ + F + I + G4 for cyt b tree and TPM2μ + F + R for d-loop based on Bayesian Information Criterion (BIC) using ModelFinder (Kalyaanamoorthy et al. 2017) implemented in IQ-Tree. The consensus tree was visualized and graphically edited using FigTree 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
Microsatellite data analysis
Intrapopulation genetic diversity of microsatellite loci was estimated by calculating the mean number of alleles per locus (A), allelic richness (AR), observed (HO) and expected (HE) heterozygosity and fixation index (FIS) using Arlequin and Fstat 2.9.3.2 (Goudet 2001). The deviation of observed genotype proportion from that expected under Hardy–Weinberg equilibrium (HWE) was tested for each locus-population pair based on the Fisher’s exact test following Markov chain parameters with 1000 batches and 10,000 iterations per batch (Guo and Thompson 1992), which was implemented in the Genepop 4.2 (Raymond and Rousset 1995). The existence of recent bottleneck was checked by testing the excess of expected heterozygosities under the mutation-drift equilibrium relative to those under HWE in Bottleneck 1.2.1 (Piry et al. 1999). The significance in bottleneck analysis was determined based on the Wilcoxon signed-rank test under two-phase model [TPM by combining 70% of stepwise mutation model (SMM) and 30% of infinite allele model (IAM)]. A mode-shift away (shifted mode) from the typical L-shaped distribution of allelic frequencies (Luikart et al. 1998) was examined for each population to determine the level of bottleneck impact in another way. Pairwise-FST and -RST values were calculated to estimate the level of interpopulation genetic divergence and the significances were determined through 1000 permutations in Arlequin. The existence of genetic cluster was examined based on Bayesian framework on Structure 2.3.4 (Pritchard et al. 2000). This analysis was performed under the admixture model for cluster (K) from 2 to 5, with ten independent runs, each consisting of 4 × 105 MCMC generations after burn-in of 105 MCMC generations. The most reliable number of genetic clusters was predicted using the delta K method (Evanno et al. 2005), which was implemented in Structure Harvester 0.6.94 (Earl and vonHoldt 2012).
Results
Mitochondrial analysis
An extensive mitochondrial diversity was detected in Hynobius quelpaertensis with 99, 82 and 50 unique haplotypes of COI, cyt b and d-loop, respectively (Table 2S, Table 3S and Table 4S in supplementary material). Polymorphism was observed at 198, 130, and 35 nucleotide sites in COI (1,464 bp), cyt b (907 bp) and d-loop (767 bp), respectively, 142, 101 and 22 of which were parsimoniously informative. Diversity indices were listed for each population in Table 5S, Table 6S and Table 7S in supplementary material. Common haplotype between inland populations was rarely observed, despite an active signature of interpopulational gene flow within Jeju Island (Table 2S, Table 3S and Table 4S in supplementary material). Overall, inland populations showed a higher level of mitochondrial diversity than Jeju Island populations (Table 2).
All H. quelpaertensis haplotypes formed a monophyletic group in all three phylogenetic trees reconstructed using COI, cyt b, and d-loop (Fig. 2; Fig. 1S in supplementary material). No phylogenetic structure, even between in- and island populations, was observed within the H. quelpaertensis clade in all three phylogenetic trees (Fig. 2; Fig. 1S in supplementary material). The clustering pattern among the species within the H. leechii species complex varied depending on the locus used (Fig. 2; Fig. 1S in supplementary material). H. quelpaertensis haplotypes unexceptionally were the sister to the group of HC1 and H. unisacculus (HC2) in all phylogenetic trees (Fig. 2). In the COI tree, the remaining H. leechii species complex members formed an independent clade, in which the cluster of H. leechii and H. yangi haplotypes was in a sister relationship to those of HC4, with HC3 being placed on the basal (Fig. 2a). In the cyt b tree, on the contrary, the haplotypes of H. quelpaertensis, HC1 and H. unisacculus were in a sister relationship to those of HC3 and HC4, with H. leechii and H. yangi being positioned on the basal (Fig. 2b). The haplotypes of H. quelpaertensis, HC1 and H. unisacculus were observed to be the sister to the cluster of HC3 and H. leechii haplotypes in the d-loop tree (Fig. 1S). No data were obtained for H. yangi and HC4 in our d-loop analysis. Consistent with the phylogenetic results, no clear genetic structure was observed among H. quelpaertensis haplotypes in our haplotype network analysis (Fig. 3; Fig. 2S and Fig. 3S in supplementary material). However, the haplotypes from Jeju island populations relatively tightly clustered together, though several groups were formed (Fig. 3; Fig. 2S and Fig. 3S in supplementary material). Some haplotypes of the population HY were identical or closely related to those of the Jeju Island populations (Fig. 3; Fig. 2S and Fig. 3S in supplementary material).
