Abstract
The spatial organization of polytene chromosomes and the association of their pericentromeric regions in the Drosophila melanogaster nurse cells during oogenesis have been examined by 3D-immunofluorescence microscopy. All nurse cell chromosomes are shown to contact the nuclear membrane with their pericentromeric regions, and the X chromosome additionally with its telomeric region. Three morphological types of the associations of the nurse cell chromosome pericentromeric regions in the nuclear space are observed: (1) the pericentromeric regions of chromosomes 2, 3, and 4 contact the nuclear membrane at one pole of the nucleus, while the pericentromeric region of the X chromosome does so at the other pole (morphotype I); (2) the pericentromeric regions of chromosomes X, 2, and 3 are separated from each other in the nuclear space and contact the nuclear membrane (morphotype II); and (3) the pericentromeric region of chromosome 2 contacts the nuclear membrane at one pole of the nucleus, while the pericentromeric regions of chromosomes X, 3, and 4 contact the membrane at the other pole (morphotype III). The author, therefore, proposes that the nurse cell nuclei with morphotype I are prevalent at the early stages of oogenesis, while the nurse cell nuclei with morphotype III are most abundant at the late stages. The dynamics of associations of the pericentromeric chromosome regions in the nuclear space of D. melanogaster ovarian nurse cells in the oogenesis demonstrated that there may be some functional relationship between the 3-D organization of the nurse cell chromosomes and the organization of the nucleolus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spatial organization of the interphase nucleus is an actively studied area in genetics that is of paramount importance for obtaining insight into the role of nuclear architecture in the epigenetic control of gene expression [5, 11, 24, 30, 46, 56, 61]. The nuclear architecture and the mechanisms underlying the interaction of chromosomes with the nuclear membrane have been examined in vertebrates, insects, humans, and plants [13, 22, 23, 27, 29, 51, 53]. However, the mechanisms involved in the regulation of the genome function at the level of spatial organization of the nucleus are still not clear and represent one of the major problem areas in modern genetics [6, 11, 12]. An insight into the principles determining the arrangement of genetic material within the nuclear space (the principles specifying the organization of chromosome territories) is of special importance since this, in many respects, determines its activity and transcriptional status. It is already known that defects in genome organization and nuclear architecture are responsible for a number of diseases, such as neurodegenerative disorders and muscular dystrophies; in addition, their relation to human aging has recently been shown [8, 36, 47].
Heterochromatin plays an important role in the chromosome spatial organization in the nucleus by forming associations of pericentromeric, intercalary, and telomeric chromosome regions with one another, and contacts of these chromosome regions with the nuclear membrane [37, 42, 51, 54, 55, 57]. These interchromosomal and chromosome-membrane interactions are also supported by various nuclear protein structures, such as the nuclear matrix and lamina, providing the orderliness of chromosomes and their territoriality in the nuclear space [7, 10, 11, 25, 26, 49]. On the one hand, the interchromosomal interactions based on ectopic conjugation (in particular the association of chromosomes into the chromocenter) and the chromosome contact with the nuclear membrane create certain architecture of the nucleus; on the other hand changes in the organization of transcriptionally active regions in chromosomes change their location in the nuclear space. It is not only the dynamics of certain chromosome regions within their own territory that are observed in the transcriptionally active interphase nuclei, but also the migration of chromosomes from the nuclear membrane to the center of the nucleus [14, 16,17,18, 39, 41, 62, 63]. In this process, the chromosomes residing in the central part of the nucleus become transcriptionally active, and those at the periphery, transcriptionally inactive. The nucleolus is also known to determine the spatial organization of chromosomes; its active function can change the chromosome location [9]. In particular, the nucleolus-organizing chromosome in Calliphora erythrocephala nurse cells migrates from the central part of the nucleus to its periphery with an increase in polytenization [34].
