Abstract
Quality assurance of stereotactic radiotherapy demands the use of equipment with the highest resolution and sensitivity available. This study examines the sensitivity of a commercially available liquid-filled ionization chamber array—the Octavius 1000 SRS (PTW, Frieburg, Germany) for detecting small (sub-millimetre) multi-leaf collimator (MLC) alignment errors in static square fields (side length 16–40 mm). Furthermore, the effectiveness of detecting small MLC errors in clinical stereotactic radiotherapy patient plans using the device was also evaluated. The commonly used gamma pass rate metric (of the measurements compared with treatment planning system generated results) was used. The gamma pass rates were then evaluated as a function of MLC position error (MLC error size 0.1–2.5 mm). The detector array exhibited a drop in pass rate between plans without error and those which had MLC errors induced. For example a drop in pass rate of 4.5 % (gamma criteria 3 %, 1 mm) was observed when a 0.8 mm error was introduced into a 16 mm square field. Furthermore the drop in pass rate increased as the MLC position error increased. This study showed that the Octavius 1000 SRS array could be a useful tool for applications requiring the detection of small geometric delivery uncertainties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Increasingly complex radiotherapy delivery techniques have necessitated the need for quality assurance (QA) testing of radiotherapy treatment plans on a patient-by-patient basis to ensure safe treatment. Dose measurement in an appropriate phantom, or a dose calculation independent of the treatment planning system (TPS) must be carried out to validate treatment plans that use complex techniques [1] (i.e., broadly, a complex treatment involves small fields and/or intensity modulated beams). One advantage of patient-specific QA protocols that involve dose measurements is that the entire treatment delivery chain is audited from planning, to data transfer to the linear accelerator, and delivery of the treatment. Each step offers an opportunity to discover any systematic error in the radiotherapy planning and treatment chain [2].
There are a number of commercially available detector arrays offered as alternatives to radiochromic film for two-dimension dose measurements for patient specific QA. Arrays have an advantage over film of not requiring as much post-processing to analyse results, and do not require calibration each time a measurement is taken. Arrays for QA come in a variety of designs ranging from planar ionization chambers and helically arranged diodes. Multiple studies have demonstrated the efficacy of arrays for patient-specific QA, with arrays of all types being characterised and tested to reveal errors in treatment delivery of intensity-modulated arc therapy (IMAT) [3–5].
Planning studies have established the clinical significance that a hypothetical multi-leaf collimator (MLC) error would affect on an IMAT treatment plan [6, 7]. Oliver et al. used dose volume histogram (DVH) based metrics, including evaluating a clinically significant change in the planning treatment volume (PTV) dose distribution [6]. They suggested that a 2 % change in PTVmean (i.e., the mean dose delivered over all PTV voxels) was the minimum required for clinically significant results in head and neck IMAT plans [6]. This corresponded with a 0.6 mm shift in an entire MLC bank. Furthermore, Heilemann et al. [7] took measurements with the Delta4 (Scandidos, Uppsala, Sweden) and the Octavius Seven27 (PTW, Freiburg, Germany) arrays to establish whether MLC errors of a similar magnitude—specifically, opening or closing of leaves, giving larger or smaller fields—could be effectively detected for IMAT delivery using the common gamma analysis technique described by Low et al. [8]. It was found that the smallest MLC shifts which would produce a failure of the chosen >90 % criterion for gamma-index of 2 %/2 mm were a 1 mm opening of the leaves; and a 0.5 mm and 1 mm closing of leaves (for prostate and head-and-neck plans respectively) [7]. It was inferred that clinically significant changes to the PTV dose distribution would not be detected from data measured with the Delta4 and Seven27 arrays using a gamma analysis criteria of 2 %/2 mm.