The maximum likelihood trees of Hynobius salamander species reconstructed for COI (a) and cyt b (b) sequences using IQ-Tree 1.3.10. All of the H. quelpaertensis populations are shown in color, and blue and green were used to represent inland and Jeju Island populations, respectively. High boostrapping values (> 94) were indicated on the node (color figure online)
Tajima’s D and Fu’s Fs were not found to be statistically significant, if estimated with all the surveyed individuals of H. quelpaertensis (Table 2). When the inland and island populations were estimated separately, the values of Jeju Island populations were all significant positive (Table 2). Mismatch distribution analysis showed a pattern of multimodal distribution for both inland and island populations (Fig. 4S in supplementary material). Unlike inland populations, however, island populations showed a highly discontinuous pattern (Fig. 4S in supplementary material). Our Bayesian skyline plots revealed that the effective population sizes of inland populations greatly increased around 100,000 years ago, whereas no visible historical change was observed in Jeju Island populations (Fig. 4).
The results of extended Bayesian skyline plot analysis showing the change of mean effective population size (y-axis: population) against geological time (x-axis: time (Mya)) performed based on the concatenated sequences of three mitochondrial loci (COI, cyt b and d-loop) for inland (a) and island (b) populations of Hynobius quelpaertensis
Microsatellite analysis
Five (Hyq046) to fifteen (Hyq026 and Hyq044) microsatellite alleles were detected per locus (Table 8S in supplementary material). Loci with a relatively large number of alleles (i.e., Hyq022, Hyq026, Hyq044) also showed relatively high HO and HE values (Table 8S in supplementary material). Some loci, Hyq002 and Hyq026 exhibited high positive FIS values but was not found to be significantly deviated from HWE (Table 8S in supplementary material). Because an active level of gene flow was revealed among Jeju Island populations in our mitochondrial analysis, those populations were reorganized into two, JJ (JA + JB + JJ) and SG (SH + SN + SS), by integrating populations in close proximity (Table 9S in supplementary material). A significantly higher FIS value was found in some inland populations (NW, HS and WD; Table 9S in supplementary material), which might be due to the possibility of sampling error (probably caused by small individual numbers analyzed for those populations) or the existence of null alleles. The signatures of genetic bottleneck were detected in quite a few inland populations (shifted mode), but not proven to be accurate, given that only ten or less individuals were used for those populations (Table 9S in supplementary material). No evidence of recent genetic bottleneck was detected in two Jeju Island populations (Table 9S in supplementary material). The Japanese H. lichenatus used as a reference taxon was clearly separated from the H. quelpaertensis populations by the large FST and RST values (Table 3). In H. quelpaertensis, island populations were slightly differentiated from inland populations (Table 3), which was also discovered in the results of our structure analysis (Fig. 5). Like our mitochondrial results, several individuals of population HY showed high genetic similarity to Jeju Island populations (Fig. 5).
Population structure of Hynobius quelpaertensis estimated from microsatellite genotyping. a Two or five were presumed as the most reliable number of clusters (K) based on Delta K method (y-axis: Delta K). b The analyses in Structure 2.3.4 were performed for all K values from 2 to 5, and the genetic differentiation between inland and Jeju island (plus some individuals of population HY) was observed
Discussion
It is biogeographically notable for six endemic species and species candidates of Hynobius to exist on the Korean Peninsula, a relatively small area on the southern tip of Far East Asia. The robust endemic diversity can be thought to have originated from H. leechii or its lineage(s), which when migrating south, that colonized various refugia in the southern part of the peninsula during Pleistocene glacial advances (Hewitt 2004; Hoarau et al. 2007; Provan and Bennett 2008). Therefore, these species and species candidates probably have historical imprints engraved by birth, change and isolation processes of refugia. In particular, the intraspecific genetic structure of H. quelpaertensis—with the widest distribution among Korean endemic Hynobius—may provide insight into the historical dispersal and colonization events which occurred on the southern region of the Korean Peninsula. Prior to this study, it was thought that H. quelpaertensis would have a low level of genetic diversity considering that it only inhabits this region. However, our mitochondrial and microsatellite results contradict this prediction. Especially, the mitochondrial haplotype diversity was enormous, with 99 COI haplotypes identified from slightly more than 200 H. quelpaertensis individuals.
