Abstract
Volumetric modulated arc therapy (VMAT) is modern rotational intensity modulated therapy used for treatment of several sites. The study aimed to analyze partial tangential arc VMAT treatment planning and delivery, including analyzing the cardiac and contralateral breast doses resulting from this technique. A total of 153 consecutively treated breast cancer (conservation as well as mastectomy) patients were taken for this dosimetric study. All patients were planned using partial arc VMAT in the Monaco treatment planning system using two partial arc beams. All patients were divided into seven different categories: (1) all the patients in the study, (2) left sided whole breast and chest wall patients, (3) left Chest wall patients, (4) left whole breast patients, (5) right sided whole breast and chest wall patients, (6) right chest wall patients, and (7) right whole breast patients. We evaluated each treatment plan for PTV coverage and doses to OARs. SPSS version 16.0 software was used for statistical analysis. There were 91 left sided and 62 right sided breast cancer patients in the overall analysis. The percentage of PTV volume receiving 95% of the prescription dose (PTV V95%, mean ± SD) varied in the range of 91.2 ± 5.2–94.8 ± 2.1% with mean dose of 92.4 ± 5.2% for all cases. The (mean ± SD) cardiac dose for all the patients was 289 ± 23 cGy. The (mean ± SD) cardiac doses were higher for left sided patients (424 ± 33.8 cGy) as compared to right sided patients (123.9 ± 80 cGy) (p < 0.001). Cardiac mean doses were higher with arc angles >30° versus 30° (324.5 ± 247.1 vs. 234.4 ± 188.4 cGy) (p = 0.001). Similarly contralateral breast mean dose was higher with arc angles >30° versus 30° (126 ± 115 vs. 88.6 ± 76.1 cGy) (p = 0.001). However cardiac V20, V30 and V40 Gy did not exhibit any statistical difference between the two groups (p = 0.26, 0.057 and 0.054 respectively). This is the first large study of its kind that assesses the dosimetric outcome of tangential partial arc VMAT treatments in a large group of mastectomy and breast conservation patients. Our study demonstrates the efficacy of this technique in dose coverage of PTV as well as in minimizing dose to OARs. Further, based on our results, we conclude that the arc length for the bi-tangential arcs should be 30° since it helps to achieve the most optimal balance between target coverage and acceptable OAR doses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiotherapy planning of carcinoma breast has evolved from evaluation of dose distribution in a single plane i.e., two-dimensional (2-D) planning to computed tomography (CT) based 3-dimensional radiotherapy (3-D CRT) planning [1,2,3,4]. Recent years have seen rapid evolution of advanced planning and delivery techniques like intensity modulated radiotherapy (IMRT) [5,6,7]. This progress in radiotherapy planning of carcinoma breast has enabled better dose uniformity for the target region of breast or chest wall as also the avoidance of excessive dose deposition in the normal tissues. It is becoming evident that even small doses to the heart and contralateral breast during radiotherapy course are important in the long term outcomes of breast cancer patients [8]. However the challenge is to further reduce the adverse effects of adjuvant irradiation in breast cancer and to innovate techniques for greater efficacy.
Volumetric modulated arc therapy (VMAT) is a complex IMRT technique that involves continuous delivery of an intensity modulated beam with a rotating gantry [6]. The VMAT technique or its variants have been widely used for several sites such as brain, head and neck, abdomen and thorax. The use of such rotational techniques in breast cancer radiotherapy, although reported by several centres, has been limited by concerns of dose spill in the contralateral breast and contralateral lung [9]. As such, techniques such as field-in-field or forward planning IMRT have become more prevalent in contemporary radiotherapy planning of carcinoma breast [5, 10, 11].
However, the question of the usefulness of VMAT in breast radiotherapy planning and delivery has not been settled. At our institution we have been using this technique for treating post mastectomy radiotherapy (PMRT) and for breast conservation therapy (BCT) patients. In the present study, we analyzed our technique of partial tangential arc VMAT treatment planning and delivery, including analyzing the cardiac and contralateral breast doses resulting from this technique.