The Octavius 1000 stereotactic radiosurgery (SRS) array (PTW, Freiburg) is a plane detector made up of a liquid-filled ionization chamber array mounted in a motorized cylindrical phantom (Octavius 4DTM, PTW, Freiburg) which rotates during treatment—so the detector is perpendicular to the beam direction for all gantry angles of a rotational treatment beam. This array has been well characterized as a dosimeter, with investigation revealing short- and medium-term reproducibility of 0.1 and 0.2 % respectively, and dose linearity and dose rate dependence within 3 % (which is within the manufacturer specified dose range) [9, 10].
As its name suggests, the Octavius 1000 SRS array was designed for SRS QA, but to date there is little in the literature testing the suitability of this device for picking up very small MLC position errors that may impact upon a patient’s treatment. It is therefore the aim of this study to assess sensitivity of the Octavius 1000 SRS array in detecting small errors in the delivery of SRS. The study is concerned with whether the QA system as a whole would flag any potential MLC issues with the linac/TPS for a gamma analysis criteria set for a given QA protocol (e.g. QA protocol is set to 3 %/1 mm and a 95 % pass rate is required for all SRS measurements—can we detect clinically significant errors with this? i.e., does pass rate drop appreciably with MLC error).
Method and materials
Octavius 1000 SRS
The Octavius 1000 SRS detector is an array of 977 liquid-filled ionization chambers within a 10 × 10 cm2 area (see Fig. 1). Designed with small field dosimetry in mind, the 1000 SRS features a detector spacing of 2.5 mm for high resolution. Each detector has dimensions of 2.3 mm × 2.3 mm × 0.5 mm, with an active volume of 0.003 cm3. The array itself is housed within the Octavius phantom: a rotational device which turns in unison with the gantry. This matched rotation is achieved by attaching an inclinometer to the gantry which communicates with the array over BluetoothTM. Associated PTW software VeriSoft provided an interface to the device and was used to collate all data and gamma analysis of measured and planned dose distributions.
Image of Octavius 1000 SRS (above), and a diagram representing the detector placement with the device; detectors are spaced 2.5 mm apart in the central 5 × 5 cm square of the array, and 5 mm spacing in the periphery (Diagram not to scale, reproduced from Poppe et al. [10])
Controlled square fields
The treatment planning system iPlan (BrainLab AG, Feldkirchen, Germany.) was used to estimate dose distributions a number of square rotational fields and cranial patient plans. A CT data set (Toshiba Aquilion, 120 kV, 2 mm slice thickness) of the Octavius system was imported into iPlan and used as the phantom for the study. 40 mm and 16 mm square arc fields were created with iPlan and the calculated dose distribution exported and used as the control for gamma analysis of any measured fields. Dose distributions were calculated using the iPlan pencil beam algorithm that had been commissioned clinically for cranial SRS.
The arcs created in iPlan were then manipulated such that there was a systematic MLC leaf offset from the nominal field size (i.e., 40 or 16 mm) ranging between 0.1 and 2.5 mm for the 40 mm square field size and from 0.1 to 0.8 mm for the 16 mm field. This had the effect of ‘shifting’ the entire field across the linac head in the direction of MLC travel. These altered arcs were exported and transferred to the linac via the record and verify (R&V) system MOSAIQ (Elekta, Stockholm, Sweden)
Patient plans
A similar process to for manipulating square fields was used with the clinical patient plans, with the dose distribution re-calculated with iPlan using the Octavius as a phantom for each patient plan. All iPlan dose distributions were calculated with a 1.0 mm dose grid resolution. Each arc in each plan was then adjusted to produce a ‘random’ error in an effort to replicate a machine delivery problem such as an MLC calibration error or incorrect MLC sequences sent to the R&V system. To solicit such an error, half the MLC leaves on the X1 bank were moved out by up to 0.5 mm, and the opposing half of the MLC leaves in the X2 bank were moved out by up to 0.5 mm. Although more realistic ‘random’ errors could be created by the user by manipulating individual leaves, a controlled change in MLC position, as illustrated in Fig. 2, was applied to all treatment beams.