A contributing factor to this tremendous mitochondrial haplotype variation appears to be the extreme genetic variance among populations, especially in the inland region. Given the ecological characteristics of H. quelpaertensis (e.g., slow to move, strict dependence on wet and sheltered habitat conditions for survival), this species is unlikely to frequently migrate to regions inhabited by other distinct populations, even if the regions are in close proximity. In fact, it is difficult to trace the behavioral radius or migration range of the Korean Hynobius salamanders that generally appear for spawning only in early spring (Yang et al. 2001). A lack of gene flow and the resultant genetic differentiation between populations has been observed in many other amphibian species (Semlitsch 2002; Monsen and Blouin 2003; Wang 2009); taking this into consideration, the average effective population size of H. quelpaertensis, similar to most other amphibian species, can be considered very small compared to the enormous genetic diversity of this species. If attempting to restore the populations or establish conservation strategies for this species in the future, these observations should be taken seriously, and focus should be placed on the formation of habitat links to ensure gene flow between populations.
In our study, the mitochondrial haplotype diversity of Jeju Island H. quelpaertensis populations was fairly low compared to that of inland populations, even considering the difference in number of individuals analyzed. Jeju Island has insufficient areas to provide suitable habitats for large numbers of salamander populations to flourish, which is probably because Jeju Island is an island formed at the tip of the continental shelf with many volcanic eruptions (Sohn and Park 2004). Mainly in the center of the island, large forests are concentrated along the north to south, and there is no suitable place for H. quelpaertensis to survive and reproduce on the east and west coastal areas. Given such a habitat distribution pattern, the relatively active signature of gene flow among populations in Jeju Island is logical. From this point of view, maintenance of the existing forest areas is the most essential aspect of preserving the H. quelpaertensis populations in Jeju Island.
Our phylogenetic trees reconstructed based on different mitochondrial loci revealed different patterns of interspecific relationship within the H. leechii species complex. In the cyt b tree, HC3 and HC4 was more closely related with H. quelpaertensis, HC1 and H. unisacculus rather than H. leechii and H. yangi. A different clustering pattern was shown in the COI tree, however, where HC3 and HC4 were primarily clustered with H. leechii and H. yangi. Importantly, these two different phylogenetic results would not lead to different biogeographical conclusions since the distribution of HC3 and HC4 are extremely narrow, such that only a single geographic population has been identified to date. As mentioned above, a huge lineage derived from H. leechii existed and migrated south, and individuals belonging to this lineage have probably occupied various refugia throughout the south. In isolated refugia, populations could grow, leading to the birth of H. quelpaertensis, HC1 and H. unisacculus. The results of the cyt b tree suggest that HC3 and HC4 might have been differentiated in a small isolated refugia of this lineage. H. yangi, apart from this lineage, might have originated from a small group of H. leechii (or its descendants) which arrived to the south independently. From the results of the COI tree, it should be concluded that HC3 and HC4 also originated from this ancestor. However, it is premature to determine which possibility is correct based on the present information; a comprehensive analysis using more genetic information is urgently needed.
No distinct phylogenetic structure was observed among the H. quelpaertensis haplotypes. In particular, no evidence of clear genetic differences was detected between the inland and Jeju Island populations. When the sea levels decreased before or after the peak of the last glacier between 25,000 and 10,000 years ago, the entire coast of Jeju Island had occasionally been connected to the peninsula (Sohn and Park 2004; Woo et al. 2013), allowing amphibians intermittent opportunities to migrate between the two regions. Despite the existence of historical links between inland regions and Jeju Island, the isolation signature appeared more clearly in the microsatellite loci, most likely due to the relatively short timeframe and small geographic scales; together, these aspects revealed that mitochondrial loci were somewhat ineffective at revealing the genetic structure between inland and island populations. It is possible, or even likely, that the Jeju Island populations formed from a small number of individuals crossing the sea had a relatively small effective population size leading to frequent historical genetic drift. This evolutionary process may have led to differences in microsatellite loci between inland and Jeju Island. Unfortunately, our microsatellite analysis did not yield an estimate of historical demographic status. However, when comparing inland and island populations, the values of pairwise-FST were slightly greater than or equal to the pairwise-RST values, though the pairwise-RST values were normally higher for the other comparisons. This likely indicates that demographic changes contributed to the differentiation between the two regions during colonization of Jeju Island.