The Dipteran ovarian nurse cells with polytene chromosomes are a unique model of the interphase nuclei and readily lend themselves to the study of interchromosomal associations and chromosome-membrane interactions in the nuclear space [28, 40, 51, 55]. A specific feature of the formation of polytene chromosomes in the nuclei of ovarian nurse cells in fruit flies is the buildup (endocycle stages S3–S4) of compact polytene chromosomes without band pattern. In the early follicles, thin elongated chromosomes (S2) start to take shape, followed by an increase in polytenization in more mature follicles and the formation of compact, poorly banded chromosomes (S3–S4). At the later stages of oogenesis, blob-like chromosomes are formed to further decompact and give large endopolyploid nuclei with a reticular structure (S8) [1, 19]. The chromatin in nurse cell nuclei is also transcriptionally active on the background of an increase in polytenization; most likely, certain transcriptionally active chromosome regions– or the whole chromosomes–dynamically change in the nuclear space, similar to the interphase diploid nuclei [32, 33, 35]. The data on how the spatial polytene chromosome organization is formed in the nurse cell nuclei and to what degree their organization is dynamic in Diptera are so far inconclusive.
We have previously demonstrated that the species-specificity of D. melanogaster nurse cell nuclear architecture resides in the facts that the chromocenter is absent and the chromosome pericentromeric regions contact the nuclear membrane. The following pattern in the association of nurse cell chromosome pericentromeric regions has been frequently observed: the pericentromeric regions of chromosomes X, 3, and 4 reside at one pole of the nucleus, while the pericentromeric region of chromosome 2 is located at the other pole [59]. However, we have not studied in detail the associations of the pericentromeric regions in the D. melanogaster nurse cell chromosomes during oogenesis. It should be emphasized that we have obtained the above described results by microscopic analysis of semisquash preparations of D. melanogaster nurse cell polytene chromosomes stained with lacto-aceto-orcein. In this variant, the nurse cell nuclei become flattened, despite retaining their integrity. As such, the comprehensive study of the spatial organization of chromosomes and the association of their pericentromeric regions in the D. melanogaster nurse cell nuclei during oogenesis was somewhat problematic. Correspondingly, the goal of our work was to analyze the 3D arrangement of the chromosomes in the D. melanogaster nurse cells with the progression of oogenesis.
Materials and methods
The ovarian nurse cells of the female D. melanogaster laboratory strain Canton’S at an age of 24–36 h after hatching from the puparium was the analyzed material.
3D immunofluorescence localization of the antibodies to the proteins lamin Dm0, HP1, and fibrillarin in the intact D. melanogaster ovarian nurse cell nuclei
The D. melanogaster ovaries were separated in EBR (Ephrussi Beadle Ringer) solution (130 mM NaCl, 5 mM KCl, 2 mM CaCl2, and 10 mM HEPES pH 6.9) at 0 °C into individual ovarioles and fixed with 100 µL devitelinizing buffer (6% formaldehyde, 16.7 mM KH2PO4/K2HPO4 pH 6.8, 75 mM KCl, 25 mMNaCl, and 3.3 mM MgCl2) and 600 µL heptane for 10 min with gentle stirring. The further stages of immunostaining were performed according to the standard protocol [60] using the following primary antibodies: (1) monoclonal mouse anti-lamin Dm0 antibodies (DSHB, United States), used at a dilution of 1:200; (2) monoclonal rabbit anti-HP1 antibodies (DSHB, United States), used at a dilution of 1:350; and (3) monoclonal mouse anti-fibrillarin antibodies (Abcam, United States), used at a dilution of 1:300. After all treatments, the ovarioles were placed on a glass slide into a chamber filled with DAPI-Vectashield (Vector Laboratories, Inc., Germany). This process of preparation avoids cell deformation which allows for a microscopic analysis of the intact nuclei. The preparations were examined using an AxioImager Z1 luminescent microscope equipped with an ApoTome module (Carl Zeiss, Germany), which facilitates the obtaining of optical sections with a high resolution; a digital CCD camera AxioCamMRm; and AxioVision Rel. 4.7 software (Carl Zeiss, Germany). The 3D model of D. melanogaster ovariole was obtained by reconstructing 40 optical sections; 20–25 optical sections were used to construct the model of a nurse cell nucleus. As a result, the spatial organization of polytene chromosomes was analyzed in 595 D. melanogaster nurse cell nuclei.
The data were quantified using MS Excel 2010.
