Abstract
The effect of melt processing temperature on the isothermal crystallization behavior of poly(3-hydroxybutyrate-co-4-hydroxybutyrate)[P(3HB-co-4HB)] is studied using differential scanning calorimetry and polarizing microscopy. P(3HB-co-4HB) undergoes thermal degradation above its melting temperature. The degree of degradation increases with increase in the 4HB content in copolymer. The formation of low molecular weight P(3HB-co-4HB) from thermal degradation reduces the crystallization temperature. Thus, the isothermal crystallization rate and crystallinity decrease at a processing of 190 ℃ compared with 180 ℃ where less thermal degradation occurs. This is more evident in the case of P(3HB-co-4HB) of high 4HB content. The P(3HB-co-4HB) crystals formed in the isothermal crystallization process have different perfectness in the 4HB rich phase and the 3HB rich phase depending on the melt processing temperature. This results in the appearance of two distinct melting peaks with different melting temperatures and melting enthalpies. Our results indicated that thermal history in the isothermal crystallization process will affect these crystals differently.
Graphical abstract

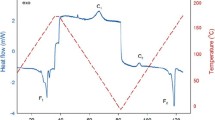
A 10 °C difference in the processing temperature of P(3HB-co-4HB) results in a clear difference in the temperatures where crystallization is possible. Samples processed at 180 °C have a higher crystallizable temperature and crystallize more readily during the cooling stage compared with those process ed at 190 °C. As a result, the effect of thermal degradation on the crystallization behavior is minimized for the samples processed at 180 °C, while it is greater when thermal degradation retards crystallization and the crystallization temperature decreases for the samples processed at 190 °C
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Most of the waste plastic is disposed of in landfills but recently the amount of plastic discarded in marine environments is dramatically increasing [1]. Biodegradable polymers to preserve both environments need specific enzymes from microorganisms. Since polyhydroxyalkanoate[PHA] is made by microorganisms, it has an excellent biodegradation rate in marine environments compared to commercially available biodegradable polymers produced from petroleum or biomass [2, 3].
Among biodegradable PHAs, poly(3-hydroxybutyrate-co-4-hydroxybutyrate) [P(3HB-co-4HB)] is a biodegradable aliphatic polyester developed to improve the low crystallization rate, high crystallinity, and low thermal stability of poly(3-hydroxybutyrate)[P(3HB)] [4,5,6,7,8]. Due to the low crystallization rate of P(3HB), big spherulites are formed which impinge to form an interface and make P(3HB) brittle [9,10,11,12]. Thermal degradation of P(3HB) occurs near the melting point to make products by melt processing very difficult. To overcome these issues, 4-hydroxybutyrate (4HB) is biosynthetically copolymerized with 3-hydroxybutyrate (3HB) to prepare P(3HB-co-4HB) [13,14,15,16].
When 3HB and 4HB are copolymerized, random copolymers are formed and its chain irregularity results in more difficult crystallization compared with P(3HB). Above a certain 4HB content, a rubbery non-crystalline P(3HB-co-4HB) of lower strength and higher elongation is formed [17,18,19]. Our previous result [20] indicated that this is related to rheological property change with increasing 4HB chain relatively flexible compared with the 3HB chain. Along with the change in this microstructure, the incorporation of the more flexible 4HB may even increase the crystallization rate [21,22,23]. The P(3HB-co-4HB) crystals formed in the isothermal crystallization are dependent on the flexibility as well as the randomness and they determine the properties of P(3HB-co-4HB). Therefore, research on the effect of 4HB content on the isothermal and dynamic crystallization of P(3HB-co-4HB) copolymers and the resulting crystal structure and crystallinity is necessary.