The prediction that the Jeju Island populations might have remained relatively small compared with inland populations was at least partially supported by the mitochondrial loci-based demographic estimations. Jeju Island populations showed all positive, and some significant, Tajima’s D and Fu’s Fs values, indicating that a signature of allelic deficiency remained, probably due to a historical bottleneck. It can also presumed that the allelic deficiency in Jeju Island populations can be from the discontinuous multimodal pattern in our mismatch distribution analysis. An increase in the size of inland populations was pronounced in our extended Bayesian skyline plots, whereas there was no significant change in Jeju Island populations, at least partially supporting our demographic assumption.
The results presented here provide insight into the past biogeographic inland-to-island migration pathway. Both mitochondrial and microsatellite data reveal that among the inland populations, only HY contained alleles similar or identical to those of Jeju Island populations. Such a result was very unexpected given that population HY is found in the central south of the Korean Peninsula. Since all the coasts of Jeju Island are connected to the well-developed continental shelf on the west and southwest coasts of the Korean Peninsula (Woo et al. 2013), it is thought that the continental shelf contributed to the migration route of H. quelpaertensis to Jeju Island. Therefore, it is unreasonable to propose that population HY—found in the central south—is the starting point for migrating across the sea. From our data, there is no direct evidence revealing how this population alone could have Jeju Island alleles. It is simply presumed that the alleles of population HY and Jeju Island populations existed in low frequency in other regions, but not at present, assuming that the Jeju Island populations originated from the populations geographically close to the well-developed region of the continental shelf. If the effective population size of H. quelpaertensis had been small on average, as claimed earlier, the loss of alleles was likely a frequent and widespread occurrence. In addition, if only a small number of individuals migrated and formed populations in Jeju Island, it may well be hypothesized that these alleles, which were present at low frequency in the inland region, flowed into the island by chance. However, a comparative study using other vertebrate species is necessary to test this hypothesis.
The findings of our genetic study are summarized as follows. First, historical inferences about how the endemic diversity of Hynobius on the Korean Peninsula was shaped were established using a phylogenetic analysis. Second, although no distinct population structure was revealed within H. quelpaertensis, there was a weak trace of geographic isolation between inland and Jeju Island populations. According to evidence from our demographic analyses, it is likely that Jeju Island populations have undergone historical genetic drift, which might have strengthened the genetic differences between inland and island populations. Finally, our results revealed that only a single inland population, HY, had similar or identical alleles to those of Jeju Island populations. However, it was difficult to estimate the biogeographic migration route to Jeju Island by this information alone.
References
Baek HJ, Lee MY, Lee H, Min MS (2011a) Mitochondrial DNA data unveil highly divergent populations within the genus Hynobius (Caudata: Hynobiidae) in South Korea. Mol Cells 31(2):105–112
Baek HJ, Song JY, Lee H, Min MS (2011b) Species identification of a new candidate taxon HC2 (Caudata: Hynobiidae) using mitochondrial CO1 gene. Korean J Herpetol 3(1):25–32
Choi SK, Lee JE, Kim YJ, Min MS, Voloshina I, Myslenkov A, Oh JG, Kim TH, Markov N, Seryodkin I, Ishiguro N, Yu L, Zhang YP, Lee H, Kim KS (2014) Genetic structure of wild boar (Sus scrofa) populations from East Asia based on microsatellite loci analyses. BMC Genet 15:85
d’Alpoim Guedes J, Austermann J, Mitrovica JX (2016) Lost foraging opportunities for East Asian hunter-gatherers due to rising sea level since the last glacial maximum. Geoarchaeology 31(4):255–266
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29(8):1969–1973
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4(2):359–361
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14(8):2611–2620
Fouquet A, Vences M, Salducci MD, Meyer A, Marty C, Blanc M, Gilles A (2007) Revealing cryptic diversity using molecular phylogenetics and phylogeography in frogs of the Scinax ruber and Rhinella margaritifera species groups. Mol Phylogenet Evol 43(2):567–582
Fu Y (1997) Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147(2):915–925
Goudet J (2001) FSTAT, a program to estimate and test gene diversity and fixation indices (version 2.9.3). http://www2.unil.ch/popgen/softwares/fstat.htm. Accessed May 2019