Four clinical cranial SRS plans were measured in a ‘no-error’ form and alternatively with an error in MLC position. The four patients were chosen at random, and the treatment sites and prescriptions are summarised in Table 1. The clinical plans were changed to a static MLC delivery (rather than the dynamic MLC modulation in the clinical plans). Each plan was originally formed with up to five non-coplanar arcs; however each arc was measured individually with the Octavius at a couch angle of 0°. The above simplifications to the patient plans were made to isolate the results to the sensitivity of the detector array itself in picking up positional uncertainty in the treatment fields.
In order to evaluate clinical significance of the introduced errors, the dose distributions were re-calculated with iPlan after making the adjustments to the MLC leaves. Specifically, the dose distribution to the PTV in the MLC shift plans calculated by the TPS was analysed, with the new PTVmean value and percentage change in PTVmean between the control plans and those with MLC error recorded. The values for PTVmean for all plans were taken directly from the DVH display of the PTV in the iPlan TPS. Looking into the change in PTVmean due to the MLC errors will give an indication to the potential clinical impact such an error may have, and what the associated change in gamma analysis results are.
In addition to using DVH statistics for plan evaluation, the Radiation Therapy Oncology Group (RTOG) proposes the use of the Conformity Index (CI) to quantitatively evaluate the level of conformity and isodose coverage of a treatment volume in SRS. The CI is defined as the ratio of the volume of the reference isodose (VRI) and the target volume (TV) [11]
The ideal CI value is 1. RTOG defines guidelines for SRS plan quality control using the CI, where values between 1 and 2 comply with guidelines; an index between 2 and 2.5, or 0.9 and 1, are considered to be minor violations, with values outside this range a major violation of the protocol. Part of this study will identify any changes to plan conformity due to the controlled MLC positioning errors introduced to the treatment plans. As with the analysis of changes to PTVmean due to the MLC positioning errors created in the SRS treatment plans, differences in CI as calculated by the iPlan TPS were recorded.
Delivery and analysis
All beams were delivered on an Elekta Axesse linear accelerator with Beam Modulator collimation (i.e., an MLC system with fixed jaws and 40 leaf pairs of 4 mm width at isocentre) with the Octavius device setup at isocentre on the treatment couch. The in-room lasers were used to align the device correctly to isocentre. The array was not moved for the duration of measurements in order to isolate uncertainty in machine delivery and MLC alignment from any uncertainty associated with experimental set-up.
The VeriSoft (PTW, Freiburg) patient plan verification software was used to perform gamma analysis between the measured and calculated data sets, using the default factory array calibration provided by the manufacturer. The factory calibration was verified simply by measuring square fields and comparing to a reference 2D distribution exported from iPlan TPS. All analyses were of relative dose normalized to region of interest (ROI) dose maximum of 100 %. All gamma analysis was performed with assessment criteria of 3 % dose difference and 1 mm distance-to-agreement. The percentage of points within a 10 × 10 cm ROI above a 10 % dose threshold (i.e., points below 10 % of ROI maximum dose are not calculated) which passed these criteria was recorded. The same gamma analysis criteria were used for all treatment deliveries. Keeping this constant allowed for investigation into how the QA system would perform overall in clinical practice.
For all the squares fields, the gamma analysis pass rates of the arcs with the induced MLC error were compared with the calculated gamma analysis pass rates of the ‘correct’ square field from iPlan. Similarly, patient plans altered with an MLC shift were compared with the actual clinical plans exported from the TPS.
Results
Square fields
Measurement of the unmodified 40 and 16 mm square rotational fields with Octavius yielded a gamma pass rate of 97.1 and 99.6 % respectively when compared against the TPS dose distribution. Once a positional uncertainty was introduced, the pass rates dropped as a function of MLC shift (see Fig. 3). For example a 0.8 mm uncertainty caused a reduction in the gamma pass rate of 5.7 and 4.5 % for the 40 and 16 mm field sizes respectively.
Patient plans
Across all four patient plans, and for all arcs, the average drop in gamma pass rate was found to be 3.9 %, ranging from a decrease of 10.1 % to an increase in pass rate of 1.5 %.
