Abstract
Food protein hydrolysates contain peptide sequences with dipeptidyl peptidase IV (DPP-IV) inhibitory properties which may find use to improve serum glucose regulation in type 2 diabetics. Four plant protein isolates from hemp (H), pea (P), rice (R) and soy (S) were hydrolysed with three enzyme preparations: Corolase L10 (Cor), Promod 144MG (Prom) and Protamex (Prot). From the 12 hydrolysates generated after 4-h incubation, 7 yielded DPP-IV inhibitory activity <2.0 mg protein equivalent mL−1. Their IC50 values ranged from 0.73 ± 0.11 to 3.54 ± 0.24 mg dry weight (dw) hydrolysate mL−1 for the 4 h P_Prot and 4 h H_Cor hydrolysates, respectively. Simulated gastrointestinal digestion (SGID) of the intact proteins yielded DPP-IV IC50 values between 1.85 ± 0.34 and 4.50 ± 0.55 mg dw hydrolysate mL−1 for the R and H, respectively. The DPP-IV inhibitory potency of the 4 h hydrolysates subjected to SGID (DPP-IV IC50 ranging from 1.00 ± 0.42 to 3.83 ± 0.36 mg dw hydrolysate mL−1 for the SGID of the 4 h S_Prom and 4 h H_Prot, respectively) was generally higher than that of the corresponding intact protein subjected to SGID, with the exception of the SGID of R and that of the 4 h R_Prot hydrolysate which had similar IC50 values (P > 0.05). To our knowledge, this is the first study reporting that H and P protein hydrolysates inhibit DPP-IV in vitro. This study also demonstrates the potential benefit, in some instances, of hydrolysing plant protein substrates prior to oral ingestion with the view of releasing DPP-IV inhibitory peptides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dipeptidyl peptidase IV (DPP-IV) is a ubiquitous enzyme which is involved in the degradation of the incretin hormones, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) [1]. Degradation of GLP-1 and GIP by DPP-IV results in a loss in their ability to induce insulin secretion in pancreatic beta cells following nutrient intake [2, 3]. Inhibition of DPP-IV has been employed as a means to increase incretin half-life in order to maintain insulinotropic activity during the postprandial phase. In this context, various DPP-IV inhibitory drugs, known as gliptins, have been developed for the treatment of type 2 diabetes (T2D). Gliptins generally have DPP-IV half maximum inhibitory concentration values in the nanomolar range [4].
Natural alternatives to DPP-IV inhibitory drugs may exist in the diet. These include DPP-IV inhibitory peptide fragments which can be released following the hydrolysis of food proteins [5]. Based on in silico analyses, a wide range of food proteins have been reported to contain known DPP-IV inhibitory peptide sequences [6–8]. To date, milk proteins are the most studied substrate for the generation of DPP-IV inhibitory peptides [9–14]. Other food protein sources of DPP-IV inhibitory peptides include rice (R) [15], barley [16], amaranth [17], seaweed [18], tuna cooking juice [19], a dried-cured ham water soluble extract [20], Atlantic salmon [21] and porcine skin gelatin [22, 23]. DPP-IV inhibitory peptides from food proteins generally have IC50 values several orders of magnitude higher than those of gliptins. A well-known DPP-IV inhibitory peptide, Ile-Pro-Ile, which was originally discovered in microbial cultures of Bacillus cereus, has an IC50 value in the micromolar range [24]. This peptide sequence, known as diprotin A, can also be found within certain food proteins such as κ-casein, chicken egg ovotransferrin and the phycoerythrin β subunit from Palmaria palmata [7].
While different dietary proteins have been investigated for their DPP-IV inhibitory properties, most substrates described in the literature to date appear to originate from animal proteins. Plant proteins are readily available and are generally more competitively priced compared to animal proteins. In addition, they can be consumed by people who do not eat or restrict animal protein intake in their diet (vegetarian/vegan, Kosher, Halal diet, etc.). Plant proteins have been shown to have a wide range of bioactivities mainly for their antioxidant and antihypertensive properties [25–28]. DPP-IV inhibition has been reported with R protein hydrolysates and several dipeptides with a Pro residue at the C-terminus have been identified as being responsible for the observed DPP-IV inhibition [15]. In addition, several studies have demonstrated the central role of Trp-containing peptides in DPP-IV inhibition [7, 29–31]. Hemp (H), pea (P), R and soy (S) contain Trp concentrations above the requirements for an adult (6 mg g−1 protein day−1 as defined by the FAO guidelines [32], Supplementary Table S1). Therefore, they are potential dietary protein sources for the generation of hydrolysates with Trp-containing peptides.
