Abstract
Study Objective
To evaluate contained bag electromechanical morcellation for removal of myomas and uterus with myomas, laparoscopically (Study group B), and compare it with uncontained laparoscopic morcellation (Control group A) in patients with similar parameters done earlier.
Design
Retrospective Cohort Comparative Study (Canadian Task Force 2-1).
Setting
Advanced Gynaecologic MAS, university recognized tertiary centre, Mumbai, India.
Patients
720 women had laparoscopic removal of myomas or large uterus with myomas during a study period of 6 years (from 13 May 2012 to 14 August 2018) with contained bag electromechanical or conventional morcellation.
Interventions
Laparoscopic hysterectomy, laparoscopic myomectomy, conventional uncontained morcellation, contained in-bag morcellation.
Main Outcomes Measures
Laparoscopic contained in-bag morcellation was compared with conventional morcellation of myomas and uterus with large myomas during a study period of 6 years. Parameters assessed were operating time, time for insertion of bag, morcellation of tissues and removal of bag, blood loss, complications, conversion to open surgery and histopathologic findings of tissues. In Group A, in the first 3 years, 355 women underwent uncontained morcellation. Myoma size and weight varied from 5 cm to 26 cm and 200 g to 3740 g respectively. The myoma number ranged from 1 to 18. No case of leiomyosarcoma was reported. In Group B, in the next 3 years, 365 women underwent contained bag morcellation in 196 myomectomy cases and 169 hysterectomy cases. Myoma size and weight varied from 4 cm to 20 cm and 200 g to 2100 g respectively. The number of myomas varied from 1 to 17.
Results and Conclusion
Laparoscopic contained bag morcellation for myomas and uterus with large myomas were evaluated. In myomectomy group both conventional and in bag laparoscopic morcellation were comparable in terms of duration of the surgery and blood loss. When all cases ( hysterectomy and myomectomy combined together) and cases of hysterectomy with large fibroid were studied, laparoscopic in bag morcellation took less operative time and there was statistically significant difference in operative time . No case of leiomyosarcoma was found in our study of 720 cases of myomas or uterus with large myomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After laparoscopic morcellation for fibroid and large uterus was accepted [1], a controversy erupted when one case of leiomyosarcoma was diagnosed in a patient of laparoscopic hysterectomy in which morcellation lead to spread of sarcoma with significant morbidity. This brought a halt in laparoscopic morcellation followed by restricted use with black box warning on laparoscopic morcellation by USFDA in 2014 [2].
Though the risk of myomectomy or removal of large uterus involves many steps before morcellation, which are same with open or laparoscopic surgery yet, morcellation is blamed for spread of cells.
In view of USFDA caution with main objective of acceptance and safety, we undertook a comparative study at Total Health Care Centre, Mumbai, India, to evaluate 365 cases of laparoscopic contained bag morcellation of myomas or large uterus with myoma from 14 May 2015 to 14 August 2018 (Group B) with the earlier technique of uncontained laparoscopic morcellation from 13 May 2012 to 13 May 2015 in 355 similar cases (Group A) (Table 1).
Materials and Methods
Patients
Group A
One hundred and fifty-one cases of laparoscopic myomectomy and 204 cases of laparoscopic hysterectomy with more than 14-weeks size uterus were included.
Group B
One hundred and ninety-six cases of laparoscopic myomectomy and 169 cases of laparoscopic hysterectomy with more than 14-weeks size uterus were included.
All cases, laparoscopic myomectomy or hysterectomy for large uterus with fibroids, required morcellation as an essential step for effective surgery.
All patients in both groups gave consent in writing prior to recruitment in the study. Due approval of Institutional Ethics Committee was taken, which was granted.
All patients had standard preoperative work-up, sonography and Color Doppler prior to surgery for diagnosis and mapping of myomas. MRI was not done as a routine due to cost factor and limited utility.
Inclusion Criteria for the Study
-
1.
Patients with fibroids not responding to medical treatment for infertility or excessive bleeding requiring myomectomy to conserve the uterus.
-
2.
Patients needing hysterectomy with uterus more than 14-weeks size with fibroid or hysterectomy in previous caesarean section, needing bisection, coring or cutting the uterus for vaginal removal.
-
3.
Patients keen for minimal access laparoscopic surgery.