Another drawback of P(3HB) is that the thermal stability is significantly lowered by the formation of crotonic acid through the random degradation via β-elimination [24,25,26]. The chain scission of 3HB in P(3HB-co-4HB) is suppressed by the presence of methylene groups where chain scission occurred at 4HB units in the random degradation of 3HB and 4HB units. Cyclization and transesterification by OH-end groups in degraded 4HB units also occur to increase the onset temperature of thermal degradation to increase the thermal stability with the addition of 4HB units in the P(3HB-co-4HB) [27, 28]. The thermal degradation of P(3HB-co-4HB) changes with the 4HB content and is known to exhibit different thermal degradation mechanisms [29, 30]. Contrary to this, at low degrees of thermal degradation addition of small amounts of 4HB results in an increase in the thermal degradation with 4HB content, but when the 4HB content exceeds a certain amount, especially when the copolymer is non-crystalline, a significant increase in the thermal stability has been confirmed [31, 32]. We have recently reported a study on the effect of thermal degradation on the properties of P(3HB-co-4HB) and suggested reducing the thermal degradation by melt blending of crystalline P(3HB-co-4HB) and non-crystalline P(3HB-co-4HB) [33]. The thermal degradation characteristics of P(3HB-co-4HB) are expected to depend on the melt processing temperature and time and the crystallization behavior, but research on this has not been reported.
In this study, the effect of the melt processing temperature of P(3HB-co-4HB) on the isothermal crystallization behavior is investigated. Specifically, this research is focused on the effect of thermal degradation of P(3HB-co-4HB) of different 4HB content exhibiting different thermal stability on the crystallization behavior. The change in the crystal structure of P(3HB-co-4HB) processed at different temperatures is also studied.
2 Experimental
2.1 Materials
Two laboratory-grade crystalline P(3HB-co-4HB) with different 4HB content were used in this study. PHA2.1 whose 4HB content is low as 2.1% and the molecular weight is 200,000 g/mol and PHA10 whose 4HB content is high as 10% and the molecular weight is 600,000 g/mol, provided by CJ Cheiljedang Corp.
2.2 Analysis
The effect of 4HB content on the thermal stability of the provided P(3HB-co-4HB) is studied on a thermogravimetric analyzer (TGA TA, Q20, Delaware, USA) by measuring the weight change after isothermal exposure to a 180–200 ℃ environment for 20 min.
The isothermal and dynamic crystallization behaviors are investigated on a differential scanning calorimeter (DSC, TA Q20, Delaware, USA). To evaluate dynamic crystallization, the sample is preheated from − 50 ℃ to the melt processing temperatures of 180–200 ℃ at 20 ℃/min. quenched to − 50 ℃ at 10–50 ℃/min., then heated again from − 50 ℃ to 200 ℃ at 20 ℃/min. to measure the crystallization temperature(Tc)and enthalpy(ΔHc). To evaluate isothermal crystallization behavior, the sample is preheated from 25 ℃ to melt processing temperatures of 180–200 ℃ at 20 ℃/min. and kept at that temperature for 5 min., quenched at 50 ℃/min., then heated again to the isothermal crystallization temperature of 40–110 ℃. The effects of 4HB content, processing temperature, and isothermal crystallization temperature on relative crystallinity(Xc: J/g) obtained from the change in the enthalpy with time and the half-time of crystallization is studied. The isothermally crystallized sample is again heated from 0 ℃ to 190 ℃ at 20 ℃/min. to observe the effect of isothermal crystallization on the melting temperature and enthalpy.
The structure of the spherulites is studied by observing with a CCD camera attached to a polarizing microscope(Olympus BX51, Tokyo, Japan) equipped with a hot stage(Mettler FP82HT, Greifensee, Switzerland). The same temperature profile as that used in the dynamic and isothermal crystallization on the DSC is used to obtain the optical micrographs of the change in the Maltese cross of spherulites with time.
3 Results and discussion
The weight change in the isothermal thermal degradation of P(3HB-co-4HB) at the melt processing temperature of 180–200 ℃ for 20 min is shown in Fig. 1. With the increase in processing temperature, the weight decreases by 0.3–2.2% on thermal degradation, and degradation increases with an increase in the 4HB content. The formation of low molecular weight P(3HB-co-4HB) is expected based on the weight loss in thermal degradation. The thermal degradation of P(3HB-co-4HB) occurs through random degradation of 3HB and 4HB units and an increase in the 4HB content minimizes the thermal degradation of 3HB units due to the presence of the methylene group on the degraded 4HB unit which also decreases degradation through cyclization and transesterification [27, 28]. This is also observed in our dynamic TGA results where the onset temperature of thermal degradation increases with an increase in the 4HB content [33]. Contrary to dynamic experiments, isothermal thermal degradation results in less than 2.2% weight loss after a designated time, and an increase in the 4HB content increases the weight loss, as can be seen in Fig. 1.