To our knowledge, apart from the previously mentioned plant proteins (R, amaranth, soybean, black bean, wheat and barley), no other plant proteins appear to have been evaluated for their in vitro DPP-IV inhibitory properties. The aim of this study was to investigate a range of plant protein isolates, i.e. from H, P, R and S for their ability to act as substrates for the generation of DPP-IV inhibitory peptides. This was achieved by enzymatically hydrolysing the plant protein isolates with different food-grade enzyme preparations in order to release DPP-IV inhibitory peptides. An in vitro simulated gastrointestinal digestion (SGID) protocol was utilised to (a) release DPP-IV inhibitory peptides from intact proteins with digestive enzymes and (b) assess the stability to digestive enzymes of the DPP-IV inhibitory peptides within prehydrolysed samples. Finally, an assessment of the presence of Trp-containing peptides within the hydrolysates was conducted using a specific fluorescence detection-based chromatographic technique.
Materials and Methods
Reagents
Hemp (H, 47.0 % (w/w) protein), pea (P, 81.7 % (w/w) protein), brown rice (R, 80.0 % (w/w) protein) and soy (S, 90.0 % (w/w) protein) protein isolates from Pulsin (Gloucester, UK) were purchased in a local food store. Trifluoroacetic acid (TFA), tris(hydroxymethyl)aminomethane (TRIS), sodium phosphate monobasic, sodium phosphate dibasic, Gly-Pro-pNA, Leu, Trp, diprotin A (Ile-Pro-Ile) and porcine DPP-IV (≥10 U mg−1 protein) were obtained from Sigma-Aldrich (Dublin, Ireland). 2,4,6-Trinitrobenzenesulfonic acid (TNBS) was from Pierce Biotechnology (Medical Supply, Dublin, Ireland). Hydrochloric acid (HCl), high-performance liquid chromatography (HPLC)-grade water and acetonitrile (ACN) were from VWR (Dublin, Ireland).
Enzymatic Hydrolysis of the Plant Protein Isolates
The plant protein isolates were resuspended in water at a concentration of 30 g dry weight (dw) L−1 with the exception of the H protein isolate which was resuspended at 60 g dw L−1. The protein isolates were allowed to hydrate for 1 h at 50 °C. A control sample was removed from the protein dispersion and maintained at 50 °C for the duration of the hydrolysis reaction (4 h). Three different enzyme preparations were used to hydrolyse the plant proteins, including a Bacillus proteinase, Protamex® (Prot, Novozymes, Bagsvaerd, Denmark) and two proteinase preparations from Carica papaya latex, i.e. Corolase® L10 (Cor, AB Enzymes, Darmstadt, Germany) and PromodTM 144MG (Prom, Biocatalysts, Cefn Coed, Wales, UK). The enzyme preparations were added to the protein solutions previously adjusted to pH 7.0 at an enzyme to substrate ratio (E/S) of 2 % (w/w for Prot or w/v for Prom and Cor). Hydrolysis was conducted at 50 °C at constant pH (7.0) using a pH stat (Titrando 843, Tiamo 1.4 Metrohm, Dublin, Ireland). At defined times of hydrolysis (1, 2 or 4 h), samples were withdrawn from the reaction vessel and the enzyme was inactivated by heating at 90 °C for 20 min. Hydrolysates were then freeze-dried (FreeZone 18L, Labconco, Kansas City, USA) and stored at −20 °C until further analysis. Because the hydrolysates were generated with plant protein isolates with different protein contents, their potency on a protein equivalent basis was determined to select the samples for regeneration. The IC50 value expressed in protein equivalent was calculated by multiplying the IC50 value in mg dw hydrolysate mL−1 per the protein content of the starting substrate. The hydrolysis reaction for the seven protein hydrolysates which displayed DPP-IV IC50 values <2.0 mg protein equivalent mL−1 after 4-h hydrolysis (Table 1) was performed in duplicate (n = 2).
In Vitro Simulated Gastrointestinal Digestion of the Plant Protein Isolates and Hydrolysates
The plant protein isolates and the hydrolysates generated after 4-h incubation were subjected to in vitro SGID. This was conducted as described by Nongonierma and FitzGerald [33] following the method of Walsh et al. [34]. Briefly, the plant protein isolates or the freeze-dried plant protein hydrolysates were resuspended in water with gentle stirring at 1 % (w/v) on a protein equivalent basis at 37 °C for 30 min. The samples were incubated at 37 °C, pH 2.0 with pepsin (Biocatalysts) for 90 min and then at pH 7.5 with Corolase PP (AB Enzymes), a pancreatic enzyme preparation, for another 150 min. The enzymes were inactivated by heating at 90 °C for 20 min, and the samples were subsequently freeze-dried and stored at −20 °C until utilisation.
Dipeptidyl Peptidase IV Inhibition Assay
The protein hydrolysates were dispersed in HPLC-grade water at concentrations ranging from 31.3 × 10−3 to 5.0 mg dw hydrolysate mL−1 (final concentration). The DPP-IV inhibition assay was carried out as described by Nongonierma and FitzGerald [10]. Briefly, the test samples (25 μL) were pipetted onto a 96-well microplate (Sarstedt, Dublin, Ireland) containing Gly-Pro-pNA (final concentration 0.200 mM). The negative control contained 100 mM Tris-HCl buffer pH 8.0 (25 μL) and Gly-Pro-pNA. The reaction was initiated by the addition of DPP-IV (final concentration 0.0025 U mL−1). Diprotin A was used as a positive control. Each sample was analysed in triplicate. The microplate was incubated at 37 °C for 60 min in a microplate reader (Biotek Synergy HT, Winoosky, VT, USA), and absorbance of the released pNA was monitored at 405 nm. The DPP-IV IC50 values expressed in mg dw hydrolysate mL−1 were determined by plotting the percentage inhibition as a function of the test compound concentration.
