Abstract
Bioprosthetic aortic valves (BAVs) are becoming the prostheses of choice in heart valve replacement. The objective of this paper is to assess the effects of leaflet geometry on the mechanics and hemodynamics of BAVs in a fluid structure interaction model. The curvature and angle of leaflets were varied in 10 case studies whereby the following design parameters were altered: a circular arch, a line, and a parabola for the radial curvature, and a circular arch, a spline, and a parabola for the circumferential curvature. Six different leaflet angles (representative of the inclination of the leaflets toward the surrounding aortic wall) were analyzed. The 3-dimensional geometry of the models were created using SolidWorks, Pointwise was used for meshing, and Comsol Multiphysics was used for implicit finite element calculations. Realistic loading was enforced by considering the time-dependent strongly-coupled interaction between blood flow and leaflets. Higher mean pressure gradients as well as von Mises stresses were obtained with a parabolic or circular curvature for radial curvature or a parabolic or spline curvature for the circumferential curvature. A smaller leaflet angle was associated with a lower pressure gradient, and, a lower von Mises stress. The leaflet curvature and angle noticeably affected the speed of valve opening, and closing. When a parabola was used for circumferential or radial curvature, leaflets displacements were asymmetric, and they opened and closed more slowly. A circular circumferential leaflet curvature, a linear leaflet radial curvature, and leaflet inclination toward the surrounding aortic wall were associated with superior BAVs mechanics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bioprosthetic heart valves (BHVs) are increasingly the prosthesis of choice in valve replacement procedures.27 Both prosthesis and patient related factors determine the choice of prostheses. Two types of prosthetic valves are currently available, namely mechanical valves and bioprosthetic valves. Unlike mechanical valves, bioprosthetic valves do not require lifelong anticoagulation and are inherently prone to early deterioration.34
While recent evidence show superior freedom from hemorrhagic and thromboembolic complications in BHVs up to 15 years,36 freedom from reoperation was significantly lower in patients with bioprosthetic valves in comparison to mechanical valves. Consequently, the use of BHVs in the younger patient population continues to be a topic of discussion and has more recently been complicated by the introduction of valve in valve percutaneous procedures which would avoid reoperation in bioprosthetic valve replacement.
Bioprosthetic valves themselves vary in design characteristics with variable freedoms from reoperation reported depending on the type of prosthesis.43 Calcific and structural valve deterioration are the major indications for BHV replacement.34 The stress experienced by BHV leaflets has been shown to initiate both calcific deterioration and structural failure of BHVs.33,39 Moreover, transvalvular gradients have been shown to vary with comparable valves of different types.8 Valve design as such is determinant in both durability and post-operative left ventricular function. As the indication for bioprosthetic valves increases despite the risk of reoperation, an understanding of the mechanics and hemodynamics of BHVs is essential to a critical choice amongst available prosthetic valve designs and in understanding postoperative outcomes.
The mechanics of BAVs include stress distribution, leaflet displacement and transvalvular pressure gradients which are affected by several factors. Importantly, the material properties of leaflets and supporting frame, leaflet angle (inclination of the leaflets towards the surrounding aortic wall), the height of the valve and the area of coaptation are major factors in valve mechanics and hemodynamics. There have been structural and computational fluid dynamics simulations on the effects of leaflet shape23 and valve design44 in the optimization of bioprosthetic valves.10,15 However, these studies either considered a closed valve or were limited in that they did not consider fluid structure interaction which would be more representative of dynamic cardiac function. Additionally, the hemodynamic consequences of leaflet geometry on pressure gradients could not be studied in purely structural models.
The clinical outcome of bioprosthetic valve replacement has been well documented.34 To date there has been no report explaining the role of the design parameters during the ejection phase of the left ventricle. During the ejection phase, the loads on the leaflets are a consequence of blood flow and leaflet interaction, and as such, it is a solution dependent factor, and not a constant load as applied in a structural analysis. While it is generally accepted that valve size is related to hemodynamics and mechanics, this study investigated the effects of valve design on hemodynamics and mechanics, namely leaflet angle and curvature on transvalvular gradients and leaflet stresses in dynamic fluid structure interaction models.
