Abstract
Zinc gallate (ZnGa2O4) has been introduced in various studies as a good host phosphorescent material, and the change of its optical properties by ion doping has received much attention. However, ZnGa2O4 is mostly synthesized through solid-state reactions, the reaction conditions of high temperature and pressure were required, and its low dispersibility often limited its applications. In this study, we synthesized normal spinel structured ZnGa2O4 nanocrystals through a colloidal method. Lanthanide doping specifically with europium and terbium resulted in red (614 nm, photoluminescence quantum yield (PLQY): 2.2%) and green (545 nm, PLQY: 13.6%) emissions under 254 nm UV excitation. By applying the novel co-doping system of europium and terbium to the synthesis of colloidal ZnGa2O4 for the first time, we were able to achieve various color conversions including green, red, and white by adjusting amount and ratio of lanthanide ions.
Graphic Abstract
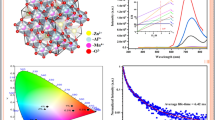
PL emission spectra by adjusting the ratios of Eu3+ and Tb3+ doping and the CIE 1931 chromatography diagram of Eu3+ and Tb3+ co-doping system of ZnGa2O4 nanocrystals

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Zinc gallate (ZnGa2O4) is a phosphorescent material with a normal spinel (Fd3m space group, JCDPS: 38–1240) crystal structure [1]. In this spinel structure, zinc (Zn2+) ions occupy the tetrahedral sites, while gallium (Ga3+) ions occupy the octahedral sites. It is a well-known wide-bandgap material with a bandgap of approximately 4.4 eV, which allows it to emit light in the blue region when excited by UV light [2]. ZnGa2O4 has been reported in various studies as a good host material for phosphors. When blue emission from the host is transferred to transition metals or rare earth ions, such as lanthanides, it results in the emission of the characteristic colors of these elements [3]. This property has potential applications in fields, such as field emission displays (FED), LED encapsulant and bioimaging [4,5,6,7,8]. Several studies have reported dopant emission from ZnGa2O4. Transition metals, such as Mn2+ and Cr3+, are used to achieve green and red emissions, [9,10,11] respectively. Lanthanide ions, such as Eu3+ and Tb3+, can be utilized as phosphorescent materials, emitting red and green light, respectively [12]. These materials exhibit a characteristic narrow emission spectrum through the 4f–4f or 4f–5d transitions [3]. Furthermore, the state of charge transfer corresponding to each photoluminescence (PL) peak has been precisely identified [11]. Color-tunable PL can be achieved by combining two or more elements. Combinations of Dy3+, Tb3+and Mn3+/ Ho3+, Bi3+ and Yb3+/ Er3+, Tb3+ and Yb3+ allow tuning of the emission color by varying the dopant composition [13,14,15]. In addition, a previous study reported that the co-doping of ZnGa2O4 with Eu3+ and Tb3+ achieved red, green, and white emissions [16] .
There are several methods for the synthesis of ZnGa2O4, including solid-state reactions, [8] sol–gel methods [10], polyol methods [12], hydrothermal methods [17], and co-precipitation and annealing [18]. Among these methods, the solid-state reaction is the most commonly used for synthesizing ZnGa2O4, which typically results in microsized particles with low dispersibility [19]. Other synthesis methods also lead to crystal aggregation and poor dispersibility in organic solvents. Colloidal ZnGa2O4 nanocrystals, which exhibit good dispersity in solvents, [3] have been reported as an improved method. Generally, colloidal method can provide dispersibility in organic solvent to the synthesized nanocrystals due to hydrophobic interaction between organic solvents and long alkyl chain of a surfactant attached to the surface of a nanoparticle. Enhancing dispersibility offers processability for display device fabrication and the possibility of a biomarker. However, the previous study focused on the single doping of europium and terbium, it was not possible to achieve color tunability by adjusting the lanthanide doping ratios. Therefore, we suggested the idea of double-doped ZnGa₂O4, which can be dispersed in organic solvents and exhibits color tunability by adjusting the doping ratios of lanthanides.