Greenan TM, Griffiths CL, Santamaria CA (2018) Molecular approaches uncover cryptic diversity in intertidal Ligia isopods (Crustacea, Isopoda, Ligiidae) across the southern Africa coastline. PeerJ 6:e4658
Guo SW, Thompson EA (1992) Performing the exact test of Hardy–Weinberg proportion for multiple alleles. Biometrics 48(2):361–372
Hamilton MB, Pincus EL, Di Fiore A, Fleischer RC (1999) Universal linker and ligation procedures for construction of genomic DNA libraries enriched for microsatellites. Biotechniques 27(3):500–507
Helms BS, Vaught RC, Suciu SK (2015) Cryptic diversity within two endemic crayfish species of the Southeastern US revealed by molecular genetics and geometric morphometrics. Hydrobiologia 755(1):283–298
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc B 359(1442):183–195
Hoarau G, Coyer JA, Veldsink JH, Stam WT, Olsen JL (2007) Glacial refugia and recolonization pathways in the brown seaweed Fucus serratus. Mol Ecol 16(17):3606–3616
Jo Y, Kim T, Choi B, Oh H (2012) Current status of terrestrial mammals on Jeju Island. J Species Res 1(2):249–256
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14(6):587–589
Kim JB, Min MS, Matsui M (2003) A new species of lentic breeding Korean salamander of the genus Hynobius (Amphibia, Urodela). Zool Sci 20(9):1163–1169
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948
Lee YS, Markov N, Voloshina I, Argunov A, Bayarlkhagva D, Oh JG, Park YS, Min MS, Lee H, Kim KS (2015) Genetic diversity and genetic structure of the Siberian roe deer (Capreolus pygargus) populations from Asia. BMC Genet 16:100
Leigh JW, Bryant D (2015) PopART: full-feature software for haplotype network construction. Methods Ecol Evol 6(9):1110–1116
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25(11):1451–1452
Luikart G, Allendorf F, Cornuet J, Sherwin W (1998) Distortion of allele frequency distributions provides a test for recent population bottlenecks. J Hered 89(3):238–247
Min MS, Baek HJ, Song JY, Chang MH, Poyarkov NA Jr (2016) A new species of salamander of the genus Hynobius (Amphibia, Caudata, Hynobiidae) from South Korea. Zootaxa 4169(3):475–503
Monsen KJ, Blouin MS (2003) Genetic structure in a montane ranid frog: restricted gene flow and nuclear-mitochondrial discordance. Mol Ecol 12(12):3275–3286
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32(1):268–274
Oh DJ, Chang MH, Oh HS, Jung YH (2007) The complete mitochondrial DNA sequence of the Jeju salamander, Hynobius quelpaertensis, and the phylogenetic relationships among the Hynobiidae. Korean J Genet 29(3):331–341
Oliver PM, Adams M, Lee MS, Hutchinson MN, Doughty P (2009) Cryptic diversity in vertebrates: molecular data double estimates of species diversity in a radiation of Australian lizards (Diplodactylus, Gekkota). Proc Biol Sci 276(1664):2001–2007
Piry S, Luikart G, Cornuet J (1999) BOTTLENECK: a computer program for detecting recent reductions in the effective population size using allele frequency data. J Hered 90(4):502–503
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155(2):945–959
Provan J, Bennett KD (2008) Phylogeographic insights into cryptic glacial refugia. Trends Ecol Evol 23(10):564–571
Raymond M, Rousset F (1995) GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86(3):248–249
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386
Semlitsch RD (2002) Critical elements for biologically based recovery plans of aquatic-breeding amphibians. Conserv Biol 16(3):19–629
Slatkin M, Hudson RR (1991) Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129(2):555–562
Sohn YK, Park KH (2004) Early-stage volcanism and sedimentation of Jeju Island revealed by the Sagye borehole, SW Jeju Island, Korea. Geosci J 8(1):73–84
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123(3):585–595
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729
Wang IJ (2009) Fine-scale population structure in a desert amphibian: landscape genetics of the black toad (Bufo exsul). Mol Ecol 18(18):3847–3856
Woo KS, Sohn YK, Yoon S, Ahn US, Spate A (2013) Jeju Island geopark—a volcanic wonder of Korea. Springer, Berlin
Yang SY, Kim JB, Min MS, Suh JH, Kang YJ (2001) Monograph of Korean amphibia. Academy Books, Seoul
Acknowledgements
We thank HT Kim and YM Go for providing field assistance.
Funding
This research was performed with the support of the grants from the National Research Foundation of Korea (Grant no. NRF-2016R1D1A1B03934071) and from the National Institute of Biological Resources, Ministry of Environment (Grant no. NIBR201403201).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suk, H.Y., Bae, HG., Kim, DY. et al. Genetic and phylogenetic structure of Hynobius quelpaertensis, an endangered endemic salamander species on the Korean Peninsula. Genes Genom 42, 165–178 (2020). https://doi.org/10.1007/s13258-019-00886-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-019-00886-8