Determination of the Free Amino Group Content of the Hydrolysates
The free amino group content of the hydrolysates was determined following the method of Adler-Nissen [35] using TNBS. Absorbance values were measured at 340 nm (Shimadzu UV mini 1240, Kyoto, Japan) which allowed determination of the free amino group (AN) content using the following formula:
with AN1, the amino group content of the unhydrolysed protein isolate (mg N g−1 protein), and AN2, the amino group content of hydrolysed proteins (mg N g−1 protein).
Gel Permeation and Reverse-Phase High-Performance Liquid Chromatography of the Hydrolysates
Gel permeation (GP-) HPLC was used to determine the molecular mass distribution of the peptides within the hydrolysates by the method described by Spellman et al. [36]. Samples were resuspended at 0.25 % (w/v) in 0.1 % TFA and 30 % HPLC-grade ACN in HPLC-grade water. A 600 × 7.5-mm I.D. TSK G2000 SW column mounted with a 75 × 7.5-mm I.D. TSKGEL SW guard column (Tosoh Bioscience, Stuttgart, Germany) was used for separation. A 20-μL sample volume was injected onto the column. The absorbance was monitored at 214 nm.
The peptide profile of different samples was determined as per Nongonierma and FitzGerald [37] using reverse-phase (RP-) HPLC (Waters, Dublin, Ireland) equipped with a 250 × 4.6-mm I.D., 5.0-μm Jupiter C18 column coupled to a C18 guard column (4 × 3 mm I.D., Phenomenex, Cheshire, UK). The absorbance was monitored at 280 and 214 nm. Samples were resuspended at 0.8 % (w/v) in 0.1 % TFA in HPLC water. A 20-μL sample volume was injected onto the column.
Detection of Trp and Trp-containing peptides was carried out as described in Nongonierma and FitzGerald [37] using fluorescence detection (model 2475 multi λ fluo detector, Waters) of indole groups at excitation and emission wavelengths of 280 and 340 nm, respectively. Trp was used for calibration; it was injected (20 μL) in triplicate at different quantities ranging from 0 to 200 nmol (Supplementary Fig. S1).
Statistical Analysis
A one way ANOVA followed by a Student Newman-Keuls post hoc test was carried out at a significance level P < 0.05 using SPSS (version 22, SPSS Inc., Chicago, IL, USA).
Results
DPP-IV Inhibitory Activity of the Plant Protein Hydrolysates
Thirty-six plant protein hydrolysates were generated using four different plant protein isolates from H, P, R and S hydrolysed with three proteo-/peptidolytic enzyme preparations (Cor, Prom and Prot) at three hydrolysis times (1, 2 and 4 h). These were subsequently evaluated for their ability to inhibit DPP-IV. The hydrolysates with the highest DPP-IV inhibitory potency (IC50 < 2.0 mg protein equivalent mL−1 after 4-h hydrolysis) comprised R_Prot, P_Prot, S_Prot, S_Prom, H_Prot, H_Cor and H_Prom. Their DPP-IV IC50 values are reported in Table 1 for the two independent duplicates of these hydrolysates. The DPP-IV IC50 values ranged from 0.73 ± 0.11 to 5.71 ± 1.11 mg dw hydrolysate mL−1 for the 4 h P_Prot and the 1 h H_Cor hydrolysates, respectively. For most of the samples, the IC50 values did not significantly differ between the 1, 2 and 4 h hydrolysates. This may arise from the fact that once released, the peptides were not further degraded by the enzymes following longer incubation times. The H protein hydrolysates generally had higher DPP-IV IC50 values (expressed as mg dw hydrolysate mL−1) than P, R and S protein hydrolysates, which could be explained by the fact that they contained ∼50 % less protein than P, R and S.
The DPP-IV IC50 values obtained for the independent duplicate of the 4 h hydrolysates were not significantly different from each other with the exception of the S_Prot and H_Cor hydrolysates. The significant differences which were seen in the IC50 values of certain duplicate hydrolysates, especially at incubation time ≤2 h (Table 1) may arise from the fact that the plant protein isolates were not fully solubilised in water even after the hydration step (1 h at 50 °C) applied prior to enzymatic hydrolysis. The limited water solubility of the plant protein substrates may have restricted access of the enzyme to the protein substrate in certain instances. However, the IC50 values of the duplicate samples were of the same order. This suggested that generation of the hydrolysates was generally reproducible in terms of their bioactive properties.