Methods
Geometry
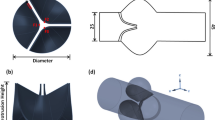
To illustrate the alteration in leaflet angle, the geometry of a typical leaflet is shown in Fig. 1. For the purpose of this study, there are five geometrical parameters that comprise the leaflet. The first parameter is the valve diameter. We assumed the diameter of the valve to be fixed at 19 mm since valve diameter is specified by the annulus size of the patient and is not as such a design parameter. The second parameter is the height of the leaflets (H 1) comprising the valve. Leaflet heights were assumed fixed at 11 mm. It should be noted that the leaflet height (H 1) and the angle of the fixed edge in respect to the vertical axis (θ 1) are mutually exclusive. The reason is that as shown in Fig. 1c, once the diameter of the valve is known, the length of the line \( {\text{AA}}^{\prime} \) will be fixed, and for a defined leaflet height, consequently the angle of the fixed edge will be fixed (Fig. 1b). The third parameter is the leaflet angle in respect to the vertical axis as shown in Fig. 1b (θ 2). This angle and the leaflet tip height (H2) are also mutually exclusive. To assess the effects of leaflet angle (θ 2), seven cases were considered with different leaflet angles. The fourth parameter is the curvature of the fixed edge. Three cases with different fixed edge curvatures were considered (1 d). The fifth parameter is the radial curvature. To assess the effects of the radial curvature, three curves were used in the radial direction (1 day). The loft command in Solid Work was used to make the surfaces of the leaflets utilizing the fixed edge, the free edge of the leaflets, and the radial curvature which was used as the guide curve. Table 1 summarizes the specifications of the 10 Cases.
Geometrical specifications of the models: (a) 3-dimensional geometry of one leaflet and three leaflets; (b) the specifications of a leaflet: θ 2 is the leaflet angle; (c) the distance between the commissures which is mutually exclusive with the valve diameter; (d) the two curves that represent the radial (left) and circumferential (right) curvatures.
Computational Modeling
Comsol Multiphysics (version 5.1, Comsol Ltd., London, UK) was used for the calculations. Comsol uses an implicit finite element package with faster solutions compared to explicit packages. The meshes in all cases were created in Pointwise (Pointwise V17.3, Pointwise Inc). Pointwise is a mesh generation software package that imports the geometry of a model and discretizes the imported geometry into computational elements (Pointwise V17.3 manual). After the meshes were created, they were exported to Comsol for finite element calculations. A two-way time-dependent three-dimensional strongly-coupled Arbitrary Lagrangian–Eulerian method fluid structure interaction analysis was conducted. The leaflets made the solid domain whereas blood was the fluid domain. In all cases, we assumed the inlet and outlet geometries as straight cylinders (surrounding aortic wall). Leaflets were assumed as isotropic linear elastic materials with Young’s modulus and Poisson’s ratio of 10 MPa and 0.4, respectively.35,45 Blood was assumed as a Newtonian incompressible fluid with the dynamics viscosity and density equal to 0.003 Pa.s and 1000 kg/m3, respectively. The leaflets fixation at the connection to aortic wall was the solid domain boundary conditions. The inlet boundary condition was a physiologically-relevant velocity wave (Fig. 2) based on recorded normal aortic flows in the literature,4 and the outlet boundary condition was a zero pressure.
Results
The pressure gradient (Fig. 3) was altered by the leaflet curvature and angle. The time of maximum leaflet displacement was not noticeably affected by the leaflet curvature and angle; however, the speed of leaflet displacement was dependent on these parameters (Figs. 4, 5). The slowest cases happened when the circumferential curvature was parabolic (Case 1-Fig. 4) and the radial curvature was parabolic (Case 4-Fig. 4). As well, the leaflets experienced asymmetric deflections in these cases (Fig. 7), although the geometry, loads and boundary conditions were symmetrical for all leaflets.
The mean and maximum values of pressure gradient are shown for the case studies. When a parabola was used for the circumferential (Case 1) or radial curvature (Cases 4), the pressure gradients were the highest. Cases 1–3 show the effects of the circumferential curvature. Cases 3, 4 and 5 show the effects of radial curvature. Cases 3, 6–10 show the effects of leaflet angle on pressure gradient. A circular circumferential curvature, a linear radial curvature, and a leaflet inclined toward the wall showed superior pressure gradients.