In this study, ZnGa2O4 nanocrystals were synthesized using a previously reported colloidal method in an organic solvent, [3] rather than a solid-state method. Organic ligands were used to achieve dispersibility in organic solvents. Eu 3+ and Tb3+ were added to the ZnGa2O4 during the colloidal synthesis process, and the PL changes were analyzed. Moreover, the co-doping of Eu3+ and Tb3+ to ZnGa2O4 during the colloidal synthesis process was performed for the first time, and changes in the photoluminescence quantum yield (PLQY) and color tunability based on the doping ratio were elucidated using CIE chromatography diagrams.
2 Experimental
2.1 Materials
All chemicals, Zn(II) acetylacetonate hydrate (Zn(acac)2, Sigma-Aldrich), Ga(III) acetylacetonate (Ga(acac)3, 99.99% trace metal basis, Sigma-Aldrich), Eu(III) acetate hydrate (Eu(OAc)3·xH2O, REacton®, 99.9%, Alfa), Tb(III) acetate hydrate(Tb(OAc)3·xH2O, REacton®, 99.9%, Alfa) 1,2-hexadecanediol (HDD, 90%, Technical grade, Sigma-Aldrich), benzyl ether (98%, Sigma-Aldrich), oleic acid (OA, 90% technical grade, Sigma-Aldrich), and oleylamine (OLA, 70% technical grade, Sigma-Aldrich) were used without further purification.
2.2 Synthesis of zinc gallium oxide (ZnGa2O4)
For the synthesis of pristine and lanthanide-doped ZnGa2O4, a previously reported colloidal synthesis method was used [3]. 1 mmol of Zn(acac)2, 2 mmol of Ga(acac)3, 5 mmol of HDD, 6 mmol of OA, and 6 mmol of OLA were added to 10 mL of benzyl ether and degassed in a three-neck reaction flask. By substituting the appropriate amount (0 to 0.3 mmol) of Eu(OAc)3·xH2O and Tb(OAc)3·xH2O for Ga(acac)3, lanthanide-doped ZnGa2O4 could be synthesized. After degassing, it was filled with N2 gas and heated to 200 °C for 30 min. The solution was heated to 280 °C for 30 min at N2 flow. After the completion of the reaction, the solution was cooled to RT and diluted with hexane. The successfully synthesized ZnGa2O4 nanocrystals were precipitated by adding ethanol and collected by centrifugation at 8000 rpm for 10 min. After purification, ZnGa2O4 nanocrystals were dispersed in hexane for optical characterization.
2.3 Material characterization
Absorption spectra were measured using a Scinco PDA S-3100 UV–vis spectrophotometer. XRD studies were performed using a Rigaku Ultima diffractometer equipped with a Cu Kα radiation source (λ = 0.15418 nm) at the Ajou University Computing Center. TEM images were obtained using a JEM-2100F (JEOL) system operated at 200 kV at the Korea Advanced NanoFab Center. The absolute PLQY and PL emission spectra were measured using an FP-8500 instrument (Jasco) and an FS5 Spectrofluorometer (Edinburgh Instruments).
3 Results and discussion
3.1 Synthesis of ZnGa2O4
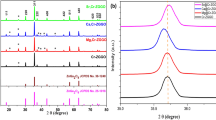
The formation of ZnGa2O4 nanoparticles was confirmed using XRD analysis. Figure 1a shows the XRD patterns of ZnGa2O4 nanocrystals obtained by the colloidal method using a benzyl ether solvent and the synthesized ZnGa2O4 in which Ga3+ ions were replaced with lanthanides (Eu3+, Tb3+). The X-ray diffraction results showed that the diffraction peaks of the synthesized colloidal ZnGa2O4 matched well with those of bulk ZnGa2O4 [3]. (JCPDS File number: 38–1240, Fd3m, Cubic) The normal cubic spinel structure of ZnGa2O4, representing the Fd3m space group, has been reported to have Zn2+ ions occupying the tetrahedral sites and Ga3+ ions occupying the octahedral sites [1]. Among the measured diffraction peaks, the main diffraction peaks from (311) to (511) and (400) at 34.12°, 58.66°, and 62.86°matched well regardless of whether the Eu3+ or Tb3+ ions were doped. Since the diffraction peaks of pristine and lanthanide-doped ZnGa2O4 very slightly shift even in the bulk size, [16] the positions of the diffraction peaks in the nanosized pristine and lanthanide-doped ZnGa2O4 nanocrystals appear almost identical. The broader diffraction peaks compared to those of bulk ZnGa2O4 were attributed to the lower crystallinity resulting from the synthesis of nanosized crystals, as shown in Fig. 1b. Figure 1b shows the TEM images of Tb3+-doped ZnGa2O4. Figure 1c also shows that the synthesized ZnGa2O4 nanocrystals exhibited an approximate size of 2.69 nm.