This discrepancy is due to the fact that above the degradation temperature of P(3HB-co-4HB) of 240 ℃, the increase in the 4HB content enhances thermal stability according to the above degradation mechanism proposed by Omura and Nakamura. [31, 32] On the other side, prolonged exposure to lower temperatures of 180–200 ℃ results in relatively more degradation of 4HB units whose bonding strength is lower than 3HB units. An increase in the 4HB content results in a decrease in the melting temperature and exposure to temperatures near the melting temperature induces slight degradation due to the inherently low thermal stability of biodegradable polymers with degradation being accelerated by an increase in temperature. When non-crystalline P(3HB-co-4HB) is formed above a critical 4HB content, a decrease in thermal degradation is observed at the melt processing temperatures due to the above degradation mechanism [33]. Therefore, research on the effect of a small amount of low molecular weight P(3HB-co-4HB) on crystallization behavior and properties is needed.
To observe the crystallization behavior of P(3HB-co-4HB) processed at different temperatures, the samples are heated above their melting temperatures to 180–200 ℃, cooled, and then heated to obtain the DSC thermograms in Fig. 2. At the relatively low processing temperature of 180 ℃ near the melting temperature, crystallization occurs on cooling and double melting peaks can be seen in the second heating thermogram. In the case of samples melt processed at 190 ℃, PHA2.1 shows a crystallization behavior in the cooling stage similar to those processed at 180 ℃, but the high 4HB content PHA10 does not crystallize in the cooling stage and shows both a cold crystallization peak and melting peaks in the second heating. In the case of both samples melt processed at 200 ℃, crystallization does not occur in the cooling stage and the second heating thermogram shows a cold crystallization peak and melting peaks.
The presence of the cold crystallization peak in the second heating stage reflects the fact that imperfect crystals are formed in the cooling stage and that further crystallization occurs during the second heating stage to result in relatively more perfect crystals. This phenomenon is observed at a processing temperature difference as small as 10 ℃ leads to a significantly different crystallization behavior. The effect becomes greater as the 4HB content of P(3HB-co-4HB) increases. This can be considered to be due to the increase in the low molecular weight P(3HB-co-4HB) from thermal degradation which changes the crystallization behavior. The presence of the double melting peaks reflecting the crystal structure along with the cold crystallization peak suggests that recrystallization of imperfect crystals is occurring or that a 4HB rich phase where imperfect crystals are formed and a 3HB rich phase where relatively more perfect crystals are formed [34]. Thus, the imperfect crystals in the 4HB rich phase melt first at a lower temperature, and then more perfect crystals in the 3HB rich phase melt at a higher temperature to display a double melting peak.
The crystallization temperature and enthalpy measured from the DSC thermograms of samples processed at different temperatures are shown in Fig. 3. PHA2.1 processed at higher temperatures of 180 ℃ exhibit lower crystallization temperatures and enthalpies. An increase in the cooling rate results in a lower crystallization temperature and crystallization enthalpy does not occur during the cooling stage in the case of the sample processed at 200 ℃. For PHA10 with higher 4HB content, the crystallization peak is observed only in the sample processed at the lower temperature of 180 ℃, and the crystallization temperature and enthalpy both decrease with an increase in the cooling rate. This suggests that the effect of the low molecular weight P(3HB-co-4HB) from degradation discussed in Fig. 1 on the crystallization of P(3HB-co-4HB) is greater when the processing temperature is higher and when the cooling rate is higher. This effect on crystallization is greater, especially in the case of P(3HB-co-4HB) of high 4HB content and thus having higher irregularity in the chain.