Displacement of the tip of one leaflet in the radial direction. Cases 1–3 show the effects of the circumferential curvature, cases 3, 4 and 5 show the effects of radial curvature, and cases 3, 6–10 show the effects of leaflet angle. The leaflets opened more slowly in Cases 1, 2, 4 and 5 where the curvatures were not linear (radial) or circular (circumferential).
The value of von Mises, first principal and third principal stresses were dependent on the leaflet angle and curvature (Fig. 6). However, the patterns of these parameters were independent of leaflet angle and curvatures (Figs. 7, 8 and 9). When the valve was fully-opened, the peak von Mises stress occurred at the vicinity of the nodulus of Aranatii, the belly and the basal attachment (Fig. 7). The peak first principal stress (Fig. 8) occurred around nodulus of Aranatii (aortic side), and the belly and basal attachment regions (ventricle side). The largest values of compressive stress (the third principal stress) occurred almost at the same locations where the first principal stress happened but occurred in an opposite fashion: within the leaflets, where the aortic side experienced highest tensile stresses, the ventricular surface experienced the highest compressive stresses, and vice versa (Figs. 8 Case1 A vs. 9 Case 1 V). The values of reaction forces were dependent on leaflet angle and curvature (Fig. 10). The reaction forces increased when a parabola or spline was used for the circumferential curvature (Cases 1 & 2) or a circular or parabolic curvature was used for the radial curvature (Cases 4 & 5). Moreover, when the leaflet angle increased, the reaction forces also increased as can be seen in a comparison of cases 3, 6, 7, 8, 9 and 10.
von Mises, first principal and third principal stresses when the valve was fully-opened (Time = 0.19 s-peak inlet flow). *The value of third principal stress was multiplied by -1. Cases 1–3 show the effects of the circumferential curvature, cases 3, 4 and 5 show the effects of radial curvature, and cases 3, 6–10 show the effects of leaflet angle. Note that in Cases 8–9, unlike the first and third principal stresses, von Mises stress increased monotonically.
The distribution of von Mises stress (MPa). The pattern of von Mises stress was similar for the all cases. The three leaflets showed similar patterns of the von Mises stress except for Cases 1 and 4 where the leaflets deformations were not symmetrical. This view is from the aortic side. Time = 0.19 s (peak inlet flow).
The distribution of the first principal stress (MPa). The pattern of first principal stress was similar for the all cases. The three leaflets showed similar patterns of the first principal stress except for Cases 1 and 4 where the leaflets deformations were not symmetrical. V stands for ventricle view, and A stands for aortic view. Time = 0.19 s (peak inlet flow).
The distribution of the third principal stress (MPa). The pattern of third principal stress was similar for the all cases. The three leaflets showed similar patterns of the third principal stress except for Cases 1 and 4 where the leaflets deformations were not symmetrical. V stands for ventricle view, and A stands for aortic view. Time = 0.19 s (peak inlet flow).
The total reaction force (N) for the three leaflets at the junction of the leaflets and the annulus, in the direction parallel to the flow (vertical to the plane in Fig. 1c). As shown, the reaction force was affected by the leaflet curvature and angle. The peaks in the reaction forces were sharper when the leaflets had a nonlinear radial curvature or a non-circular circumferential curvature (Cases 1, 2, 4 and 5).
Discussion
The effect of leaflet design on dynamic mechanics of BAVs was studied using fluid structure interaction models. According to the best of our knowledge this is the first study that, considering the complex interaction between fluid and structure, systematically examines the mechanics of BAVs for different leaflet angles and curvatures. We assumed a relatively small valve diameter (19 mm), as hemodynamics of valves with a smaller diameter is inferior compared to valves with larger diameters.17 Moreover, the higher gradients associated with the 19 mm valve is of major concern and often result in aortic root enlargement to prevent patient prosthesis mismatch.