Materials and methods
A total of 153 consecutively treated breast cancer [conservation as well as mastectomy] patients were taken for this dosimetric study. Mean age of the patients was 51.2 ± 11.9 years These patients had received treatment at our center between December 2014 to November 2016. All these patients had been recommended adjuvant radiotherapy by our multidisciplinary tumor board, in consistence with standard guidelines for adjuvant radiotherapy for breast cancer. The patients were positioned supine on the CT simulator using an inclined all-in-one (AIO) breast set.. Both arms were kept in abducted position holding the rod near the patient’s head. All patients were planned using partial arc volumetric modulated arc therapy (VMAT) using Monaco (V5.00.04) (Elekta CMS, Sunnyvale, CA). Treatments were delivered in Elekta Synergy (Elekta, Crawley, UK) linear accelerator with 6 MV photons.
Simulation and contouring
Under laser guidance, the patient was suitably aligned in the CT simulator. A set of three laser marking fiducials (small mm balls made of lead) was placed in anterior and two lateral positions (approximately in the plane passing through craniocaudal centre of the chest wall or breast). Suitable wires/markers were placed over breast/mastectomy scars to aid in contouring. Axial scans of thickness 3 mm were taken from hyoid to 8 cm below the ipsilateral (in case of conservation) or contralateral (in case of mastectomy) infra-mammary fold. Each patient was contoured by an experienced radiation oncologist for delineating the chest wall planning target volume (PTV) or the breast planning target volume PTV. The contours were done in the Monaco Sim (V5.00.04 CMS Elekta, Sunnyvale, CA) contouring workstation and were pushed to the Monaco workstation for treatment planning.
Radiotherapy planning
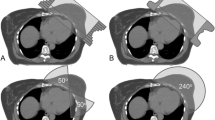
All patients were planned using partial arc volumetric modulated arc therapy (VMAT) in the Monaco treatment planning system (TPS). A typical left breast dose distribution and beam arrangement is shown in Fig. 1: panel a.
a Left beast treatment planning, beam setup and isodose distribution. b Geometrical relationship between the isocentre, arc start angle and arc length. Start angle was decided on the basis of the classical three dimensional half beam portals. ACB represent the half beam block portal. D represents the isocentre
Isocentre was decided on the basis of the geometrical relationship between three parameters: isocentric depth from the anterior surface, arc length and arc start angle. We used two partial arc beams geometrically resembling the breast or chest wall tangential portals since any other beam arrangement would increase the doses to organs at risk [OARs] with no or marginal improvement in the PTV dose. We started our breast VMAT program with variable arc lengths ranging from 25° to 45° depending on the size of the PTV. With gain in experience we soon standardized the arc length to 30° where each partial arc beam consisted of two arcs spanning 30° in the clockwise direction followed by another 30° in the anti-clockwise direction. In our technique the choice of start and end gantry angle was dependent on the conventional 3-D CRT tangential beam arrangement and was chosen in the following way: First the CT slice with the largest thickness of the breast or chest wall PTV contour was identified. On this slice, a half-beam medial tangential field was placed in such a way its central axis was just deep enough (line AB in Fig. 2) to adequately cover the PTV. The isocentre for the VMAT beams was placed at the point I that equally divided the PTV thickness. Points A and I were joined to mark the central axis line for the VMAT beam. A line CD perpendicular to AB was drawn passing through I to meet the horizontal line from A, drawn parallel to the couch top, at D (Fig. 1 panel a). Lines ID, CI and AD were marked as x1, x2 and x3 respectively. In Fig. 1 panel a, angle CAD is θ and the line IA divided its opposite angle into θ1 and θ2. Angle θ depends upon tangential line and tissue thickness, thus it is fixed for a particular patient and θ1 and θ2 are variables which are dependent on the location of isocenter location. From equal and opposite angle property it can be written as:
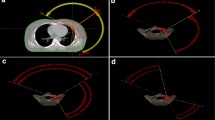
PTV dose coverage in different subgroup of patients. Foot note a PTV volume receiving 95% of the prescription dose. b PTV mean dose in Gy. c Percentage PTV volume receiving prescription dose. d Percentage PTV volume receiving 105% of the prescription dose. e Percentage PTV volume receiving 107% of the prescription dose. f MU to dose ratio (modulation factor). Error bar shows the standard deviation. Where OVERALL indicate all patients in the study. LWBCW left sided whole breast and chest wall patients. LCW left chest wall patients. LWB left whole breast patients. RWBCW right sided whole breast and chest wall patients. RCW right chest wall patients. RWB Right whole breast patient
The distances x1, x2 and x3 can be measured with the measuring tool in the planning system. By geometry
From sine rule, we have
From θ2 we determined the medial tangential arc start arc angle [angle nearest to the contralateral breast] as \(270 ^\circ + \theta _{2}\) given by
Lateral tangential start angle was taken as the conjugate reciprocal of medial tangential angle. Lateral arc length was the reciprocal of the medial arc except few cases before standardization of the arc length to 30°. To avoid the motional miss of the breast tissue from the field boundary, a 2 cm margin in air beyond the breast tissue was considered during optimization (called as surface/flash margin).