To study the effect of thermal degradation at the processing temperatures on the crystallization behavior of P(3HB-co-4HB), isothermal crystallization thermograms of samples processed at different processing temperatures are shown in Fig. 4. As seen in Fig. 3, the isothermal crystallization temperature range depends on cooling rate, processing temperature, and the 4HB content, thus, isothermal crystallization temperature is set at 90 ℃ where PHA2.1 and PHA10 both crystallize, and the isothermal crystallization curves are shown in Fig. 4. As can be seen in the figure, an increase in the processing temperature results in an increase in the time to the end of enthalpy change in the isothermal crystallization suggesting slower crystallization. When processed at 180 ℃, the 4HB content does not have a significant effect on the crystallization behavior, but when processed at 190 or 200 ℃ increase in the 4HB content results in a significant decrease in the crystallization rate and also a decrease in the crystallization enthalpy. These results indicate that a small change in the melt processing temperature range of 10–20 ℃ results in a significant change in the crystallization behavior, thus control of the crystallization appears to be possible through changes in the melt processing temperature as well as changes in the 4HB content. Especially, P(3HB-co-4HB) has low thermal stability inevitable biodegradable polymers need to be processed at lower temperatures, and the consequent effect on the crystallization behavior is expected to affect the processability and the ultimate properties.
The effect of the melt processing temperature on the half-time of crystallization and the crystallization enthalpy obtained from the isothermal crystallization thermogram of Fig. 4 is shown in Fig. 5. For the samples processed at a low temperature of 180 ℃ close to the melting temperature of P(3HB-co-4HB), relatively less thermal degradation occurs, and thus the half-time of crystallization is short suggesting a very fast crystallization rate with subsequently high crystallinity, regardless of the 4HB content. In the case of PHA2.1, the crystallization rate decreases slowly and the crystallinity decreases slightly with an increase in the processing temperature in the range of 185–195 ℃. For PHA10 of higher chain randomness and flexibility from the higher 4HB content in copolymer, the crystallization rate decreases drastically and the crystallinity decreases much more with an increase in the processing temperature in the range 180–185 ℃. This indicates that the melt processing temperature affects the isothermal crystallization of the copolymer, especially in the case of the high 4HB content copolymer which is more irregular and flexible, and this behavior appears to be related to the increase in the low molecular weight chains from thermal degradation.
An increase in the 4HB content of P(3HB-co-4HB) results in a decrease in the regularity of the polymer chain to hinder crystallization but also increases the flexibility of the chain to enhance crystallization. In the range of 4HB content of this study, the irregularity and chain flexibility did not affect the crystallization behavior in the case of samples processed at low temperatures, but when the melt processing temperature is higher the increase in the 4HB content results in a significant decrease in the crystallization rate and also a decrease in the crystallinity. This suggested that the crystallization behavior is influenced more by the chain irregularity compared with flexibility. The low molecular weight from thermal degradation also affects the crystallization behavior. On the other hand, the processing temperature does not greatly affect the crystallinity of PHA2.1 but affects the crystallinity of PHA10 whose randomness is relatively higher with the highest change occurring when the melt processing temperature is raised from 180 ℃ to 190 ℃.
Based on these results, it appears that making a 4HB copolymer of P(3HB) to decrease the crystallinity and melt processing at lower temperatures to increase the crystallization rate and thus decrease the spherulite size appears to be a possible method to minimize the disadvantageous brittle properties of P(3HB). By melt processing at lower temperatures, the inevitable thermal degradation problem of biodegradable polymers can be minimized and also increase the crystallization rate and crystallinity.
The Maltese cross patterns displayed by the spherulites in the isothermal crystallization at 90 ℃ on a polarizing optical microscope are shown in Fig. 6 for the samples processed at 180 ℃ and 190 ℃. In the case of PHA2.1, more nuclei are formed to result in faster impingement of growing spherulites in samples processed at 180 ℃ compared with 190 ℃. In the case of PHA10, the spherulites formed by the samples processed at 180 ℃ and 190 ℃ in the isothermal crystallization at 90 ℃ show a very different spherulite structure. Nucleation is faster at both temperatures compared with PHA2.1, but at 180 ℃ more nuclei are formed and crystal growth is more rapid resulting in impingement of small spherulites, while at 190 ℃ the nuclei formed grow slowly to result in impingement of larger spherulites. This indicated that 4HB in PHA10 acted as a nuclei but in the case of samples processed at 190 ℃ the difference between crystallization temperature and melting temperature increases due to the lower temperature at which crystallization can occur from the thermal degradation resulting in a significantly smaller number of nuclei to slowly form larger spherulites compared with sample processed at 180 ℃. This suggests that the brittle character of the P(3HB-co-4HB) from the interface formed by the spherulites can be minimized by processing PHA10 at a lower temperature of 180 ℃.