The results confirm previous numerical and experimental reports. A computational structural study reported high compressive stresses in the vicinity of the fixed edge, in an open valve,21 which corresponds to peak third principal stresses around the fixed edge in our computations (compare Fig. 9 Cases 1–10A with Fig. 4b in Ref. 21). Examining explanted failed valves (due to structural deterioration) and valves tested in vitro by an accelerated set up, Sacks and Schoen reported that the integrity of leaflet material deteriorates most frequently within a region that extends from the nodulus of Aranatii to the basal attachments (Fig. 5 in Ref. 33). The leaflets experienced highest values of cyclic von Mises, tensile and compressive stresses in the vicinity of nodulus of Aranatii, in the belly, and in basal attachment regions, as our simulations showed (Figs. 8, 9 and 10). Since the locations of high cyclic stresses correspond to the locations of tissue degradation reported by, Ref. 33 the mechanism of buckling failure (which is caused by cyclic tensile and compressive loading41) is confirmed by our results. The calculated pressure gradients (Fig. 3) were within the range of pressure gradients reported in the literature for 19 mm prosthetic valves.37
A lower pressure gradient was not associated with all measures of stress, in all cases. The von Mises stress is a criterion to characterize yielding caused by shear stresses.19 The lowest von Mises stress happened in Case 6 which also experienced the lowest pressure gradient. As well, the highest von Mises stress happened in Case 4 which also experienced the highest pressure gradient. However, the lowest and the highest first and third principal stresses did not occur in Cases 6 and 4. Therefore, the pressure gradient and stresses do not have a straightforward relation. A computational study is necessary to examine the changes in stresses when the design parameters of a BAV change. Selection of appropriate failure criteria has a determinant role for assessment of BHVs.
Examination of the pressure gradient and stresses for valves with the same curvatures and only with different leaflet angles shows that when the leaflets are more inclined toward the surrounding aortic wall, pressure gradient decreases and so does the von Mises stress (Figs. 3, 6, Cases 3, 6–10). An explanation for this result can be found by examining the leaflet displacements and reaction forces (Figs. 5, 10). When the leaflets are inclined toward the surrounding aortic wall, they encounter a lower resistance during opening, which is represented in lower reaction forces (Fig. 10, Cases 6–10).
The curvature of the attachment (circumferential curvature) had a profound role in valve mechanics (Fig. 6). When the radial curvature was not linear, or when the circumferential curvature was not circular, high stresses happened (Cases 1, 2, 4 and 5 vs Cases 3, 6–9). When the nonlinear radial curvature was used (Cases 4 & 5), the stresses were higher compare to other cases. Note that in Case 10 leaflets highly deviated from the surrounding aortic wall, which might explain why in Case 10 stresses were comparable to Cases 4 & 5.
Effects of leaflet curvature on leaflets dynamics were apparent in Cases 1 & 4 in which slow opening and closing were seen (Fig. 4). The native aortic valve closes and opens so fast that the time interval between the fully-opened and the fully-closed configurations is much shorter in comparison to the duration of the fully-opened and fully-closed configurations.38 Cases 3, 6–10 showed a similar pattern in leaflet opening and closing, but leaflet dynamics in cases 1, 2, 4 and 5 was not similar to native valve. The slowest opening and closing happened when a parabola was used for either the circumferential (Case 1) or radial curvature (Case 4) (Fig. 4). Interestingly, when a spline was used for the circumferential curvature (Case 2), the leaflet opening was the highest. Yet, Case 2 showed neither low pressure gradient nor low stresses compared to some other cases with circular circumferential curvature. The relation between leaflets opening, pressure gradient and stresses is not straightforward. A design with larger opening might have higher pressure gradient and/or stresses. A design with linear radial and/or circular circumferential curvature will better emulate the native valve.
As well, the curvature of the leaflets caused asymmetry in leaflets displacement in some of the cases (more apparent in Cases 1 & 4 in Fig. 7). The effects of circumferential and radial curvature on the asymmetry in leaflets displacements could be attenuated or amplified by other parameters such as leaflet angle. The explanation for asymmetry in leaflets displacement might be as follows: when the leaflets open fast enough, the blood flow does not generate asymmetric vortexes, and it flows in the axial direction. When the leaflets do not open fast enough (as can be seen in Fig. 4, Cases 1 & 4), the blood generates asymmetric vortexes. The leaflets deform in an asymmetric way as a result of the asymmetric vortexes. In other words, the speed of leaflet opening has a key role in blood flow pattern and the leaflets dynamics. In Cases 1 & 4, the resistance against leaflet displacement is so high (Fig. 10) that the leaflets open slowly (Fig. 4), and as a result of flow and structure interaction, the flow pattern causes asymmetric leaflets displacement. This result also clarifies why a merely structural analysis is incapable of reproducing some crucial aspects of valve dynamics.