To identify the site specific statistical characteristics, all patients were divided into seven different categories: (1) all the patients in the study (OVERALL), (2) left sided whole breast and chest wall patients (LWBCW), (3) left chest wall patients (LCW), (4) left whole breast patients (LWB), (5) right sided whole breast and chest wall patients (RWBCW), (6) right chest wall (RCW) patients, and (7) right whole breast (RWB) patients. We evaluated each treatment plan for PTV coverage and doses to OARs.
Prescriptions used was 40 Gy in 15 fractions for the conservative breast. A 12.6 Gy (in five fractions) sequential cavity boost was added for intact breast patients and delivered using multiple beam 3DCRT technique. For chest wall patients a dose of 40 Gy in 15 fractions was used in all patients.
Results
The patient’s characteristics and basic planning statistics are shown in Table 1. There were 91 left sided and 62 right sided breast cancer patients in the overall analysis. Of these, 35 patients (22.8%) patients were below age of 40 years, 78 patients (50.9%) were between 40 and 60 years and 40 patients (26.1%) were above age of 60 years. For 73 patients arc lengths were 30° in both the VMAT fields and the remaining 31 patients had different arc lengths at least in one field.
While a typical left sided breast or chest wall radiotherapy planning required 3–4 iterative optimizations on an average consuming about 3.5 h of planning time, a typical right sided chest wall or breast plan took 2–3 optimizations and a planning time of about 2 h.
The percentage of PTV volume receiving 95% of the prescription dose (PTV V95%, mean ± SD) varied in the range 91.2 ± 5.2–94.8 ± 2.1% with mean dose of 92.4 ± 5.2% for all cases.
The (mean ± SD) cardiac dose for all the patients was 289 ± 23 cGy. The (mean ± SD) cardiac doses were higher for left sided patients (424 ± 33.8 cGy) as compared to right sided patients (123.9 ± 80 cGy) (p < 0.001). The dosimetric parameters for PTV and OARs for both left and right sided patients with subgroups analysis are presented in Table 2. The other PTV related dosimetric parameters for various subgroups analysis can be seen in Fig. 2.
Mean liver dose for right and left sided patents were 4647 ± 260.1 and 34.3 ± 28.1 cGy respectively. Maximum liver dose for right breast /chest wall patients were 4417.7 ± 978.2 cGy. Paddik conformity index (PI) at 95% prescription dose for left breast and chest wall were 0.88 ± 0.04 and 0.83 ± 0.1 respectively [12]. PI for right breast and chest wall was 0. 81 ± 0.06 and 0.87 ± 0.1 respectively. Cardiac mean doses and 2 cc dose were higher with arc angles >30° versus 30° (324.5 ± 247.1 vs. 234.4 ± 188.4 cGy) (p = 0.001) and (1852 ± 1417.5 vs. 2100.2 ± 1643.7 cGy) (p = 0.001) respectively. Similarly contralateral breast mean dose was higher with arc angles >30° versus 30° (126 ± 115 vs. 88.6 ± 76.1 cGy) (p = 0.001). However cardiac V20, V30 and V40 Gy did not exhibit any statistical dependence between the two groups (p = 0.26, 0.057 and 0.054 respectively). Dosimetric differences with arc angles have been given in Table 3.
The mean number of monitor units (MUs) for OVERALL was 581.5 ± 215.5 cGy (mean ± SD). The modulation factor was calculated as the ratio of required MU per fraction to the dose per fraction. OVERALL modulation factor (for all patients) was 2.58 ± 0.68 cGy/MU.