The isothermal crystallization thermograms at different temperatures of P(3HB-co-4HB) processed at 180 ℃ and 190 ℃ are shown in Figs. 7 and 8. As seen in Fig. 3, the crystallization of samples processed at 180 ℃ occurs in the range 90–110 ℃ compared with samples processed at 190 ℃ which crystallize in the 40–90 ℃ range. The effect of the isothermal crystallization temperature on the crystallization behavior is clearly different for the samples processed at 180 ℃ and 190 ℃. The crystallization rate and crystallinity of samples processed at 180 ℃ decrease with an increase in the isothermal crystallization temperature, while the crystallization rate of samples processed at 190 ℃ shows a maximum of around 60 ℃ and decreases when the isothermal crystallization temperature is lower or higher. The increase in irregularity with an increase in the 4HB content has an effect only on the crystallinity in the samples processed at 180 ℃ but affects both the crystallization rate and the crystallinity in the samples processed at 190 ℃.
The effect of the isothermal crystallization temperature on the half-time of crystallization and the relative crystallinity of the samples processed at 180 ℃ and 190 ℃ are shown in Fig. 9. As can be seen in the figure, a 10 ℃ difference in the processing temperature results in a clear difference in the temperatures where crystallization is possible. Samples processed at 180 ℃ have a higher crystallizable temperature and crystallize more readily during the cooling stage compared with those processed at 190 ℃. As a result, the effect of thermal degradation on the crystallization behavior is minimized for the samples processed at 180 ℃, while it is greater when thermal degradation retards crystallization and the crystallization temperature decreases for the samples processed at 190 ℃. Evaluating the crystallization rate based on the half-time of crystallization suggests that the crystallization rate is faster for the samples processed at 180 ℃ compared with those processed at 190 ℃ and the crystallinity is highest when the isothermal crystallization temperature is 80–90 ℃ and decreases on further increase in temperature, irrespective of the processing temperature.
The effect of higher 4HB content on the crystallization behavior is manifested as only a slight increase in the crystallization rate but a drastic decrease in the crystallinity for the samples processed at 180 ℃. For the samples processed at 190 ℃, the crystallization rate increases with 4HB content at the lower isothermal crystallization temperatures of 40–60 ℃ but decreases as the temperature is further increased showing that the isothermal crystallization temperature has a significant effect on the crystallization behavior. Crystallinity is not affected by the 4HB content at lower isothermal crystallization temperatures, but at higher isothermal crystallization temperatures it is drastically decreased with an increase in the 4HB content. From these results, it appears that the crystallization behavior of P(3HB-co-4HB) can be controlled by a change in the irregularity through the change in 4HB content, the isothermal crystallization temperature, and the melt processing temperature. To overcome the brittle property of P(3HB) both an increase in the crystallization rate and a decrease in crystallinity are needed and it appears that this may be achieved through processing crystalline P(3HB-co-4HB) of high 4HB content at lower processing temperatures and solidifying products at higher crystallization temperatures.