Results and discussion
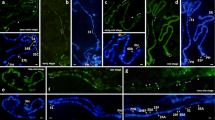
Performing 3D immunofluorescence-based localization of the antibodies to lamin Dm0 and HP1 in the intact D. melanogaster nurse cell nuclei allowed us to visualize the nuclear membrane and the pericentromeric regions of all chromosomes (Fig. 1). We examined 11 D. melanogaster ovarioles and analyzed three follicles in each (Fig. 1a). It is known that each follicle comprises one oocyte and 15 nurse cells, which carry polytene chromosomes at endocycle stages S2–S5 [1, 19]. During these stages, identifiable [57, 58] polytene chromosomes with different degrees of polytenization [19] are formed in the nurse cell nuclei of the three analyzed D. melanogaster follicles. In this work, we have shown that the pericentromeric regions of all chromosomes as well as the X chromosome telomeric region contact the nuclear membrane (Fig. 1). We have also discovered that the polytene chromosomes differing in their morphology (thin elongated chromosomes, poor banded compact chromosomes, and blob-like chromosomes) display differences in the associations of pericentromeric regions. Examination of the spatial organization of the nurse cell nuclei from three follicles in ovarioles demonstrates different variants of the associations of polytene chromosome pericentromeric regions at different stages of oogenesis. Thus, three morphotypes of the associations of chromosome pericentromeric regions within the nurse cell nuclear space have been distinguished: morphotype I is represented by the pattern wherein the pericentromeric regions of chromosomes 2, 3, and 4 contact the nuclear membrane at one pole of the nucleus, while the pericentromeric region of the X chromosome does so at the other pole (Fig. 1b, b′);in morphotype II, the pericentromeric regions of chromosomes X, 2, and 3 are separated from each other in the nuclear space and contact the nuclear membrane (Fig. 1c, c′); and in morphotype III, the pericentromeric region of chromosome 2 contacts the nuclear membrane at one pole of the nucleus, while the pericentromeric regions of chromosomes X, 3, and 4 contact the membrane at the other pole (Fig. 1d, d′). All three morphotypes of the spatial polytene chromosome organization in nurse cell nuclei were observed in the three examined follicles (S2–S5) of each ovariole (Fig. 1a).
3D orientation of the chromosomes in ovarian nurse cell nuclei at different stages of Drosophila melanogaster oogenesis. a A fragment of ovariole; b, b′, c, c′, d, d′ 3D models of ovarian nurse cell nuclei; red, DAPI stained chromosomes; green, immunofluorescence localization of anti-HP1 antibodies; yellow, immunofluorescence localization of anti-lamin Dm0; X, 2, 3, 4 are the corresponding chromosomes; c, pericentromeric regions of polytene chromosomes; t, telomeric region of the X chromosome. Scale bar = 10 µm (color figure online)
The frequencies of these three morphotypes of the associations of polytene chromosome pericentromeric regions in the nurse cell nuclei were quantitatively analyzed in each follicle of ovarioles (Fig. 2).
Frequencies of three morphological types of associations between pericentromeric regions of polytene chromosomes in the nurse cell nuclei of D. melanogaster ovarian follicles. a Morphotype I; b morphotype II; c morphotype III; vertical lines in histogram denote the standard error of the proportion; 1—the first follicle of ovariole; 2—second follicle of ovariole; 3—third follicle of ovariole
The frequency of the nurse cell nuclei displaying morphotype I of the chromosome pericentromeric region associations in the first follicle (S2) of ovarioles was 65.0 ± 3.7%; in the next follicle (S3), these nuclei were less abundant as compared with the first follicle, accounting for 40.0 ± 3.8%; and their rate decreased to 24.0 ± 3.3% in the third follicle (S4–5) (Fig. 2).
The nurse cell nuclei with morphotype II in the first follicle of the ovarioles accounted for 28.0 ± 3.5% their rate increased to 41.0 ± 3.8% in the second follicle but somewhat decreased (34.0 ± 3.7%) in the third follicle as compared with the second one (Fig. 2).
The nurse cell nuclei of morphotype III in the first follicle of ovarioles were observed at a rate of 7.0 ± 2.0%, increasing to 19.0 ± 3.0% in the second follicle and to 42.0 ± 3.8% in the third one, exceeding the rates observed in the first and second follicles (Fig. 2).
Correspondingly, the nurse cell nuclei of morphotype I are the most abundant in the first follicle (65.0 ± 3.7%), which is characteristic of the early stages of chromosome polytenization. The nuclei displaying morphotype II are the most abundant (41.0 ± 3.8%) in the second follicle, while the nuclei with morphotype III are most numerous in the third follicle, accounting for 42.0 ± 3.8% (Fig. 2).