Discussion
Our study was a dosimetric study for all the consecutively treated breast cancer patients at our radiation oncology centre. All these patients had been recommended adjuvant radiotherapy by our multidisciplinary tumour board, in consistence with standard guidelines for adjuvant radiotherapy for breast cancer. It is well known that as the number of beams for a target volume goes up, the conformity may increase. It is with this rationale multiple fields are used while planning for 3-D CRT as well as other modern techniques like IMRT. VMAT is an extreme example of using such multiple number of beams in which the gantry is continuously treating while intensity modulated beams are being targeted to the PTV region. The pitfall of such techniques as compared to techniques with limited fields is the increase in the volume of low dose area to the tissue in vicinity to the target volume [9]. In the context of breast cancer, the critical organs at risk include contralateral breast, heart and lungs. VMAT techniques that use full rotational arc around the patient are likely to increase radiation received by these structures, albeit with the lower isodoses. But even this low doses can be detrimental to heart and the normal breast in the long term. It is for this reason that in our VMAT technique we used only small partial tangential arcs, with tight control over dose deposition to vulnerable OARs. The other accompanying benefit of VMAT delivery with partial arcs is the shorter duration of overall treatment time.
Recent evidence has established the importance of controlling loco regional disease with respect to overall survival of breast cancer patients [13,14,15,16]. Simultaneously, there is emerging data of some detriment by radiation to critical OARs such as the heart and contralateral breast [8]. It is therefore crucial to plan carcinoma breast patients meticulously by radiotherapy to attain good loco regional control and spare side effects.
Our study employed a predominantly bi-tangential arc technique of VMAT delivery. It is important to note [as a corollary of equation 1 in material and methods] that if the isocentre is posteriorly placed [deep inside the breast tissue; towards lung] then the start angle will go toward anterior direction. If the choice of isocentre is close to skin surface then arc start angle will move toward lateral direction (towards G = 270° for right breast and towards G = 90° for the left breast cases) and vise-versa. The movement of the start angle depending on the isocentre position is consequence of avoidance of contralateral breast.
Although some centres practice it, it is well known that full rotational arc therapies are likely to improve conformity of the breast coverage at the cost of increasing ipsilateral lung, contralateral lung, contralateral breast and heart doses. Our technique was aimed to get the benefits out of both worlds: the well-proven static tangential pair technique and as well as the emerging arc therapy technique. Our mean cardiac doses were <3 Gy in the overall analysis, which is well below the values reported by authors around the world in modern series [17]. Further, our mean contralateral breast doses have been below 1.1 Gy, which is less compared to the range reported in seminal publications [18].
For a VMAT based breast/chest wall radiotherapy planning it is most crucial to decide placement of the start and end angle of the arc (hence the arc length). A wrong choice of the arc position and angle can lead to a significant increase in the doses to OARs and increase in optimisation time. Initially we tried with arc lengths 25°–50° in few points before we quickly standardized it to 30°. As Table 3 reveals, most of the reported dose volume characteristics had unfavorable heart, ipsilateral lung and contralateral breast doses with larger or lesser arc angles. Based on our study, which is the largest of its kind, we therefore recommend that 30° as the most optimal arc length when planning with bi-tangential dual arcs technique.
With the tangential VMAT technique, significantly higher cardiac avoidance, dose coverage and dose homogeneity were achieved when compared with the field-in-field or tangential IMRT techniques (p < 0.01). VMAT technique also decreased the high dose areas (above 20 Gy) of ipsilateral lung. Another study of 20 left sided breast conservation patients compared the dosimetry outcomes using five different radiotherapy techniques [19]. The patients were planned using five different radiotherapy techniques, including: (1) conventional tangential wedge-based fields (TW); (2) field-in-field (FIF) technique; (3) tangential inverse planning intensity-modulated radiation therapy (T-IMRT); (4) multi-field IMRT (M-IMRT); and (5) volumetric modulated arc therapy (VMAT). T-IMRT plan improved the PTV dose homogeneity index (HI) by 0.02 and 0.03 when compared to TW plan and VMAT plan, and decreased the V5, V10 and V20 of all PRV-OARs. In all five plans, the dose volume of coronary artery area showed a strong correlation to the dose volume of the heart (the correlation coefficients were 0.993, 0.996, 1.000, 0.995 and 0.986 respectively) [19].