Mercer et al. measured the dimensions of the native aortic valve leaflets, and concluded that the basal attachment can be considered as a parabola.28 Their report implies that a parabolic basal attachment is an optimal shape for the leaflets. However, their conclusion is not in agreement with our results, as our simulations showed that a circular basal attachment is superior compared to a parabolic shape. An explanation to this discrepancy could be as follows: when the native valve is closed the basal attachment could have a parabolic shape; however, the basal attachment shape becomes an arc (circular shape) when the valve is fully-opened. Experimental measurement of the basal attachment dimensions when the valve is fully-opened is necessary to justify our explanation.
This study is the first report on examining effects of leaflet angle and curvature on the dynamics of BAVs with fluid structure interaction. We used a strongly-coupled Arbitrary Lagrangian–Eulerian method fluid structure interaction analysis which has been validated in the literature.1,14,40 Fluid–structure interaction analysis is complicated18 because of factors such as the 3-dimensional complex leaflets geometry, transient fluid and structural parameters, and interaction of blood flow and leaflets motion.25,30 There are many studies on dynamic structural modeling of BAV but only the leaflets were modeled not the interaction between leaflets and blood flow.11,16,44 These studies failed to consider realistic loads and boundary conditions applied on the leaflets by blood flow. Axisymmetric and two dimensional fluid structure interaction models have been used to analyze mechanical as well as bioprosthetic heart valves.2,22,31,32 These models, however, fail to consider the asymmetry of the blood flow. Nicosta et al. developed a 3-dimensional fluid structure interaction model of heart valves using explicit computations,29 which might require more computational cost in terms of time and convergence of the solution. In a study that aims to compare several case studies, computational efficiency becomes particularly important.
One of the limitations of this study was that it did not consider coaptation between leaflets. As a result of coaptation, the contacts between leaflets play an important role in the dynamics of the leaflets. The motions of a leaflet could encounter a resistive force applied by adjacent leaflets, which is caused by the contacts between leaflets. Modeling the load-bearing portions of the leaflets, this study still provides important insights regarding the effects of leaflet angle and curvature on the BAV dynamics. We are working on including coaptation in our modeling methodology.
Leaflets were assumed as linear elastic materials.12,26,44 This assumption can affect the stress distribution in the leaflets which have orthotropic material behavior with nonlinear constitutive equations. However, this assumption could be justified for the goals of this project. Our primary goal was to provide a computational platform to examine the effects of geometrical parameters on pressure gradient of BAVs. Using the same material is rationalized as long as the effects of geometrical parameters are the goal of study.44
The sinus of Valsalva was not considered in this study. Based on our goal, the surrounding aortic geometry was assumed to be a cylinder, which had the same role in valve mechanics for all case studies. Using a cylinder was preferred due to computational efficiency. Assuming the surrounding geometry as a cylinder has also been implemented in the literature.10 It should be noted that the modeling framework presented in this paper is capable of considering the sinus of Valsalva.
We assumed the inlet and outlet cylinders to be rigid, which means the pulse propagated within them instantaneously. Our goal was to study the roles of valve parameters in valve mechanics. Since the same inlet and outlet cylinders were used for all cases, these cylinders were assumed to be rigid for computational efficiency. This assumption, which also has been used in the literature,6,10,30 is justified based on the goal of this study.
Since we aimed to study the pressure gradient (not the exact values of pressures), the outlet boundary condition was set to a zero pressure, based on the literature.9,10,20,42 This zero pressure outlet boundary condition was associated with better computational efficiency. The reason is that a realistic outlet pressure would require a high inlet pressure to achieve the same blood flow. Consequently, excessive element distortions would happen, due to too high stresses within the leaflets. Lacking a realistic outlet boundary condition does not affect the goal of this study which was studying effects of geometrical parameters on the mechanics of aortic valves because the same boundary conditions were used for all case studies. The values of stresses within the leaflets would be higher if realistic outlet boundary conditions were used.