DPP-IV Inhibitory Activity of the Unhydrolysed and Hydrolysed Plant Proteins Following SGID
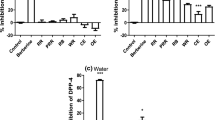
The DPP-IV IC50 values for the unhydrolysed protein and those of the seven 4 h hydrolysates subjected to SGID are illustrated in Fig. 1a. None of the unhydrolysed protein substrates was able to inhibit DPP-IV (data not shown). Following SGID of the proteins, the resulting hydrolysates displayed DPP-IV inhibitory activity with IC50 values ranging from 1.85 ± 0.34 to 4.50 ± 0.55 mg dw hydrolysate mL−1 for the R and H SGID samples, respectively. Interestingly, the IC50 values obtained with P, R and S subjected to SGID were not significantly different (P > 0.05).
a Dipeptidyl peptidase IV (DPP-IV) half maximum inhibitory concentration (IC 50 ) of simulated gastrointestinal digests (SGID) of hemp (H), pea (P), rice (R) and soy (S) proteins hydrolysed for 4 h with Corolase L10 (Cor), Promod 144MG (Prom) or Protamex (Prot) and their associated SGID samples. b Free amino group content of the hydrolysates. Each hydrolysate was independently generated twice (trial 1 and 2). IC50 values expressed in mg dw hydrolysate mL−1 represent the mean ± SD of three replicates (n = 3). Data with different letters are significantly different (P < 0.05)
When the seven 4 h hydrolysates were subjected to SGID, their IC50 value did not significantly change with the exception of the 4 h R_Prot (1.21 ± 0.08 vs. 1.60 ± 0.21 mg dw hydrolysate mL−1 for the 4 h R_Prot before and after SGID, respectively) and 4 h H_Prot (3.09 ± 0.68 vs. 3.82 ± 0.36 mg dw hydrolysate mL−1 for the 4 h H_Prot before and after SGID, respectively) which showed a significantly (P < 0.05) higher IC50 value following SGID. Generally, the DPP-IV IC50 values for the 4 h H, P, R and S protein hydrolysates subjected to SGID were significantly lower (P < 0.05) than for their associated starting protein substrate subjected to SGID. In contrast, the IC50 values for the 4 h R_Prot after SGID and that of the R protein isolate subjected to SGID were not significantly different (Fig. 1a).
Physicochemical Characteristics of the Plant Protein Hydrolysates
The amino group content of the seven DPP-IV inhibitory hydrolysates generated at 4 h and their duplicate samples are shown in Fig. 1b. The amino group content for these samples ranged from 11.3 ± 0.8 to 20.0 ± 0.5 mg N g−1 sample for the 4 h S_Prom and 4 h P_Prot, respectively. For each duplicate, the values obtained for the hydrolysate were not significantly different, with the exception of the 4 h S_Prot and 4 h H_Prom. The lower amino group content for the duplicates of the 4 h S_Prot and 4 h H_Prom may be linked to the higher IC50 value seen with the 4 h S_Prot; in contrast, there was no such effect seen with the 4 h H_Prom. Overall, this showed reproducibility in the enzymatic hydrolysis process.
The peptide profile at 214 nm of the seven 4 h plant protein hydrolysates is illustrated in Fig. 2. The peptide profiles significantly differ. When considering 4 h hydrolysates generated with the same protein isolate, the peptide profile obtained was enzyme-dependent. This can be seen with peptide peaks which are only present in S_Prom at 30.6 min (Fig. 2f) and S_Prot at 23.5 and 26.1 min (Fig. 2g). Similarly, certain peptide peaks are only seen in H_Cor at 27.9 min (Fig. 2a), H_Prom at 17.2 min (Fig. 2b) and H_Prot at 28.3 min (Fig. 2c). This suggested that different peptides may be responsible for the DPP-IV inhibitory properties seen with these samples.
Reverse-phase high-performance liquid chromatographic (RP-HPLC) profiles (absorbance at 214 nm vs. elution time) of hemp (H), pea (P), rice (R) and soy (S) proteins hydrolysed for 4 h with Corolase L10 (Cor), Promod 144MG (Prom) or Protamex (Prot). a H_Cor, b H_Prom, c H_Prot, d P_Prot, e R_Prot, f S_Prom and g S_Prot. On each panel, the profile of the hydrolysate (black), its simulated gastrointestinal digested (SGID, dark grey) sample and the SGID of the corresponding starting protein substrate (light grey) are depicted. The broken lines correspond to the ACN gradient. ACN acetonitrile. For the hydrolysates, the arrows show peptide peaks which differ between substrates. For the SGID samples the numbers (1, 2 and 3) show common peptide peaks in the different samples
The molecular mass distribution of the 4 h hydrolysates together with their unhydrolysed starting protein isolate is shown in Fig. 3a. As expected, the plant isolates mainly comprised large molecular mass components. However, all isolates also had a certain proportion of low molecular mass constituents less than 1 kDa (>14 %) which may be composed of free amino acids and short peptides (Fig. 3a). Following hydrolysis, all the intact proteins were significantly broken down to smaller peptides and/or free amino acids as can be seen by the significant reduction of the proteinaceous components (>5 kDa) in all hydrolysate samples (Fig. 3a). Within samples generated with the same starting substrates, protein breakdown yielded hydrolysates with very different molecular mass distributions. Hydrolysis of R, S and P with Prot for 4 h yielded samples with the highest proportion of low molecular mass peptides (<1 kDa), while for H this was the case on hydrolysis with both Prom and Prot.