Thus, the dynamics of the associations between pericentromeric chromosome regions in the nuclear space are observed with the maturation of follicles in ovarioles and the increase in polytenization in the nurse cell nuclei. At the early stages of polytenization of nurse cell chromosomes, the pericentromeric regions of autosomes reside at one pole of the nucleus, while the X chromosome is localized to the opposite pole of the nucleus (Fig. 3a). With an increase in polytenization, the pericentromeric regions of chromosomes separate from each other (Fig. 3b); at later stages of polytenization, the pericentromeric region of chromosome 2 resides at one pole of the nucleus and the pericentromeric region of chromosomes 3, 4, and X, at the opposite pole (Fig. 3c). Presumably, the pericentromeric region of chromosomes 3 and 4 migrate from the pericentromeric region of chromosome 2 to the X chromosome pericentromeric region. Since it is shown that the X chromosome is, over all stages of oogenesis fixed in the nuclear space—being bound to the nuclear membrane not only with its pericentromeric region, but also with its telomeric one—it is likely that this chromosome markedly migrates in the nuclear space and retains its territorial position. We have shown in our early studies that the homologs disjoin and unwind into numerous fibers, rather “stably” bound to the nuclear membrane, in the pericentromeric region of chromosome arm 2Rin D. melanogaster nurse cells [50]. This suggests that the pericentromeric region of chromosome 2 as well as that of chromosome X does not markedly migrate within the nuclear space. It is also known that the pericentromeric region in chromosome 3 has a thin chromatin cord common with the pericentromeric region of chromosome 4 that contacts the nuclear membrane [50]. Presumably, this chromatin cord does not interfere with the changes in spatial orientation of the chromosome 3 pericentromeric region in the nurse cell nuclei during oogenesis. Based on our observations, it is reasonable to consider that only chromosome 3 would be able to migrate within the nuclear space during all stages of oogenesis so that the three morphotypes described above are observable (Fig. 3).
Scheme of the dynamics of the associations pericentromeric regions of polytene chromosomes in the nurse cell nuclear space during D. melanogaster oogenesis. a Morphotype I of nurse cell nuclei; b morphotype II of nurse cell nuclei; c morphotype III of nurse cell nuclei; c—pericentromeric regions; X, 2, 3, 4—the corresponding chromosomes; t—telomeric region of the X chromosome
Conceivably, these dynamics of the spatial arrangement of pericentromeric regions of chromosomes 3 and 4 in the nurse cell nuclei during polytenization are directly associated with the functional role of nurse cells in the oogenesis. A known characteristic of the nutrimentary egg development, taking place in Diptera, is that nurse cells take on the function of synthesis of the main bulk of ribosomal RNA necessary for oocyte development, whereas the oocyte is almost inactive in this respect; correspondingly, the nucleoli actively function in the nurse cells [1]. The nucleolus is rapidly growing in the Drosophila nurse cells during oogenesis [15]. In general, it is known that the intranuclear compartment, the nucleolus, plays an important role in the spatial chromatin organization in the interphase nucleus [9]. In particular, the migration of chromatin domains may, in some cases be associated with the work of the nucleolus [17, 34]. Our early studies of the nurse cell nuclear architecture in some D. melanogaster species detected—with the help of Ag staining—two nucleoli, one of which was directly connected with the pericentromeric region of the nucleolus-organizing X chromosome and the other one, with the pericentromeric regions of chromosomes 3 and 4 [52, 59]. Presumably, the formation of nucleoli between the pericentromeric regions of chromosomes X, 3, and 4 can explain the association of pericentromeric regions of these chromosomes at one pole of the nucleus, observed at later stages of polytenization. In order to test this assumption, we conducted immunofluorescence staining of the nucleolus using the antibodies to the protein fibrillarin in the intact nuclei of D. melanogaster ovarian nurse cells (Fig. 4). As a result, we examined the formation of the nucleolus as a putative factor underlying the dynamics of spatial association of the pericentromeric regions of D. melanogaster nurse cell polytene chromosomes at different stages of oogenesis (Fig. 4). Thus, we have studied the formation of the nucleolus in the nurse cell nuclei displaying three different morphological types of spatial association between chromosome pericentromeric regions. It has been shown that the nuclei with morphotype I form one nucleolus, which contacts the pericentromeric region of the nucleolus-organizing X chromosome (Fig. 4a, a′, a″). The nurse cell nuclei with morphotype II, in addition to the nucleolus contacting the X chromosome, display a distinct second nucleolus, which contacts the pericentromeric regions of chromosomes 3 and 4 (Fig. 4b, b′, b″). As for the nurse cell nuclei with morphotype III, the amount of nuclear material there was considerably increased and two nucleoli fused into a single large nucleolus (Fig. 4c, c′, c″).