The synthesized nanocrystals showed unique optical properties. Figure 1d and e show the PL excitation spectrum and the energy diagrams of the luminescence mechanisms of pristine and Tb3+-doped ZnGa2O4. ZnGa2O4 is generally reported to be an ultrawide bandgap material (approximately 4.4 eV) [2]. The synthesized ZnGa2O4 nanocrystals emitted broad blue light at approximately 460 nm under 254 nm UV light [20]. The sharp peak observed at 254 nm in the PL excitation spectrum was identified as the charge–transfer band, where energy was transferred from O2− to Ga3+ ions occupying octahedral sites [3]. As shown in the energy diagram on the right, the transferred energy leads to the emission of blue light between 412 and 467 nm through 5D to 7F transitions. In the case of terbium (lanthanide) doping, energy transfer from 5D3 to 5D4 occurs, leading to green emission through transitions to the 7F band [20]. In the case of europium doping, the energy diagram is illustrated in Figure S1 [21]. The emission bands for each charge–transfer band are shown in Figs. 2 and 3.
3.2 Optical properties after lanthanide doping
Figure 2 illustrates the changes in optical properties resulting from the doping of ZnGa2O4 with Tb3+. The PL emission and PL absorption spectra of ZnGa2O4 doped with 0.3 mmol of Tb3+ are presented in Fig. 2a. In the PL emission spectrum, a broad host-emission PL of ZnGa2O4 at approximately 450 nm and four sharp PL emissions of Tb3+ charge transfer can be observed. The sharp PL emission due to Tb3+ doping resulted in changes in the CIE 1931 chromatography coordinates from (x: 0.1850, y: 0.2027) of pristine ZnGa2O4 to (x: 0.30234, y: 0.51861) in Fig. 2b. Figure 2c show the PL excitation spectrum of Tb3+-doped ZnGa2O4. The energy transfer from 5D4 to 7Fx, as indicated in the energy diagram of the emission mechanism in Fig. 1e, is reflected in the PL emission peaks shown in the emission spectrum in Fig. 2d [3, 21]. When Tb3+-doped ZnGa2O4 exhibiting these optical properties was excited at 254 nm, a PLQY of 13.6% was measured.
Figure 3 illustrates the changes in optical properties resulting from doped ZnGa2O4 with Eu3+, similar to the case with Tb3+. The PL emission and PL absorption spectra of ZnGa2O4 doped with 0.3 mmol of Eu3+ are presented in Fig. 3a. The PL emission spectrum showed a broad host emission PL of ZnGa2O4 at approximately 450 nm and five sharp PL emissions of Eu3+ charge transfer. Figure 3c show the PL excitation spectrum of Eu3+-doped ZnGa2O4.The charge–transfer bands from 5D0 to 7Fx are indicated for each PL emission peak in Fig. 3d [2, 3]. Doped ZnGa2O4 with Eu3+ also changed the CIE 1931 chromatography coordinates from zinc gallate to (x: 0.48311, y: 0.28751) in Fig. 3b. Comparing the charge transfer between Eu-doped- and Tb-doped zinc gallate with emission spectrum, Tb3+ zinc gallate demonstrate larger transfer property than Eu3+ zinc gallate. In addition, the PLQY of the Eu3+-doped ZnGa2O4 was 2.2%, which was 10% lower than that of the Tb3+-doped ZnGa2O4.