Figure 10 shows the changes in the melting peaks in DSC heating thermograms of P(3HB-co-4HB) samples processed at 180 ℃ and 190 ℃ then quenched to the respective isothermal crystallization temperature and crystallized. Excluding the PHA10 processed at 180 ℃, double melting peaks are observed. Two melting peaks appear as a result of both the crystallization of the 4HB rich phase with the recrystallization of imperfect crystals by second heating of DSC scanning and the crystallization of the 3HB rich phase in the random copolymer. The double melting peak appears in PHA2.1 regardless of the processing temperature, except at the high isothermal crystallization temperature of 110 ℃ with processing at 180 ℃. When PHA10 is processed at 180 ℃, the single melting peak related to imperfect crystals in the 4HB rich phase is obtained. This means that at low processing temperature, the crystallization of 4HB rich phase is dominated in PHA10. Thus, it is understood that the crystallization behavior of PHA10 at 180 °C which many nuclei are formed and grow rapidly to end crystallization as seen in Fig. 6b is due to the crystallization of 4HB rich phase. From this result, it can be seen that crystallization behavior is affected by 4HB content, processing temperature, isothermal crystallization temperature, recrystallization of imperfect crystals formed in the cooling process, and the crystallization of the 3HB rich phase or 4HB rich phase.
The effect of the processing temperature and the isothermal crystallization temperature on the melting temperature and enthalpy obtained from Fig. 10 is shown in Fig. 11. PHA2.1 exhibits high melting temperatures of the double melting peaks regardless of the temperature it was processed, suggesting that more perfect crystals are formed in the 4HB rich and 3HB rich phases compared with PHA10. PHA2.1 processed at 180 ℃ isothermally crystallized at relatively high temperatures and does not show much change in the double melting peak with isothermal crystallization temperature compared with PHA10. The melting peak related to the crystal of 3HB rich phase even disappeared in PHA10 processed at 180 ℃. This indicates that the crystallization of PHA10 processed at 180 ℃ is due to the crystallization at the 4HB rich phase. On the other hand, when processed at 190 ℃, PHA2.1 shows a decrease in only the melting temperature of the 4HB rich phase, but in the case of PHA10 the two melting temperatures both decrease with a slight increase with increasing of isothermal crystallization temperature. This suggests that an increase in chain irregularity with an increase in 4HB content and the formation of low molecular weight chains at higher processing temperatures are factors hindering the crystallization of P(3HB-co-4HB) as a result, the lowering of the perfectness of crystal formed.
The change in the melting enthalpy is shown in Fig. 11b. In the case of PHA2.1, an increase in the isothermal crystallization temperature results in an increase in the imperfect crystals in the 4HB rich phase and a decrease in the crystals in the 3HB rich phase regardless of the processing temperature. PHA10 processed at 180 ℃ shows a similar tendency of crystal formation although the total melting enthalpy is relatively low due to high 4HB content. On the other hand, in the case of PHA10 processed at 180 ℃, only imperfect crystals in the 4HB rich phase are present, and an increase in the isothermal crystallization temperature results in a decrease in these imperfect crystals. The melting enthalpy of 3HB rich phase decreases while that of the imperfect crystals in the 4HB rich phase increases with an increase in the isothermal crystallization temperature, suggesting that crystals obtained at higher isothermal crystallization temperatures become imperfect. That is, for samples processed at 190 ℃, the crystallization rate at low isothermal crystallization temperatures is low but the crystals become more perfect. Contrary to this, for the samples processed at 180 ℃, more imperfect crystals in the 4HB rich phase are formed irrespective of the 4HB content. From these results, it is found that the processing temperature, 4HB content, and isothermal crystallization temperature affect the crystallization behavior and also may be a factor in determining the perfectness of the crystals formed.
4 Conclusions
The formation of low molecular weight P(3HB-co-4HB) by thermal degradation during the melt processing affects the isothermal crystallization behavior of P(3HB-co-4HB). When processed at 180 ℃ closed the melting temperature, less thermal degradation occurs, as a result, high crystallization rates and crystallinity can be obtained with crystallization occurring at 90–110 ℃. When processed at higher temperatures, thermal degradation occurs to decrease the range of crystallization to 40–90 ℃, and the crystallization rate decreases resulting in low crystallinity. The effect of thermal degradation on the isothermal crystallization behavior of P(3HB-co-4HB) is greater in the case of copolymers of high 4HB content. The effect of the melt processing temperature on the crystallization behavior also affects the perfectness of the crystals. It is confirmed that the crystallization behavior in melt processing of P(3HB-co-4HB) can be controlled by the control of not only the 4HB content but also the melt processing temperature.