Formation of the nucleoli in the nurse cell nuclei with different morphotypes of the associations pericentromeric regions of polytene chromosomes at different stages of D. melanogaster oogenesis. a, a′, a″ Morphotype I of nurse cell nuclei; b, b′, b″ morphotype II of nurse cell nuclei; c, c′, c″ morphotype III of nurse cell nuclei; green, the nucleolus stained with anti-fibrillarin antibodies; X, 2, 3, 4—the corresponding chromosomes; c—pericentromeric regions; N1—first nucleolus; N2—second nucleolus; N—nucleolus. Scale bar = 10 µm
Thus, it is demonstrated that the nurse cell nuclei at the early stages of oogenesis develop one nucleolus in the region of the nucleolus-organizing X chromosome, which resides separately from the pericentromeric regions of chromosomes 2, 3, and 4 in the nuclear space (Fig. 5a). The second large nucleolus, tightly contacting the pericentromeric regions of chromosomes 3 and 4, is formed at the subsequent stages of oogenesis, when the pericentromeric regions of chromosomes 2, 3, and X are distant in the nuclear space (Fig. 5b). At the later stages of oogenesis when the pericentromeric regions of chromosomes X and 3 are in close proximity to one another in the nuclear space, the nucleoli formed by these chromosomes fuse into a single large nucleolus (Fig. 5c). In this process, chromosomes 3 and 4 migrate in the nuclear space towards the X chromosome, since this chromosome was demonstrated to be fixed in the nuclear space owing to its “stable” attachment to the nuclear membrane, whereas chromosome 3 in this sense is rather free. In this context, it is appropriate to refer to the study that has shown that the nucleolus-organizing chromosome 6 of the Calliphora erythrocephala ovarian nurse cells experiences large-scale migrations in the nuclear space from its central part to the periphery with the progression of polytenization [35].
Scheme of formation of the nucleoli with changes in the spatial of the association pericentromeric regions of polytene chromosomes of the nurse cell nuclei during D. melanogaster oogenesis. a Morphotype I of nurse cell nuclei; b morphotype II of nurse cell nuclei; c morphotype III of nurse cell nuclei; X, 2, 3, 4—the corresponding chromosomes; c—pericentromeric regions; t—telomeric region of the X chromosome; N1—first nucleolus; N2—second nucleolus; N—nucleolus
Thus, this work shows that chromosomes 3 and 4 in the D. melanogaster nurse cell nuclei are—in addition to the X chromosome—also associated with nucleolus organizing although they develop the second large nucleolus only at certain stages of oogenesis. We presume that these processes underlie the dynamics of spatial orientation of the polytene chromosome pericentromeric regions in the nurse cell nuclei during D. melanogaster oogenesis. It is known that the nucleolus plays a certain role in the architecture of the interphase nucleus [9, 17, 44]. It has also been demonstrated that the centromeric regions of some chromosomes are also located in a nonrandom manner in the interphase nuclear space of human and animal cells [2, 20]. In particular, it is shown that the centromeric regions of nucleolus-organizing chromosomes 2 and 4 in some Arabidopsis species most frequently reside in the nuclear space in close proximity to each other [4, 21, 45, 48]. In this work, we have observed an analogous arrangement of the centromeric regions of nucleolus-organizing chromosomes X, 3, and 4 in the space of the D. melanogaster nurse cell nuclei with morphotype III (Figs. 1, 2) and that this configuration was most frequently observed at the later stages of oogenesis.