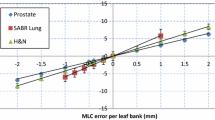
Minimal difference between the PTVmean values for the treatment plans with and without MLC errors were identified, as shown in Table 2. No patient plan approached the 2 % change to PTVmean which represents a clinically significant cut off point as stated in the Background section. However, larger changes to the CI between plans were apparent, as illustrated in Fig. 4. A distinct relationship between the change in gamma pass rate and the change in CI is difficult to establish as in two cases (patients’ C and D) the index increased with MLC error. An illustration of gamma pass rates and CI for all the treatment plans is given in a plot in Fig. 4.
It should be noted that there was no case where the CI moved to values which would violate the RTOG guidelines for SRS conformity.
Discussion
Testing of the square fields revealed a fall in gamma pass rate which changed as the MLC offset introduced increased. However, for a simple 16 mm square field geometry, offset 0.8 mm in the MLC leaf direction only a 4.5 % reduction in pass rate was detected. This meant that although the MLC error amounted to 5.4 % of the field dimensions, measurements with the Octavius system (and a gamma criteria of 3 %, 1 mm) could still ‘pass’ a QA procedure with a Gamma pass rate limit as high as 95 %, and no linac or planning faults would be flagged. Therefore it is recommended that before clinical implementation, each user should decide what gamma criteria would be acceptable to their practice, and subsequently what gamma criteria pass rates are required to meet these. Whilst it has been shown in this study that the Octavius 1000 SRS has the ability to pick up small positional uncertainty, it is beyond the scope of this study to suggest specific gamma criteria for SRS QA including pass rates.
The results for the patient plans were less straightforward, with gamma pass rates changing in a less consistent manner than the square rotational fields, as indicated by the large range in change of gamma between the two sets (10.1 % decrease to 1.5 % increase). This was possibly due to the greater complexity of these beams, compared with a static square field. The one instance where the gamma pass rate actually improved with the MLC shift, although small (1.5 %) created a potential false positive situation. However, this could have been caused by the MLC shift—in conjunction with machine delivery uncertainty—actually moving the beam into a more ‘correct’ position.
This current study contained an introductory series of basic tests which tested the feasibility of using the Octavius 1000 SRS detector array for SRS QA. Further investigations with Octavius could be performed based on detecting errors with larger impact on the plan itself e.g. a larger change in DVH for a given OAR. An average 0.2 % change in PTVmean was found in this study for 0.5 mm MLC shifts, with a maximum change of 0.16 Gy occurring for patient C (16.5 Gy prescription dose). This small change in PTV dose distribution and the CI (−0.02 for patient C) in the TPS manifested itself as a 5.0 % decrease in gamma pass rate due to the MLC shifts introduced to the plan (see Fig. 4). This change in gamma pass rate could potentially move a result below a tolerance for passing points of interest (e.g. 95 % of points with gamma <1), even though there were small changes in plan metrics such as CI and PTV dose. The largest change in CI was −0.17, occurring in patient A (20 Gy prescription), which elicited a 2.8 % decrease in mean gamma pass rate. The results collected made a relationship between gamma pass rate and change in PTVmean and CI difficult to establish (as per Table 2; Fig. 4), even though there was some success in identifying an issue with the plan (i.e., the controlled MLC shifts).
Further work on the QA system sensitivity would perhaps defining a larger change in DVH first—then finding the MLC leaf error required to produce such a change in dose distribution—would elicit stronger results from QA measurements. This would follow from the method of Oliver et al. [6], where the ‘tipping point’ of clinical significance for SRS could be found in terms of a mm MLC shift—such as the 0.6 mm figure found for fractionated head and neck treatments, and then test to see if the Octavius can detect such an error with reliability in an SRS context, rather than prostate or head and neck IMAT. However, such detailed tests are beyond the scope of this initial study.