Blood was assumed as a Newtonian fluid because in large arteries blood has a Newtonian behavior.3,7,24 As well, this assumption has been used in the literature for modeling blood flow through heart valves.2,5,6,13
This study investigated the effects of leaflet curvatures and angle on the mechanics of BAVs. Transvalvular pressure gradient and leaflet stresses were analyzed for different curvatures and leaflet angles. A circular circumferential curvature, a linear radial curvature and more leaflet inclination toward the surrounding aortic wall provided superior valve mechanics. Results could lead to better understanding of the mechanics, the failure mechanisms, and better designs of BAVs.
References
Al-Atabi, M., D. M. Espino, and D. W. L. Hukins. Computer and experimental modelling of blood flow through the mitral valve of the heart. J. Biomech. Sci. Eng. 5(1):78–84, 2010.
Bahraseman, H. G., K. Hassani, A. Khosravi, M. Navidbakhsh, D. M. Espino, D. Kazemi-Saleh, et al. Estimation of maximum intraventricular pressure: a three-dimensional fluid-structure interaction model. Biomed. Eng. Online 22:12, 2013.
Barbee, J. H., and G. R. Cokelet. Prediction of blood flow in tubes with diameters as small as 29 microns. Microvasc. Res. 3(1):17–21, 1971.
Bogren, H. G., R. H. Klipstein, D. N. Firmin, R. H. Mohiaddin, S. R. Underwood, R. S. Rees, and D. B. Longmore. Quantitation of antegrade and retrograde blood flow in the human aorta by magnetic resonance velocity mapping. Am. Heart J. 117(6):1214–1222, 1989.
Bonomi, D., C. Vergara, E. Faggiano, M. Stevanella, C. Conti, A. Redaelli, G. Puppini, G. Faggian, L. Formaggia, and G. B. Luciani. Influence of the aortic valve leaflets on the fluid-dynamics in aorta in presence of a normally functioning bicuspid valve. Biomech. Model. Mechanobiol. 14(6):1349–1361, 2015.
Borazjani, I., and F. Sotiropoulos. The effect of implantation orientation of a bileaflet mechanical heart valve on kinematics and hemodynamics in an anatomic aorta. J. Biomech. Eng. 132(11):111005, 2010.
Caro, C. G., T. J. Pedley, R. C. Schroter, and W. A. Seed. The Mechanics of the Circulation. Oxford: Oxford University Press, 1978.
Cevasco, M., S. L. Mick, M. Kwon, L. S. Lee, E. P. Chen, and F. Y. Chen. True external diameter better predicts hemodynamic performance of bioprosthetic aortic valves than the manufacturers’ stated size. J. Heart Valve Dis. 22(3):377–382, 2013.
Choi, C. R., and C. N. Kim. Numerical analysis on the hemodynamics and leaflet dynamics in a bileaflet mechanical heart valve using a fluid-structure interaction method. ASAIO J. 55(5):428–437, 2009.
Claiborne, T. E., M. Xenos, J. Sheriff, W. C. Chiu, J. Soares, Y. Alemu, S. Gupta, S. Judex, M. J. Slepian, and D. Bluestein. Toward optimization of a novel trileaflet polymeric prosthetic heart valve via device thrombogenicity emulation. ASAIO J. 59(3):275–283, 2013.
Conti, C. A., E. Votta, A. Della Corte, L. Del Viscovo, C. Bancone, M. Cotrufo, and A. Redaelli. Dynamic finite element analysis of the aortic root from MRI-derived parameters. Med. Eng. Phys. 32(2):212–221, 2010.
Croft, L. R., and R. K. Mofrad. Computational modeling of aortic heart valves. In: Computational Modeling in Biomechanics, edited by S. De, F. Guilak, and R. K. Mofrad. Dordrecht: Springer Science + Business Media B.V., 2010, pp. 221–252.
De Hart, J., G. W. Peters, P. J. Schruers, and F. P. Baaijens. A two-dimensional fluid–structure interaction model of the aortic valve. J. Biomech. 33(9):1079–1088, 2000.
Espino, D. M., D. E. Shepherd, and D. W. Hukins. Evaluation of a transient, simultaneous, arbitrary Lagrange-Euler based multi-physics method for simulating the mitral heart valve. Comput. Methods Biomech. Biomed. Engin. 17(4):450–458, 2014.