3.3 Color tunability after lanthanide doping
Through the luminescence mechanism described in Figs. 2 and 3, color tunability was achieved by adjusting the Eu3+ and Tb3+ doping ratios in ZnGa2O4. As the Tb3+ doping ratio increased, the intensities of the PL emission peaks at 488 and 545 nm, corresponding to the 5D4 to 7F6 and 7F5 transitions, respectively, also increased. Conversely, as the Tb3+ doping ratio decreased and the Eu3+ doping ratio increased, the intensities of the PL emission peaks at 590 and 615 nm, corresponding to the 5D0 to 7F1 and 7F2 transitions, gradually increased. Figure 4 illustrates the changes in the PL emission spectrum owing to variations in the doping ratios [16].
The broad host-emission of pristine ZnGa2O4 at approximately 450 nm was transferred to sharp emission bands when singly doped with lanthanide elements because of the specific energy transfer of each element, as shown in Figs. 2 and 3. By adjusting the Eu3+:Tb3+ ratio to 1:2, 1:1, and 2:1, the intensities of the PL peaks emitted by each lanthanide element increased or decreased accordingly. While Eu3+ showed five sharp PL emission peaks and Tb3+ showed four emission peaks in the visible range, co-doped ZnGa2O4 did not exhibit a total of nine peaks in the spectrum. This is due to the spectral overlap caused by the energy transfer, resulting in the modulation of specific spectrum positions. This tendency is particularly evident in the emission peaks between 575 and 625 nm, as illustrated in Figure S2.
Figure 5 presents the photographs of the luminescence under a 254 nm UV lamp in a dark room, visually confirming the color tunability observed in the CIE 1931 chromatography diagram. Additionally, the PLQY values for ZnGa2O4 doped with Eu3+ and Tb3+ were 2.2% and 13.6%, respectively, upon 254 nm UV excitation. For co-doped ZnGa2O4, the PLQY values exhibited a linear increase as the ratio of Eu3+ decreased and the ratio of Tb3+ increased.
The color tunability could linearly cover the CIE 1931 chromatography diagram when the lanthanide concentration was fixed at 0.3 mmol. Furthermore, by adjusting the doping amounts of Eu3+ and Tb3+ based on pristine ZnGa2O4 and lanthanide-doped ZnGa2O4, a broad area can be covered. Figure 6 shows the color coordinates from pristine ZnGa2O4 to ZnGa2O4 doped with 0.1 mmol and 0.3 mmol lanthanide. Both 0.1 mmol samples of Eu3+ and Tb3+ exhibit color coordinates between those of pristine ZnGa2O4 and 0.3 mmol samples. This demonstrates a system in which colors within the marked CIE chromatography area can be obtained by varying the amount and ratio of Eu3+ andTb3+ lanthanide dopant.
The main advantage of this study can be shown in the application of ZnGa2O4 as a display material. As shown in Fig. 6, the lanthanide doping of ZnGa2O4 resulted in significant coverage on the color coordinate diagram. For display materials, the ability of a single material to exhibit color tunability is crucial for uniformity and productivity. For example, CdSe quantum dots (QDs) have attracted significant interest owing to their high PLQY and color purity in the visible spectrum [22]. From this perspective, the color tunability of co-doped ZnGa2O4, which covers the RGB range as a single source, has great potential. Specifically, co-doped ZnGa2O4 offers significant advantages in display devices in terms of process uniformity compared to using different materials for RGB. Utilizing a single material provides significant benefits in terms of uniform lifetime, affinity for processing materials, and better control over surface conditions. Furthermore, through previously reported ligand engineering techniques, ZnGa2O4 could potentially be applied in fields such as display patterning and bioimaging [23, 24].
4 Conclusions
We successfully synthesized co-doped ZnGa2O4 nanocrystals with Tb3+ and Eu3+ ions for the first time using a colloidal synthesis method. The synthesized ZnGa2O4 material exhibited distinct luminescent properties corresponding to each lanthanide element when excited using 254 nm UV light. In addition, we demonstrated the ability to control optical properties by adjusting the ratio and amount of doped lanthanide ions. As the ratio of lanthanide ions changed, the intensity of the specific emission bands varied linearly, enabling the coverage of the area in the CIE 1931 chromatography diagram. The potential applications of this material in the display field are numerous, particularly as an LED encapsulation material. The ability of doped ZnGa2O4 to emit a wide range of colors as a single material offers substantial advantages in terms of process convenience and material uniformity for device fabrication. This study paves the way for the broader utilization of ZnGa2O4 in various display technologies owing to its unique optical properties.