It is known that a single nucleolus, associated with the nucleolus organizer residing in the X chromosome pericentromeric heterochromatin, is formed in the nuclei of D. melanogaster salivary gland cells with polytene chromosomes [38]. Furthermore, the polytene nuclei of salivary gland cells may develop additional nucleoli associated with different sites of the polytene chromosomes. The rDNA of these additional nucleoli is actively transcribed and replicated [3]. The frequency of these nucleoli increases proportionally to the degree of polyteny [43]. Unlike the salivary glands, the polytene nuclei of D. melanogaster nurse cells displayed two nucleoli, one of which was directly connected with the nucleolus-organizing X chromosome, while the other tightly contacts the pericentromeric region of chromosomes 3 and 4. Presumably, the regions of the pericentromeric heterochromatin in chromosomes 3 and 4 of the D. melanogaster nurse cells are associated with active rDNA sequences (possibly extra-chromosomal) and thus develop the nucleolus directly connected with the pericentromeric region of these chromosomes. Earlier work has reported that extra-chromosomal circular rDNA sequences are involved in the process of nucleolus formation in Drosophila [31] polytene cells. Our present data also support the idea that the formation of the second nucleolus/nucleolur organizer is induced for the regulation of the spatial arrangement of the pericentromeric heterochromatin region of the chromosomes of nurse cell nuclei during oogenesis.
References
Aizenshtadt TB, Baranov VS, Borovkov AYu. The current problems in oogenesis. Moscow: Nauka; 1977.
Alcobia I, Dilao R, Parreira L. Spatial associations of centromeres in the nuclei of hematopoietic cells: evidence for cell-type-specific organizational patterns. Blood. 2000;95:1608–15.
Ananiev EV, BarskyVE Ilyin YV, Churikov A. Localization of nucleoli in Drosophila melanogaster polytene chromosomes. Chromosoma (Berl). 1981;81:619–28.
Berr A, Pecinka A, Meister A, Kreth G, Fuchs J, Blattner FR, Lysak MA, Schubert I. Chromosome arrangement and nuclear architecture but not centromeric sequences are conserved between Arabidopsis thaliana and Arabidopsis lyrata. Plant J. 2006;48:771–83.
Boikova TV, Orlando V, Lupo R, Bogachev SS. M/SAR elements of the Bithorax complex of Drosophila melanogaster. Genetika (Mosk). 2005;41:1–12.
Bolzer A, Kreth G, Solovei I, Koehler D, Saracoglu K, Fauth C, Muller S, Eils R, Cremer C, Speicher MR, Cremer T. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol. 2005. https://doi.org/10.1371/journal.pbio.0030157.
Branco M, Pombo A. Chromosome organization: new facts, new models. Trends Cell Biol. 2007;17:127–34.
Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat Rev Genet. 2006;7:940–52.
Chubb JR, Boyle S, Perry P, Bickmore WA. Chromatin motion is constrained by association with nuclear compartments in human cells. Curr Biol. 2002;12:439–45.
Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301.
Cremer T, Cremer M. Chromosome territories. Cold Spring Harb Perspect Biol. 2010. https://doi.org/10.1101/cshperspect.a003889.
Cremer T, Cremer M, Dietzel S, Muller S, Solovei I, Fakan S. Chromosome territories—a functional nuclear landscape. Curr Opin Cell Biol. 2006;18:307–16.
Croft JA, Bridger JM, Boyle S, Perry P, Teague P, Bickmore WA. Differences in the localization and morphology of chromosomes in the human nucleus. J Cell Biol. 1999;145:1119–31.
Csink AK, Henikoff S. Large-scale chromosomal movements during interphase progression in Drosophila. J Cell Biol. 1998;143:13–22.
Dapples CC, King RC. The development of the nucleolus of the ovarian nurse cell of Drosophila melanogaster. Zellforsch Mikrosk Anat. 1970;103:34–47.
De Boni U. The interphase nucleus as a dynamic structure. Int Rev Cytol. 1994;150:149–71.
De Boni U, Mintz AH. Curvilinear, three-dimensional motion of chromatin domains and nucleoli in neuronal interphase nuclei. Science. 1986;234:863–6.
Dehghani H, Dellaire G, Bazett-Jones DP. Organization of chromatin in the interphase mammalian cell. Micron. 2005;36:95–108.
Dej KJ, Spradling AC. Theendocycle controls nurse cell polytene chromosome structure during Drosophila oogenesis. Development. 1999;126:293–303.
Del Prete S, Arpon J, Sakai K, Andrey P, Gaudin V. Nuclear architecture and chromatin dynamics in interphase nuclei of Arabidopsis thaliana. Cytogenet Genome Res. 2014. https://doi.org/10.1159/000363724.
Fransz P, De Jong JH, Lysak M, Castiglione MR, Schubert I. Interphase chromosomes in Arabidopsis are organized as well defined chromocenters from which euchromatin loops emanate. Proc Natl Acad Sci USA. 2002;99:14584–9.