Fan, R., A. S. Bayoumi, P. Chen, C. M. Hobson, W. R. Wagner, J. E. Mayer, Jr, and M. S. Sacks. Optimal elastomeric scaffold leaflet shape for pulmonary heart valve leaflet replacement. J. Biomech. 46(4):662–669, 2013.
Gnyaneshwar, R., R. K. Kumar, and K. R. Balakrishnan. Dynamic analysis of the aortic valve using a finite element model. Ann. Thorac. Surg. 73(4):1122–1129, 2002.
González-Juanatey, J. R., J. M. García-Acuña, M. Vega Fernandez, A. Amaro Cendón, V. Castelo Fuentes, J. B. García-Bengoechea, and M. G. de la Peña. Influence of the size of aortic valve prostheses on hemodynamics and change in left ventricular mass: implications for the surgical management of aortic stenosis. J. Thorac. Cardiovasc. Surg. 112(2):273–280, 1996.
Guivier-Curien, C., V. Deplano, and E. Bertrand. Validation of a numerical 3-D fluid-structure interaction model for a prosthetic valve based on experimental PIV measurements. Med. Eng. Phys. 31(8):986–993, 2009.
Humphrey, J. D., and G. A. Holzapfel. Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J. Biomech. 45(5):805–814, 2012.
Kim, H., J. Lu, M. S. Sacks, and K. B. Chandran. Dynamic simulation of bioprosthetic heart valves using a stress resultant shell model. Ann. Biomed. Eng. 36(2):262–275, 2008.
Krucinski, S., I. Vesely, M. A. Dokainish, and G. Campbell. Numerical simulation of leaflet flexure in bioprosthetic valves mounted on rigid and expansile stents. J. Biomech. 26(8):929–943, 1993.
Lai, Y. G., K. B. Chandran, and J. Lemmon. A numerical simulation of mechanical heart valve closure fluid dynamics. J. Biomech. 35(7):881–892, 2002.
Lim, K. H., J. Candra, J. H. Yeo, and C. M. Duran. Flat or curved pericardial aortic valve cusps: a finite element study. J. Heart Valve Dis. 13(5):792–797, 2004.
Lipowsky, H. H., S. Usami, and S. Chien. In viva measurements of “apparent viscosity” and microvessel hematocrit in the mesentery of the cat. Microvasc. Res. 19:297–319, 1980.
Makhijani, V. B., H. Q. Yang, P. J. Dionne, and M. J. Thubrikar. Three-dimensional coupled fluid-structure simulation of pericardial bioprosthetic aortic valve function. ASAIO J. 43(5):M387–M392, 1997.
Marom, G., R. Haj-Ali, E. Raanani, H. J. Schäfers, and M. Rosenfeld. A fluid-structure interaction model of the aortic valve with coaptation and compliant aortic root. Med. Biol. Eng. Comput. 50(2):173–182, 2012.
McClure, R. S., S. McGurk, M. Cevasco, A. Maloney, I. Gosev, E. M. Wiegerinck, G. Salvio, G. Tokmaji, W. Borstlap, F. Nauta, and L. H. Cohn. Late outcomes comparison of nonelderly patients with stented bioprosthetic and mechanical valves in the aortic position: a propensity-matched analysis. J. Thorac. Cardiovasc. Surg. 148(5):1931–1939, 2014.
Mercer, J. L., M. Benedicty, and H. T. Bahnson. The geometry and construction of the aortic leaflet. J. Thorac. Cardiovasc. Surg. 65(4):511–518, 1973.
Nicosia, M. A., R. P. Cochran, D. R. Einstein, C. J. Rutland, and K. S. Kunzelman. A coupled fluid-structure finite element model of the aortic valve and root. J. Heart Valve Dis. 12(6):781–789, 2003.
Nobili, M., U. Morbiducci, R. Ponzini, C. Del Gaudio, A. Balducci, M. Grigioni, et al. Numerical simulation of the dynamics of a bileaflet prosthetic heart valve using a fluid-structure interaction approach. J. Biomech. 41(11):2539–2550, 2008.
Pedrizzetti, G., and F. Domenichini. Asymmetric opening of a simple bileaflet valve. Phys. Rev. Lett. 98(21):214503, 2007.