References
J.G.B. Derraik, The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull. 44, 842 (2022)
D. Janousek, A. Schirmer, H.G. Schlegel, Biodegradation polyhydroxyalkanoic acids. Appl. Microbiol. Biotechnol. 46, 451 (1996)
K. Kasuya, T. Ohura, K. Masuda, Y. Doi, Substrate and binding specificities of bacterial polyhydroxybutyrate depolymerases. Int. J. Biol. Macromol. 24, 329 (1999)
Y. Li, S.N. Yao, C.Y. Han, H.D. Cheng, Miscibility, crystallization and mechanical properties of poly[(3-hydroxybutyrate)-co-(4-hydroxyvalerate)]/poly(propylenecarbonate)/poly(vinyl acetate) ternary blends. Polym. Int. 70, 1544 (2021)
J.F. Zhang, L. Wang, J. Sun, S.L. Jiang, H.F. Li, S. Zhang, W.T. Yang, X.Y. Gu, H. Qiao, A novel hollow microsphere acting on crystallization, mechanical, and thermal performance of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Cryst. 4, e10204 (2014)
H. Norhafini, K.H. Huong, A.A. Amirul, High PHA density fed-batch cultivation strategies for 4HB-rich P(3HB-co-4HB) copolymer production by transformant Cupriavidus malaysiensis USMAA1020. It. J. Biol. Macromol. 125, 1024 (2019)
S. Chanprateep, K. Buasri, A. Muangwong, P. Utiswannakul, Biosynthesis and biocompatibility of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Degrad. Stab. 95, 2003 (2010)
L.J. Han, C.Y. Han, W.L. Cao, X.M. Wang, J.J. Bian, L.S. Dong, Preparation and characterization of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate)/silica nanocomposites. Polym. Eng. Sci. 52, 250 (2012)
S.G. Hong, H.W. Hsu, M.T. Ye, Thermal properties and applications of low molecular weight polyhydroxybutyrate. J. Therm. Anal. Calorim. 111, 1243 (2013)
M.S.A. Aziz, G.R. Saad, H.F. Naguib, Non-isothermal crystallization kinetics of poly(3-hydroxybutyrate) in copo-ly(ester-urethane) nanocomposites based on poly(3-hydroxybutyrate) and cloisite 30B. Thermochim. Acta 605, 52 (2015)
S.G. Hong, Y.C. Lin, C.H. Lin, Crystallization and degradation behaviors of treated polyhydroxybutyrates. React. Funct. Polym. 68, 1516 (2008)
C. Xu, Z. Qiu, Crystallization behavior and thermal property of biodegradable poly(3-hydroxybutyrate)/multi-walled carbon nanotubes nanocomposite. Polym. Adv. Technol. 22, 538 (2011)
O.Y. Yun, X. Min, Y. Li, Properties analysis of biodegradable material P(3HB-co-4HB). Open J. Adv. Mater. Res. 380, 168 (2011)
J.Q. Zhang, K. Kasuya, T. Hikima, M. Takata, A. Takemura, T. Iwata, Mechanical properties, structure analysis and enzymatic degradation of uniaxially cold-drawn films of poly[(R)-3-hydroxybutyrate-co-4-hydroxybutyrate]. Polym. Degrad. Stab. 96, 2130 (2011)
X. Chen, X. Yang, J. Pan, L. Wang, K. Xu, Degradation behaviors of bioabsorbable P3/4HB monofilament suture in vitro and in vivo. J. Biomed. Mater. Res. Part B 92, 447 (2010)
T.H. Ying, D. Ishii, A. Mahara, S. Murakami, K. Yamaoka, R. Sudesh, M. Samian, M. Fujita, T.I. Maeda, Scaffolds from electrospun polyhydroxyalkanoate copolymers: fabrication, characterization, bioabsorption and tissue response. Biomaterials 29, 1307 (2008)
K.H. Huong, C.H. Teh, A.A. Amirul, Microbial-based synthesis of highly elastomeric biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) thermoplastic. Int. J. Biol. Macromol. 101, 983 (2017)
D. Hu, A.L. Chung, L.P. Wu, X. Zhang, Q. Wu, J.C. Chen, G.Q. Chen, Biosynthesis and characterization of polyhydroxyalkanoate block copolymer P3HB-b-P4HB. Biomacromol 12, 3166 (2011)