Film is commonly used for tasks requiring a high degree of sensitivity, such as SRS QA. Similar studies have been performed using radiochromic film to pick up intentional MLC alignment errors, with MLC errors between 0.5 and 1.0 mm successfully detecting with appropriate gamma criteria [12]. This study showed that the Octavius 1000 SRS performed comparably to the highest resolution dosimeter available. However, reliable results using the gamma analysis technique hinge suitable tolerance levels being chosen. Furthermore, although the device performed well enough to detect MLC errors of a given magnitude using the gamma pass rate techniques outlined in this study, it is important to scrutinize the measurement technique used to detect such errors [13]. However, in this case, the gamma pass rates proved a convenient, well understood tool for analysing the sensitivity of the Octavius 1000 SRS detector array.
Conclusion
The measurements made with Octavius 1000 SRS detector array did produce a drop in average gamma pass rates between ‘no-error’ beams and the beams with shifted MLCs. At this stage of evaluation, identifying the capability of finding errors and indicating there is a problem with delivery to the user is more important than passing a certain pre-defined gamma pass rate threshold (e.g. 95 %) for a set of dummy plans. As such, it has been shown that the device could be a valuable tool when high resolution fluence measurements are required.
References
Low DA, Moran JM, Dempsey JF, Dong L, Oldham M (2011) Dosimetry tools and techniques for IMRT. Med Phys 38:1313–1338
Alber M, Broggi S, De Wagter C et al (2008) Guidelines for the verfication of IMRT. ESTRO, Brussels
O’Daniel J, Das S, Wu J, Fang-Fang Y (2010) Patient specific quality assurance: transition from IMRT to IMAT. J Phys 250:012050. doi:10.1088/1742-6596/250/1/012050
Gloi AM, Buchanan RE, Zuge CL, Goettler AM (2011) RapidArc quality assurance through MapCHECK. J Appl Clin Med Phys 12:39–47
Li G, Zhang Y, Jiang X, Bai S et al (2013) Evaluation of the ArcCHECK QA system for IMRT and VMAT verification. Phys Med 29:295–303
Oliver M, Gagne I, Bush K, Zavgorodni S et al (2010) Clinical significance of multi-leaf collimator positional errors for volumetric modulated arc therapy. Radiother Oncol 97:554–560
Heilemann G, Poppe B, Laub W (2013) On the sensitivity of common gamma-index evaluation methods to MLC misalignments in rapidarc quality assurance. Med Phys 40:031702
Low DA, Harms WB, Mutic S, Purdy JA (1998) A technique for quantitative evaluation of dose distributions. Med Phys 25:656–661
Markovic M, Stathakis S, Manroidis P, Jurkovic I et al (2014) Characterization of a two-dimensional liquid-filled ion chamber detector array used for verification of the treatments in radiotherapy. Med Phys 41:051704
Poppe B, Stellje TS, Looe HK, Chofor N (2013) Performance parameters of a liquid filled ionization chamber array. Med Phys 40:082106
Shaw E, Kline R, Gillin M, Souhami L et al (1993) Radiation therapy oncology group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys 27(5):1231–1239
Kim J, Park S-Y, Hak JK, Kim JH et al (2014) The sensitivity of gamma-index method to the positioning errors of high-definition MLC in patient-specific VMAT QA for SBRT. Radiat Oncol 9:167
Nelms BE, Zhen H, Tome WA (2011) Per-beam, planar IMRT QA passing rates do not predict clinically relevant patient dose errors. Med Phys 38:1037–1044
Acknowledgments
All PTW equipment used in this study was provided by Nucletron Pty Ltd (Newtown, Australia) on a trial basis to the medical physics department. Measurements taken during the trial period form the basis of this study. The authors would like to extend their gratitude to Nucletron for providing the equipment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Connor, P., Seshadri, V. & Charles, P. Detecting MLC errors in stereotactic radiotherapy plans with a liquid filled ionization chamber array. Australas Phys Eng Sci Med 39, 247–252 (2016). https://doi.org/10.1007/s13246-016-0421-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-016-0421-6