Fritz A, Barutcu AR, Martin-Buley L, van Wijnen AJ, Zaidi SK, Imbalzano AN, Lian JB, Stein JL, Stein GS. Chromosomes at work: organization of chromosome territories in the interphase nucleus. J Cell Biochem. 2016;117:9–19.
Gavrilov AA, Razin SV. Compartmentalization of the cell nucleus and spatial organization of the genome. Mol Biol. 2015;49:26–45.
Gavrilov AA, Razin SV, Iarovaia OV. C-methods to study 3D organization of the eukaryotic genome. Biopolym Cell. 2012;28:245–51.
Getzenberg RH, Pienta KJ, Ward WS, Coffey DS. Nuclear structure and the three-dimensional organization of DNA. J Cell Biochem. 1991;47:289–99.
Glazkov MV. A loop-domain arrangement of the genes in eukaryotic chromosomes. Mol Biol. 1995;29:965–82.
Gorkin DU, Leung D, Ren B. The 3D genome in transcriptional regulation and pluripotency. Stem Cell. 2014;14:762–75.
Glazkov MV. Association of chromosomes with the nuclear membrane and the orderliness of the spatial organization of genetic material in the interphase nucleus. Tsitol Genet. 1999;33:79–88.
Guo T, Fang Y. Functional organization and dynamics of the cell nucleus. Front Plant Sci. 2014. https://doi.org/10.3389/fpls.2014.00378.
Gushchanskaya ES, Gavrilov AA, Razin SV. Spatial organization of interphase chromosomes and the role of chromatin fiber dynamics in the positioning of genome elements. Mol Biol. 2014;48:386–94.
Holmquist G. Transcription rates of individual polytene chromosome bands: effects of gene dose and sex in Drosophila. Chromosoma (Berl). 1972;36:413–52.
Kiknadze II, Istomina AG, Salova TA. Functional morphology of the polytene chromosomes of the Chironomus pilicornis F. from the water bodies of cryolithozone. Tsitologiya. 2002;44:89–95.
Kokhanenko AA, Anan’ina TV, Stegnii VN. Intranuclear dynamics of chromosome 6 in nurse cells of Calliphoraerythrocephala Mg. (Diptera: Calliphoridae). Russ J Genet. 2010;46:1045–7.
Kokhanenko AA, Anan’ina TV, Stegniy VN. The changes in chromosome 6 spatial organization during chromatin polytenization in the Calliphora erythrocephala Mg. (Diptera: Calliphoridae) nurse cells. Protoplasma. 2013;250:141–9.
Kokhanenko AA, Anan’ina TV, Stegniy VN. Localization of rRNA genes in the nuclear space of Calliphoraerythrocephala Mg. nurse cells during polytenization. Protoplasma. 2014;251:93–101.
Kubben N, Adriaens M, Meuleman W, Voncken JW, van Steensel B, Misteli T. Mapping of lamin A- and progerin-interacting genome regions. Chromosoma. 2012;121:447–64.
Kulichkov VA, Zhimulev IF. Analysis of spatial organization of the Drosophila melanogaster genome based on the data on ectopic conjugation of polytene chromosomes. Genetika (Mosk). 1976;12:81–9.
Lefevre G. A photographic representation and interpretation of the polytene chromosomes of Drosophila melanogaster salivary glands. In: Ashburner M, Novitski E, editors. The genetics and biology of Drosophila, vol. 1a. New York: Academic Press; 1976. p. 31–66.
Mannuelidis L. Indications of centromere movement during interphase and differentiation. Ann N Y Acad Sci. 1985;450:205–21.
Marshall WF, Sedat JW. Nuclear architecture. Res Probl Cell Differ. 1999;25:283–301.
Marshall WF, Straight A, Marko JF, Demburg AF, Swedlow JR, Murray A, Belmont A, Agard DA, Sedat JW. Interphase chromosomes undergo constrained diffusional motion in living cells. Curr Biol. 1997;7:930–9.
Meaburn KJ, Misteli T. Cell biology: chromosome territories. Nature. 2007;445:379–781.
Mecheva IS, Semionov EP. Localization of ribosomal DNA insertion elements in polytene chromosomes of Drosophila simulans, Drosophila mauritiana and their interspecific hybrids. Genetica. 1992;85:223–9.