a Molecular mass distribution profiles of protein isolates from hemp (H), pea (P), rice (R) and soy (S) and their associated 4 h hydrolysates generated with Corolase L10 (Cor), Promod 144MG (Prom) and Protamex (Prot). b Molecular mass distribution of plant protein isolates and selected 4 h hydrolysates subjected to simulated gastrointestinal digestion (SGID)
Physicochemical Characteristics of the Unhydrolysed and Hydrolysed Plant Proteins Following SGID
Although the peptide profiles for the 4 h hydrolysates before and after SGID appear different, some common peptide peaks (1, 2 and 3) were seen in both samples (Fig. 2). Most of the hydrophobic peptides and proteins eluting at longer retention time were not seen following SGID of the 4 h hydrolysates. The peptide profiles of the plant protein isolates subjected to SGID significantly differ from that of the associated 4 h hydrolysate. Peptide peaks of varying intensities eluting at 16.8, 20.1 and 25.1 min (peaks 1, 2 and 3, respectively) were seen in all SGID samples.
In agreement with the peptide profiles, the samples subjected to SGID contained no or only low amounts of high molecular mass proteinaceous components (>5 kDa) as shown on their molecular mass distribution profiles (Fig. 3b). Significant protein breakdown was seen following SGID of the plant protein isolates, yielding peptides <1 kDa representing >60 % of the sample. In the case of the 4 h hydrolysates obtained following SGID, peptides <1 kDa represented >75 % of the sample. When comparing the molecular mass profiles of the 4 h hydrolysates before and after SGID (Fig. 3a vs. b), it was seen that SGID increased the proportion of short peptides in the hydrolysates.
Trp and Trp-Containing Peptides Within the Hydrolysates and SGID Samples
The RP-HPLC profiles obtained during fluorescence detection of Trp-containing peptides in the twelve 4 h hydrolysates generated with H, P, R and S are illustrated in Fig. 4. All hydrolysates analysed displayed numerous peptide peaks eluting over a wide range of retention times. It is interesting to note that the hydrolysates displaying the higher number of Trp-containing peptides were generally those which also displayed higher DPP-IV inhibitory properties (Fig. 4 and Table 1). This is particularly evident with the P and R substrates where hydrolysates generated with Prot had a higher number of Trp-containing peptide peaks than Cor or Prom hydrolysates. The 4 h P and R Prot hydrolysates were also more potent DPP-IV inhibitors (IC50 values <2.5 mg dw hydrolysate mL−1; Table 1) than the 4 h Cor or Prom P and R hydrolysates.
Reverse-phase high-performance liquid chromatographic (RP-HPLC) profile (relative fluorescence vs. elution time) of the Trp-containing peptides detected in the 4 h plant protein hydrolysates generated from a hemp (H), b pea (P), c rice (R) and d soy (S) using Protamex (Prot, black), Promod 144MG (Prom, dark grey) and Corolase L10 (Cor, light grey). Trp detection in fluorescence mode at excitation and emission wavelengths of 280 and 340 nm, respectively, using the method for the specific detection of indole groups as described by Nongonierma and FitzGerald [37]. The broken lines correspond to the ACN gradient. ACN acetonitrile
Figure 5 depicts the RP-HPLC profile of Trp-containing peptides of the seven most potent DPP-IV inhibitory 4 h hydrolysates (<2.0 mg protein equivalent mL−1 after 4-h hydrolysis) before and after SGID together with that of the SGID of the plant protein isolates. The hydrolysates before and after SGID showed major differences in their Trp-containing peptide profiles. This suggested that different Trp-containing peptides were present in both samples. The peak eluting at 25.1 ± 0.3 min (peak 3 in Fig. 2), corresponding to free Trp (Supplementary Fig. S1), was always more abundant in both the plant protein isolate and hydrolysate subjected to SGID compared to the hydrolysate before SGID (Fig. 5). This indicated in the case of the hydrolysates that certain Trp-containing peptides were further degraded by the proteolytic action of the gastrointestinal enzymes. Interestingly, all three 4 h H protein hydrolysates (Fig. 5a–c) contained significantly more free Trp (peak eluting at 25.1 min) than the 4 h R, S and P protein hydrolysates (Fig. 5d–g). This may be, in part, due to the high amount of free Trp found in the starting H protein isolate used in this study (Supplementary Fig. S2).