Some studies have reported partial arc VMAT for breast in supine and prone position [19,20,21,22]. Viren et all described two different VMAT techniques (240° arc and 50° + 50° arc). For shorter arc technique (50° + 50° arc) mean and V30 Gy (%) cardiac dose was 6.3 ± 3.0 Gy and 5.7 ± 6.0 (%). Similarly Yu et al. reported a mean and V30 Gy cardiac dose of 6.57 ± 3.35 Gy and 5.19 ± 4.57% respectively [21]. Liu et al. reported a mean cardiac dose of 13.7 ± 0.6 Gy [22]. Mean cardiac dose for overall and left sided cases reported in our study is 2.9 ± 2.3 and 4.2 ± 2.3 Gy respectively.V30Gy (%) for overall and left sided cases was 1.4 ± 2.7 and 2.5 ± 3.2% respectively. Both cardiac mean dose and V30 Gy reported in our study were lower than other available literature series. Ipsilateral lung mean dose reported by the different authors for short arc VMAT technique were 13.8 ± 0.85, 9.6 ± 2.1, 9.6 ± 1.4, 5.2 ± 2.0 Gy respectively [19,20,21,22]. Ipsilateral lung V5 Gy(%) were 76.5 ± 4.29, 36.9 ± 5.4, 45.0 ± 6.9 and 18.4 ± 7.2% respectively. Only for left sided patients Ipsilateral lung mean dose and V5Gy(%) were 8.3 ± 3.0 Gy and 34.8 ± 12.5% respectively. Our technique yields a lesser ipsilateral lung mean and V5 Gy(%) dose from all the available literature series except Zhao et al. [20]. A recent study of 11 patients compared VMAT and IMRT plans for intact breast radiotherapy for left sided breast cancer and evaluated the irradiated dose of planning target volume and OARs, especially focusing on heart and coronary artery [23]. For the PRV-OARs, the 1-arc VMAT had significantly higher Dmean and V5 Gy for left lung and heart, and showed worse Dmean for contralateral lung and breast. In contrast, the 2-arc VMAT and the 2-F or 4-F IMRT plans showed better results for the PRV-OARs than the 1-arc VMAT. However, for the heart and coronary artery, the 1-arc VMAT showed better V20 and V40 compared with the other plans.
In a small study recently published from our centre, Giri et al. compared Field in Field IMRT, 3DCRT and partial arc VMAT for 20 Left breast and chest wall patients [21]. They concluded that VMAT is a preferable technique yielding similar PTV coverage with much lower cardiac dose.
To summarise, our work is the first large study of its kind that assesses the dosimetric outcome of tangential partial arc VMAT treatments in a large group of mastectomy and breast conservation patients. Our study demonstrates the efficacy of this technique in dose coverage of PTV as well as in minimizing dose to OARs. Further, based on our results, we conclude that the arc length for the bi-tangential arcs as 30° since it helps to achieve the most optimal balance between target coverage and acceptable OAR doses.
Change history
12 June 2017
An erratum to this article has been published.
References
Cross P, Joseph DJ, Cant J et al (1992) Tangential breast irradiation: simple improvements. Int J Radiat Oncol Biol Phys 23:433–442
Das IJ, Cheng CW, Fein DA et al (1997) Patterns of dose variability in radiation prescription of breast cancer. Radiother Oncol 44:83–89
Munshi A, Pai RH, Phurailatpam R, Budrukkar A, Jalali R, Sarin R, Deshpande DD, Shrivastava SK, Dinshaw KA (2009) Do all patients of breast carcinoma need 3-dimensional CT-based planning? A dosimetric study comparing different breast sizes. Med Dosim 34:140–144
Kantorowitz DA (2000) The impact of dose-specification policies upon nominal radiation dose received by breast tissue in the conservation treatment of breast cancer. Int J Radiat Oncol Biol Phys 47:841–848
Donovan E, Bleakley N, Denholm E et al (2007) Randomised trial of standard 2D radiotherapy [RT] versus intensity modulated radiotherapy [IMRT] in patients prescribed breast radiotherapy. Radiother Oncol 82:254–264
Jin GH, Chen LX, Deng XW, Liu XW, Huang Y, Huang XB (2013) A comparative dosimetric study for treating left-sided breast cancer for small breast size using five different radiotherapy techniques: conventional tangential field, filed-in-filed, tangential-IMRT, multi-beam IMRT and VMAT. Radiat Oncol 8:89