A. Larrañaga, E. Lizundia, A review on the thermomechanical properties and biodegradation behaviour of polyesters. Eur. Polym. J. 121, 109296 (2019)
M. Jo, Y. Jhang, E. Lee, S. Shin, H.J. Kang, Effect of 4-hydroxybutyrate content on physical properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Polymer (Korea) 46, 661 (2022)
X.M. Che, H.M. Ye, G.Q. Chen, Effects of uracil on crystallization and rheological property of poly(R-3-hydroxybutyrate-co-4-hydroxybutyrate). Compos. Part A 109, 141 (2018)
X.P. Wang, W.F. Li, H. Zhang, D.M. Jia, Effect of sodium benzoate on the crystallization behavior of poly (3-hydroxybutyrate-co-4-hydroxybutyrate). Appl. Mech. Mater. 665, 375 (2014)
Y.C. Yu, Y. Li, C.Y. Han, L.G. Xiao, Enhancement of the properties of biosourced poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by the incorporation of natural orotic acid. Int. J. Biol. Macromol. 136, 764 (2019)
H. Ariffin, H. Nishida, Y. Shirai, M.A. Hassan, Highly selective transformation of poly[(R)-3-hydroxybutyric acid] into trans-crotonic acid by catalytic thermal degradation. Polym. Degrad. Stab. 95, 1375 (2010)
H. Ariffin, H. Nishida, Y. Shirai, M.A. Hassan, Anhydride production as an additional mechanism of poly(3-hydroxybutyrate) pyrolysis. J. Appl. Polym. Sci. 111, 323 (2009)
J.M. Clark, H.M. Pilath, A. Mittal, W.E. Michener, D.J. Robichaud, D.K. Johnson, Direct production of propene from the thermolysis of poly(β-hydroxybutyrate) (PHB). An experimental and DFT investigation. J. Phys. Chem. A 120, 332 (2016)
R. Abate, A. Ballistreri, G. Montando, G. Impallomeni, Thermal degradation of microbial poly(4-hydroxybutyrate). Macromolecules 27, 332 (1994)
K.J. Kim, Y. Doi, H. Abe, D.P. Martin, Thermal degradation behavior of poly(4-hydroxybutyric acid). Polym. Degrad. Stab. 183, 109460 (2021)
R. Abate, A. Ballistreri, G. Montaudo, M. Giuffrida, G. Impallomeni, Separation and structural characterization of cyclic and open chain oligomers produced in the partial pyrolysis of microbial poly(hydroxybutyrates). Macromolecules 28, 7911 (1995)
M. Kunioka, Y. Doi, Thermal degradation of microbial copolyesters poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 23, 1933 (1990)
T. Omura, T. Goto, A. Maehara, S. Kimura, H. Abe, T. Iwata, Thermal degradation behavior of poly[(R)-3-hydroxybutyrate-co-4-hydroxybutyrate]. Polym. Degrad. Stab. 183, 109460 (2021)
S. Nakamura, Y. Doi, M. Scandola, Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 25, 4237 (1992)
M. Jo, T. Zhang, Y. Chang, E. Lee, S. Shin, H.J. Kang, Effect of thermal degradation on physical properties of poly[3-hydroxybutyrate-co-4-hydroxybutyrate]. Polymer (Korea) 46, 757 (2022)
X. Wen, X. Lu, Q. Peng, F. Zhu, N. Zheng, Crystallization behaviors and morphology of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). J. Therm. Anal. Calorim. 109, 959 (2012)
Funding
This research was funded by CJ Cheiljedang Corp.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, T., Jang, Y., Jung, M. et al. The effect melt processing temperature on the isothermal crystallization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromol. Res. 32, 1–12 (2024). https://doi.org/10.1007/s13233-023-00205-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-023-00205-x