Nemeth A, Langst G. Genome organization in and around the nucleolus. Trends Genet. 2011;27:149–56.
Pontvianne F, Carpentier M-C, Durut N, Pavlistova V, Jaske K, Schorova S, Parrinello H, Rohmer M, Pikaard CS, Fojtova M, Fajkus J, Saez-Vasquez J. Identification of nucleolus-associated chromatin domains reveals a role for the nucleolus in 3D organization of the A. thaliana genome. Cell Rep. 2016;16:1574–87.
Razin SV. Spatial organization of the eukaryotic genome and the operation of epigenetic mechanisms. Genetika (Mosk). 2006;42:1605–14.
Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–63.
Schubert V, Berr A, Meister A. Interphase chromatin organization in Arabidopsis nuclei: constraints versus randomness. Chromosoma. 2012;121:369–87.
Schwartz M, Hakim O. 3D view of chromosomes, DNA damage, and translocations. Curr Opin Genet Dev. 2014;25:118–25.
Sharakhov IV, Wasserlauf IE, Stegnii VN. Features of polytene chromosome attachment to the nuclear envelope of ovarian pseudonurse cells in Drosophila melanogaster. Russ J Genet. 1997;33:139–44.
Shchapova AI. Spatial organization of chromosomes in the eukaryotic cell nucleus of various plant and animal species. Vestn VOGiS. 2010;14:612–21.
Shelkovnikova TA, Wasserlauf IE, Stegniy VN. The changes in nuclear architecture during the ovarian development in the Drosophila subgroup melanogaster. Vestn Tomsk Gos Univ. 2007;301:222–6.
Solovei I, Cremer M. 3D-FISH on cultured cells combined with immunostaining. Methods Mol Biol. 2010;659:117–26.
Stegniy VN. Reorganization of the structure of the interphase nuclei in the ontogeny and phylogeny of malaria mosquitoes. Dokl Ross Akad Nauk. 1979;249:1231–4.
Stegniy VN. Architectonics of the genome, systemic mutations, and evolution. Novosibirsk: Novosibirsk State University; 1993.
Stegniy VN. The evolutionary significance of chromosome architectonics as a form of the epigenetic control of ontogenesis and phylogenesis in eukaryotes. Genetika (Mosk). 2006;42:1215–24.
Stegniy VN, Sharakhova MV. Systemic restructuring of the architectonics of polytene chromosomes in the ontogeny and phylogeny of malaria mosquitoes. Specific structural features of the zones of chromosome contact with the nuclear envelope. Genetika (Mosk). 1991;27:828–35.
Stegniy VN, Wasserlauf IE. Interspecific differences in the co-orientation of primary polytene chromosomes of the Drosophila melanogaster, D. simulans, and D. mauritiana. Genetika (Mosk). 1991;27:1169–73.
Wasserlauf IE. Thedynamics of chromosome orientation in the ovarian nurse cell nuclei in closely related species of the D. melanogaster subgroup and D. virilis group. Vestn Tomsk Gos Univ. 2008;313:205–14.
Xue F, Cooley L. Kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell. 1993;72:681–93.
Zimmer C, Fabre E. Principles of chromosomal organization: lessons from yeast. J Cell Biol. 2011;192:723–33.
Zink D, Cremer T. Chromosome dynamics in nuclei of living cells. Curr Biol. 1998;8:321–4.
Zuleger N, Robson MI, Schirmer EC. The nuclear envelope as a chromatin organizer. Nucleus. 2011;2:339–49.
Acknowledgements
This research work was funded by the Tomsk State University competitiveness improvement programme. We thank Mr. Ian Barrett for editing the manuscript.
Author information
Authors and Affiliations
Contributions
IEW: analysis of the results, drawing up illustrations for the article, writing the article; KEU: setting the experiment, analyzing the results, writing the article; AKS: statistical processing of the results, analysis of the results; VNS: study design, interpretation of results, critical revision and finalization of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors of this manuscript declare that all experiments comply with the current laws of the country in which they were performed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wasserlauf, I.E., Usov, K.E., Sibataev, A.K. et al. Dynamics of the spatial orientation of the pericentromeric heterochromatin regions in the polytene chromosomes of ovarian nurse cells in the Drosophila melanogaster (Diptera: Drosophilidae) oogenesis. Nucleus 63, 7–15 (2020). https://doi.org/10.1007/s13237-019-00275-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-019-00275-2