Reverse-phase high-performance liquid chromatographic (RP-HPLC) profiles (relative fluorescence vs. elution time) of Trp-containing peptides within hemp (H), pea (P), rice (R) and soy (S) proteins hydrolysed for 4 h with Corolase L10 (Cor), Promod 144MG (Prom) or Protamex (Prot). a H_Cor, b H_Prom, c H_Prot, d P_Prot, e R_Prot, f S_Prom and g S_Prot. On each panel, the profile of the hydrolysate (black), its simulated gastrointestinal digestion sample (SGID, dark grey) and the SGID of the corresponding starting protein substrate (light grey) are depicted. Trp detected in fluorescence mode at excitation and emission wavelengths of 280 and 340 nm, respectively, using the method for the specific detection of indole groups as described by Nongonierma and FitzGerald [37]. The broken lines correspond to the ACN gradient. ACN acetonitrile
Discussion
A limited number of studies exist relating to the ability of plant protein hydrolysates to act as a source of DPP-IV inhibitory peptides [15–17]. The IC50 values obtained with H, P, R and S protein hydrolysates herein are of the same order as previous studies with other plant protein hydrolysates [15, 17]. The IC50 value of 0.73 ± 0.11 mg dw hydrolysate mL−1 for the 4 h P_Prot hydrolysate is one of the lower, and therefore more potent, values reported to date for a plant protein hydrolysate.
Hydrolysis with Prot was shown to yield relatively potent DPP-IV inhibitory hydrolysates with the four plant protein substrates studied herein (Table 1). Prot is a proteolytic preparation containing mainly a Bacillus-like subtilisin activity, which has been shown to cleave peptide bonds after large hydrophobic side chains, such as Phe [38]. SGID of H, P, R and S released peptides with DPP-IV inhibitory activity (Fig. 1a). This result is consistent with a previous study showing the release of DPP-IV inhibitory peptides following SGID of plant proteins [17]. However, there was a benefit in hydrolysing food proteins prior to their SGID, i.e. it yielded more potent DPP-IV inhibition. The similar IC50 values pre- and post-SGID may indicate that the peptides responsible for the DPP-IV inhibition were stable to SGID or that the overall effect on DPP-IV inhibition of the new peptides released after SGID was similar to that of the peptides within the original hydrolysate.
The four protein substrates studied herein are good sources of Trp (Supplementary Table S1). Recently, several Trp-containing peptides have been shown to display relatively low DPP-IV IC50 values [7, 15, 29–31, 39, 40]. Peptide alignment strategies applied to DPP-IV inhibitory peptides with an IC50 value <200 μM generally showed the presence of a Trp residue at the N-terminus and/or a Pro at position 2 of the peptide [7, 41]. Interestingly, H, P, R and S hydrolysates containing several Trp-containing peptides were generally good DPP-IV inhibitors (Fig. 4). The presence of Trp-containing peptides in a hydrolysate, however, does not directly correlate with its DPP-IV inhibitory potential as dipeptides with a C-terminal Trp generally have a low or no DPP-IV inhibition in contrast with the reverse peptide [10, 31, 42]. An increase in free Trp was observed following SGID of the intact proteins and the 4 h hydrolysates. This may, in part, be explained by the fact that chymotrypsin and pepsin can cleave post Trp. Non-Trp-containing peptides may also have contributed to the DPP-IV inhibitory properties reported herein [7]. A better knowledge of the peptide sequences present within the hydrolysates should help to determine their direct contribution to overall DPP-IV inhibitory properties.
To date, three studies have shown that administration of DPP-IV inhibitory milk protein or porcine skin gelatin hydrolysates in vivo could reduce serum glucose level [13, 14, 23]. In addition, Huang et al. [23] showed that a daily administration of 300 mg for 42 days of a porcine skin gelatin hydrolysate to streptozotocin-induced diabetic rats resulted in the inhibition of plasma DPP-IV activity.
Conclusion
It was demonstrated within this study that selected plant protein hydrolysates could inhibit DPP-IV with different potencies depending on the combination of plant protein isolate and enzyme employed. To our knowledge, the in vitro DPP-IV inhibitory activity of H and P protein hydrolysates has been reported for the first time herein. In general, Prot yielded relatively good DPP-IV inhibitory hydrolysates with the four protein substrates employed. In most instances, it was demonstrated that there was a benefit to pre-hydrolyse the plant proteins as SGID of the hydrolysates generally yielded higher DPP-IV inhibitory potency than that of the intact protein subjected to SGID alone. The possible contribution of Trp-containing peptides to DPP-IV inhibitory properties was studied. In general, hydrolysates with good DPP-IV inhibitory properties had a wider range of Trp-containing peptides compared to hydrolysates with a lower potency. Future work will involve identification of the peptides within the hydrolysates and subsequent confirmatory studies with synthetic peptides. The DPP-IV inhibitory potential of the plant-derived hydrolysates also needs to be assessed in vivo to demonstrate that the in vitro results observed herein, in particular after SGID, translate to a reduction in postprandial serum glucose level in humans.