References
R.S. Monika, A.B. Yadav, S.B. Rai, RSC Adv. 13, 20164 (2023)
T.A. Safeera, E.I. Anila, Scripta Mater. 143, 94 (2017)
H.-J. Byun, J.-U. Kim, H. Yang, Nanotechnology 20, 495602 (2009)
M.-I. Chen, A.K. Singh, J.-L. Chiang, R. Horng, D. Wuu, Nanomaterials 10, 2208 (2020)
Y. Liu, T. Zheng, X. Zhang, C. Chen, Sci. Rep. Rep. (2023). https://doi.org/10.1038/s41598-023-33498-0
T. Lecuyer, E. Teston, G. Ramirez-Garcia, T. Maldiney, B. Viana, J. Seguin, N. Mignet, D. Scherman, C. Richard, Theranostics. 6, 2488 (2016)
T. Maldiney, B. Ballet, M. Bessodes, D. Scherman, C. Richard, Nanoscale 6, 13970 (2014)
J.-H. Lee, H.-J. Park, Y. Kang, B.-W. Kim, J.C. Lee, S. Park, J. Eur. Ceram. Soc. 27, 965 (2006)
T. Minami, T. Maeno, Y. Kuroi, S. Takata, Jpn. J. Appl. Phys. 34, 684 (1995)
M.K. Hussen, F.B. Dejene, G.G. Gonfa, Appl. Phys. A (2018). https://doi.org/10.1007/s00339-018-1796-x
H. Liu, F. Ren, H. Zhang, Y. Han, H. Qin, J. Zeng, Y. Wang, Q. Sun, Z. Li, M. Gao, J. Mater. Chem. B. 6, 1508 (2018)
C.M. Rao, V. Sudarsan, R.S. Ningthoujam, U.K. Gautam, R.K. Vatsa, A. Vinu, A.K. Tyagi, J. Nanosci. Nanotechnol.Nanosci. Nanotechnol. 8, 5776 (2008)
D.P. Dutta, R. Ghildiyal, A.K. Tyagi, J. Phys. Chem. C 113, 16954 (2009)
R.S. Monika, A.R. Yadav, S.B. Rai, Sci. Rep. Rep. (2021). https://doi.org/10.1038/s41598-020-80364-4
Y. Cheng, K. Sun, Opt. 59, 756 (2020)
E. Monika, A.B. Rai, S.B. Rai, J. Solid State Chem. 325, 124159 (2023)
L. Chen, Y. Liu, Z. Lu, K. Huang, Mater. Chem. Phys. 97, 247 (2006)
G. Sridhar, D. Hebbar, S.G. Menon, P.M. Lewis, K.S. Choudhari, R.E. Kroon, H.C. Swart, S.D. Kulkarni, Opt. Mater. 123, 111919 (2021)
Y. Cheng, K. Sun, P. Ge, Opt. Mater. 83, 13 (2018)
T.A. Safeera, E.I. Anila, J. Alloy. Compd. 764, 142 (2018)
G.B. Nair, S.J. Dhoble, J. Fluoresc. 26, 1865–1873 (2016)
B.O. Dabbousi, J. Rodriguez-Viejo, F.V. Mikulec, J.R. Heine, H. Mattoussi, R. Ober, K.F. Jensen, M.G. Bawendi, J. Phys. Chem. B 101, 9463 (1997)
J. Yang, J. Yoo, W.S. Yu, M.K. Choi, Macromol. Res. 29, 391 (2021)
D.K. Lee, Y.-K. Lee, Macromol. Res. Res. 18, 641 (2010)
Acknowledgements
This research was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government. (Ministry of Science and ICT) (2023R1A2C1003608)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, K., Kim, K., Kwon, HG. et al. Lanthanide-doped zinc gallium oxide (ZnGa2O4) nanocrystals and their engineered photoluminescence. Macromol. Res. (2024). https://doi.org/10.1007/s13233-024-00312-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13233-024-00312-3