Coles CE, Moody AM, Wilson CB, Burnet NG (2005) Reduction of radiotherapy-induced late complications in early breast cancer: the role of intensity-modulated radiation therapy and partial breast irradiation. Part II—radiotherapy strategies to reduce radiation-induced late effects. Clin Oncol 17:98–110
Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P (2013) Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368:987–998
Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A (2011) Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol 84:967–996
Morganti AG, Cilla S, de Gaetano A, Panunzi S, Digesù C, Macchia G, Massaccesi M, Deodato F, Ferrandina G, Cellini N, Scambia G, Piermattei A, Valentini V (2011) Forward planned intensity modulated radiotherapy [IMRT] for whole breast postoperative radiotherapy. Is it useful? When? J Appl Clin Med Phys 12:3451
Wu S, Lai Y, He Z, Zhou Y, Chen S, Dai M, Zhou J, Lin Q, Chi F (2015) Dosimetric comparison of the simultaneous integrated boost in whole-breast irradiation after breast-conserving surgery: IMRT, IMRT plus an electron boost and VMAT. PLoS ONE 17:10
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans: technical note. J Neurosurg 93(Supplement 3):219–222
Early Breast Cancer Trialists Collaborative Group [EBCTCG] (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: an overview of randomised trials. Lancet 365:1687–1717
Early Breast Cancer Trialists’ Collaborative Group (1995) Effects of radiotherapy and surgery in early breast cancer: an overview of randomized trials. N Engl J Med 333:1444–1455
Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M et al (1999) Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 353:1641–1648
Ragaz J, Jackson SM, Le N, Plenderleith IH, Spinelli JJ et al (1997) Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 337:956–962
Wollschläger D, Karle H, Stockinger M, Bartkowiak D, Bührdel S, Merzenich H, Wiegel T, Blettner M, Schmidberger H (2016) Radiation dose distribution in functional heart regions from tangential breast cancer radiotherapy. Radiother Oncol 119:65–70
Zurl B, Stranzl H, Winkler P, Kapp KS (2013) Quantification of contralateral breast dose and risk estimate of radiation-induced contralateral breast cancer among young women using tangential fields and different modes of breathing. Int J Radiat Oncol Biol Phys 85:500–505
Virén T, Heikkilä J, Myllyoja K, Koskela K, Lahtinen T, Seppälä J (2015) Tangential volumetric modulated arc therapy technique for left-sided breast cancer radiotherapy. Radiat Oncol 10:79
Hongfu Zhao, Mingyuan He, Guanghui Cheng, Dongmei Han, Ning Wu, Dan Shi, Zhipeng Zhao, Jin J (2015) A comparative dosimetric study of left sided breast cancer after breast-conserving surgery treated with VMAT and IMRT. Radiat Oncol 10:231
Yu J, Hu T, Chen Y (2016) Small-arc volumetric-modulated arc therapy: a new approach that is superior to fixed-field IMRT in optimizing dosimetric and treatment-relevant parameters for patients undergoing whole-breast irradiation following breast-conserving surgery. Medicine 95(34):e4609
Liu H, Chen X, He Z, Li J (2016) Evaluation of 3D-CRT, IMRT and VMAT radiotherapy plans for left breast cancer based on clinical dosimetric study. Comput Med Imaging Graph 54:1–5
Giri UK, Sarkar B, Jassal K, Munshi A, Ganesh T, Mohanti B, Pradhan A (2017) Left-sided breast radiotherapy after conservative surgery: comparison of techniques between volumetric modulated arc therapy, forward-planning intensity-modulated radiotherapy and conventional technique. J Radiother Pract 16(1):101–108
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the Authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
The original version of this article was revised: the name of the third author was corrected to Satheeshkumar Anbazhagan.
An erratum to this article is available at https://doi.org/10.1007/s13246-017-0562-2.
Rights and permissions
About this article
Cite this article
Munshi, A., Sarkar, B., Anbazhagan, S. et al. Short tangential arcs in VMAT based breast and chest wall radiotherapy lead to conformity of the breast dose with lesser cardiac and lung doses: a prospective study of breast conservation and mastectomy patients. Australas Phys Eng Sci Med 40, 729–736 (2017). https://doi.org/10.1007/s13246-017-0558-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-017-0558-y