References
Drucker DJ (2006) Enhancing the action of incretin hormones: a new whey forward? Endocrinology 147:3171–3172
Bjelke JR, Christensen J, Nielsen PF, Branner S, Kanstrup AB, Wagtmann N, Rasmussen HB (2006) Dipeptidyl peptidases 8 and 9: specificity and molecular characterization compared with dipeptidyl peptidase IV. Biochem J 396:391–399
Juillerat-Jeanneret L (2014) Dipeptidyl peptidase IV and its inhibitors: therapeutics for type 2 diabetes and what else? J Med Chem 57:2197–2212
Sortino MA, Sinagra T, Canonico PL (2013) Linagliptin: a thorough characterization beyond its clinical efficacy. Front Endocrinol 4:(Article 16) 11–19
Lacroix IME, Li-Chan ECY (2014) Overview of food products and dietary constituents with antidiabetic properties and their putative mechanisms of action: a natural approach to complement pharmacotherapy in the management of diabetes. Mol Nutr Food Res 58:61–78
Lacroix IME, Li-Chan ECY (2012) Evaluation of the potential of dietary proteins as precursors of dipeptidyl peptidase (DPP)-IV inhibitors by an in silico approach. J Funct Foods 4:403–422
Nongonierma AB, FitzGerald RJ (2014) An in silico model to predict the potential of dietary proteins as sources of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Chem 165:489–498
Udenigwe CC, Gong M, Wu S (2013) In silico analysis of the large and small subunits of cereal RuBisCO as precursors of cryptic bioactive peptides. Process Biochem 48:1794–1799
Lacroix IME, Li-Chan ECY (2012) Dipeptidyl peptidase-IV inhibitory activity of dairy protein hydrolysates. Int Dairy J 25:97–102
Nongonierma AB, FitzGerald RJ (2013) Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk-derived dipeptides and hydrolysates. Peptides 39:157–163
Silveira ST, Martínez-Maqueda D, Recio I, Hernández-Ledesma B (2013) Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem 141:1072–1077
Tulipano G, Sibilia V, Caroli AM, Cocchi D (2011) Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides 32:835–838
Uchida M, Ohshiba Y, Mogami O (2011) Novel dipeptidyl peptidase-4-inhibiting peptide derived from β-lactoglobulin. J Pharmacol Sci 117:63–66
Uenishi H, Kabuki T, Seto Y, Serizawa A, Nakajima H (2012) Isolation and identification of casein-derived dipeptidyl-peptidase 4 (DPP-4)-inhibitory peptide LPQNIPPL from gouda-type cheese and its effect on plasma glucose in rats. Int Dairy J 22:24–30
Hatanaka T et al (2012) Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem 134:797–802
Connolly A, Piggott CO, FitzGerald RJ (2014) In vitro α-glucosidase, angiotensin converting enzyme and dipeptidyl peptidase-IV inhibitory properties of brewers’ spent grain protein hydrolysates. Food Res Int 56:100–107
Velarde-Salcedo AJ, Barrera-Pacheco A, Lara-González S, Montero-Morán GM, Díaz-Gois A, González de Mejia E, Barba de la Rosa AP (2013) In vitro inhibition of dipeptidyl peptidase IV by peptides derived from the hydrolysis of amaranth (Amaranthus hypochondriacus L.) proteins. Food Chem 136:758–764
Harnedy PA, FitzGerald RJ (2013) In vitro assessment of the cardioprotective, anti-diabetic and antioxidant potential of Palmaria palmata protein hydrolysates. J Appl Phycol 25:1793–1803
Huang S-L, Jao C-L, Ho K-P, Hsu K-C (2012) Dipeptidyl-peptidase IV inhibitory activity of peptides derived from tuna cooking juice hydrolysates. Peptides 35:114–121
Gallego M, Aristoy M-C, Toldrá F (2014) Dipeptidyl peptidase IV inhibitory peptides generated in Spanish dry-cured ham. Meat Sci 96:757–761
Li-Chan ECY, Hunag S-L, Jao C-L, Ho K-P, Hsu K-C (2012) Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J Agric Food Chem 60:973–978
Hsu K-C, Tung Y-S, Huang S-L, Jao C-L (2013) Dipeptidyl peptidase-IV inhibitory activity of peptides in porcine skin gelatin hydrolysates. In: Hernández-Ledesma B (ed) Bioactive food peptides in health and disease. In Tech, http://www.intechopen.com/books/bioactive-food-peptides-in-health-and-disease/dipeptidyl-peptidase-iv-inhibitory-activity-of-peptides-in-porcine-skin-gelatin-hydrolysates. DOI: 10.5772/51264, pp 205–218.