Pelliccioni, O., M. Cerrolaza, and M. Herrera. Lattice Boltzmann dynamic simulation of a mechanical heart valve device. Math. Comput. Simul. 75(1–2):1–14, 2007.
Sacks, M. S., and F. J. Schoen. Collagen fiber disruption occurs independent of calcification in clinically explanted bioprosthetic heart valves. J. Biomed. Mater. Res. 62(3):359–371, 2002.
Schoen, F. J., and R. J. Levy. Tissue heart valves: current challenges and future research perspectives. J. Biomed. Mater. Res. 47(4):439–465, 1999.
Singh, R., J. A. Strom, O. Leo, B. Joseph, and D. VanAuker Michae. Age-related changes in the aortic valve affect leaflet stress distributions: implications for aortic valve degeneration. J. Heart Valve Dis. 17:290–299, 2008.
Stassano, P., L. Di Tommaso, M. Monaco, F. Iorio, P. Pepino, N. Spampinato, and C. Vosa. Aortic valve replacement: a prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. J. Am. Coll. Cardiol. 54(20):1862–1868, 2009.
Takakura, H., T. Sasaki, K. Hashimoto, T. Hachiya, K. Onoguchi, M. Oshiumi, and S. Takeuchi. Hemodynamic evaluation of 19-mm Carpentier-Edwards pericardial bioprosthesis in aortic position. Ann. Thorac. Surg. 71(2):609–613, 2001.
Thubrikar, M. The Aortic Valve. Boca Raton: CRC Press, pp. 1–20, 1993.
Thubrikar, M. J., J. D. Deck, J. Aouad, and S. P. Nolan. Role of mechanical stress in calcification of aortic bioprosthetic valves. J. Thorac. Cardiovasc. Surg. 86(1):115–125, 1983.
Van Loon, R., P. D. Anderson, F. N. Van de Vosse, and S. J. Sherwin. Comparison of various fluid-structure interaction methods for deformable bodies. Comput. Struct. 85:833–843, 2007.
Vesely, I., D. Boughner, and T. Song. Tissue buckling as a mechanism of bioprosthetic valve failure. Ann. Thorac. Surg. 46(3):302–308, 1988.
Vigmostad, S., and H. S. Udaykumar. Algorithms for fluid-structure interaction. In: Imaged-based Computational Modeling of the Human Circulatory and Pulmonary Systems Methods and Applications, edited by K. B. Chandran, H. S. Udaykumar, and J. M. Reinhardt. Dordrecht: Springer, 2011, pp. 191–234.
Wu, J. J., M. Seco, J. B. Edelman, G. D. Eslick, M. K. Wilson, M. P. Vallely, M. J. Byrom, P. G. Bannon. Mechanical vs. bioprosthetic aortic valve replacement in patients aged 40 to 70 Years: a systematic review and meta-analysis. Ann. Thorac. Surg. 2015. In Press.
Xiong, F. L., W. A. Goetz, C. K. Chong, Y. L. Chua, S. Pfeifer, E. Wintermantel, and J. H. Yeo. Finite element investigation of stentless pericardial aortic valves: relevance of leaflet geometry. Ann. Biomed. Eng. 38(5):1908–1918, 2010.
Zioupos, P., J. C. Barbenel, and J. Fisher. Anisotropic elasticity and strength of glutaraldehyde fixed bovine pericardium for use in pericardial bioprosthetic valves. J. Biomed. Mater. Res. 28:49–57, 1994.
Acknowledgments
The authors would like to acknowledge Dr. Todd Anderson, Head of The Libin Cardiovascular Institute, University of Calgary for his support and mentorship in realizing this work. We also acknowledge the contributions and helpful comments of Professor Dr. Patrick Segers, Director, Institute of Biomedical Technology, Ghent University Hospital, Ghent, Belgium. This study was supported by Zymetrix center at the University of Calgary.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editors Karyn Kunzelman and Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Dabiri, Y., Ronsky, J., Ali, I. et al. Effects of Leaflet Design on Transvalvular Gradients of Bioprosthetic Heart Valves. Cardiovasc Eng Tech 7, 363–373 (2016). https://doi.org/10.1007/s13239-016-0279-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-016-0279-5