Huang S-L, Hung C-C, Jao C-L, Tung Y-S, Hsu K-C (2014) Porcine skin gelatin hydrolysate as a dipeptidyl peptidase IV inhibitor improves glycemic control in streptozotocin-induced diabetic rats. J Funct Foods 11:235–242
Umezawa H, Aoyagi T, Ogawa K, Naganawa H, Hamada M, Takeuchi T (1984) Diprotins A and B, inhibitors of dipeptidyl aminopeptidase IV, produced by bacteria. J Antibiot 37:422–425
Garcia Mora P, Peñas E, Frias J, Martinez-Villaluenga C (2014) Savinase the most suitable enzyme for releasing peptides from lentil (Lens culinaris var. Castellana) protein concentrates with multifunctional properties. J Agric Food Chem 62(18):4166–4174
Girgih AT, He R, Malomo S, Offengenden M, Wu J, Aluko RE (2014) Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J Funct Foods 6:384–394
López‐Barrios L, Gutiérrez‐Uribe JA, Serna‐Saldívar SO (2014) Bioactive peptides and hydrolysates from pulses and their potential use as functional ingredients. J Food Sci 79:R273–R283
Singh BP, Vij S, Hati S (2014) Functional significance of bioactive peptides derived from soybean. Peptides 54:171–179
Hikida A, Ito K, Motoyama T, Kato R, Kawarasaki Y (2013) Systematic analysis of a dipeptide library for inhibitor development using human dipeptidyl peptidase IV produced by a Saccharomyces cerevisiae expression system. Biochem Biophys Res Commun 430:1217–1222
Lan VTT, Ito K, Ito S, Kawarasaki Y (2014) Trp-Arg-Xaa tripeptides act as uncompetitive-type inhibitors of human dipeptidyl peptidase IV. Peptides 54:166–170
Nongonierma AB, FitzGerald RJ (2013) Inhibition of dipeptidyl peptidase IV (DPP-IV) by tryptophan containing dipeptides. Food Funct 4:1843–1849
FAO (2013) Dietary protein quality evaluation in human nutrition. Report of an FAO Expert Consultation. FAO Food Nutr Pap 92:1–79
Nongonierma AB, FitzGerald RJ (2013) Dipeptidyl peptidase IV inhibitory properties of a whey protein hydrolysate: influence of fractionation, stability to simulated gastrointestinal digestion and food-drug interaction. Int Dairy J 32:33–39
Walsh DJ et al (2004) In vitro generation and stability of the lactokinin β-lactoglobulin fragment (142–148). J Dairy Sci 87:3845–3857
Adler-Nissen J (1986) Enzymic hydrolysis of food proteins. Elsevier Applied Science Publishers, London
Spellman D, O’Cuinn G, FitzGerald RJ (2009) Bitterness in Bacillus proteinase hydrolysates of whey proteins. Food Chem 114:440–446
Nongonierma AB, FitzGerald RJ (2012) Tryptophan-containing milk protein-derived dipeptides inhibit xanthine oxidase. Peptides 37:263–272
Groen H, Meldal M, Breddam K (1992) Extensive comparison of the substrate preferences of two subtilisins as determined with peptide substrates which are based on the principle of intramolecular quenching. Biochemistry 31:6011–6018
Lacroix IM, Li-Chan EC (2014) Peptide array on cellulose support—a screening tool to identify peptides with dipeptidyl-peptidase IV inhibitory activity within the sequence of α-lactalbumin. Int J Mol Sci 15:20846–20858
Nongonierma AB, FitzGerald RJ (2013) Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing peptides. J Funct Foods 5:1909–1917
Tulipano G, Faggi L, Nardone A, Cocchi D, Caroli AM (2015) Characterisation of the potential of β-lactoglobulin and α-lactalbumin as sources of bioactive peptides affecting incretin function: in silico and in vitro comparative studies. Int Dairy J. doi:10.1016/j.idairyj.2015.01.008
Nongonierma AB, Mooney C, Shields DC, FitzGerald RJ (2013) Inhibition of dipeptidyl peptidase IV and xanthine oxidase by amino acids and dipeptides. Food Chem 141:644–653
Acknowledgments
The work described herein was supported by Enterprise Ireland under Grant Number TC2013-0001.
Conflict of Interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table S1
Essential amino acid contents in hemp (H), pea (P), rice (R) and soy (S) proteins, and their adult daily requirements (source: FAO [32]) and average amino acid composition as indicated by the supplier (Pulsin) of the protein isolates. (DOC 95 kb)
Fig. S1
(A) Reverse-phase high performance liquid chromatographic (RP-HPLC) profile of free Trp (200 nmol) detected in fluorescence mode at excitation and emission wavelengths of 280 and 340 nm, respectively, using the method for the specific detection of indole groups as described by Nongonierma and FitzGerald [37] and (B) Trp calibration curve obtained in fluorescence mode. ACN: acetonitrile. (DOC 143 kb)
Fig. S2
Reverse-phase high performance liquid chromatographic (RP-HPLC) profile of free Trp detected in the hemp protein isolate. Trp detection in fluorescence mode at excitation and emission wavelengths of 280 and 340 nm, respectively, using the method for the specific detection of indole groups as described by Nongonierma and FitzGerald [37]. ACN: acetonitrile. (DOC 113 kb)
Rights and permissions
About this article
Cite this article
Nongonierma, A.B., FitzGerald, R.J. Investigation of the Potential of Hemp, Pea, Rice and Soy Protein Hydrolysates as a Source of Dipeptidyl Peptidase IV (DPP-IV) Inhibitory Peptides. Food Dig. Res Curr Opin 6, 19–29 (2015). https://doi.org/10.1007/s13228-015-0039-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13228-015-0039-2