-
4.
No high-risk factors disallowing laparoscopic surgery.
-
5.
Patients who consented after duly understanding the pros, cons and risk of spread of pathology by removing tissue laparoscopically after receiving information about alternative techniques.
Exclusion
-
1.
Any case requiring morcellation for removal of uterus, vaginal or laparoscopic, but with no myomas.
-
2.
Any uterus with myomas which was removed intact vaginally, where no morcellation, bisection, coring or cutting of uterus was needed.
-
3.
Any uterus with myoma of more than 30 weeks in size or myoma more than 20 cm, in the study group.
Total Healthcare Method is Very Specific with Defined Steps
All patients were operated under general anaesthesia and in modified lithotomy position.
Technique of Laparoscopic Myomectomy—151 Cases (Group A)
A 4-port laparoscopy with 10-mm primary port being umbilical or 2 inches above the palpable size of uterus in large pathology or uppermost point of vertical scar is done. Three 5-mm accessory ports, 2 on the left, 9–10 cm apart and 1 on right, higher than left lower port are taken. Dilute vasopressin 20 units in 200 ml of normal saline is injected [3], quantity of drug injected depends on the size of fibroid. Horizontal incision on bulge of the myoma is made with monopolar spatula or active blade of harmonic scalpel. For a large fibroid, an elliptical incision is made to reduce dead space for endosuturing.
Fibroid is stabilized with myoma screw and dissected out with scissors or harmonic scalpel. Once separated, the fibroid is placed at the ileo-caecal region. A check on the number of specimens is kept.
Large myoma defects are closed in two layers with barbed sutures. Myoma less than 4 cm is sutured in single layer in interrupted fashion.
All separated myomas are removed using a 15-mm reusable morcellator from the left lower port, always under vision. Morcellation site wound is closed with port closure sutures.
Technique of Laparoscopic Hysterectomy for Large Uterus—204 Cases (Group A)
Port placement is like laparoscopic myomectomy. In all cases, the first step is clipping of the uterine artery at the origin, after opening the retroperitoneum, with 5-mm silastic clips to reduce bleeding and safeguarding ureter.
Usually harmonic is used for dissection and ligasure 5-mm vessel sealer is used for laparoscopic hysterectomy [4] for uterus with large fibroids. Steps of laparoscopic hysterectomy, viz. coagulation and cutting of round ligaments and cornual structures, opening of the utero-vesical fold of peritoneum and pushing the bladder below the cervix, dissecting posterior peritoneum till utero-sacrals and incising them, coagulation and cutting of uterine vessel complex and cardinals ligaments, colpotomy and vault closure, are standardized. In previous caesarean, lateral window approach is used to push bladder below cervix [5].
Laparoscopic morcellation is done from left lower port, widened to 15 mm. Hemostasis and ureteric peristalsis are checked.
Laparoscopic Myomectomy—196 Cases and Laparoscopic Hysterectomy—169 Cases (Group B)
The steps of laparoscopic myomectomy and laparoscopic hysterectomy are as above. After separation of fibroid or uterus, contained morcellation was done as follows:
Surgical Steps of Contained Bag Morcellation
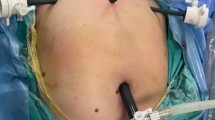
Polyurethane stomach-shaped bag (Fig. 1) is used, it is available in 3 sizes, with mouth of 13, 15 and 17 cm diameter and volume of 1.6, 2.1 and 2.6 L, respectively. Left lower port is widened by 15-mm blunt obturator of morcellator. A plastic open trocar comes with the sterile bag. Mouth of the bag is held to fit in the open plastic trocar, inserted from left lower port, assistant holds the edge of the bag’s mouth (Fig. 2), and surgeon removes the plastic open trocar. By a series of horizontal pulls on the bag, it is brought in the abdomen. Left lower port is replaced by 10-mm cannula with reducer, and if gas leaked, towel clip is applied on skin. After bag slided into the pelvis, mouth of bag is opened. Assistant stabilizes 12 O’clock position of bag, and surgeon stabilizes 6 O’clock position to keep the mouth open, facing the camera. Trendelenburg position is removed, and surgeon holds the fibroid or uterus with a tenaculum and puts the specimen inside the bag. Multiple fibroids can thus be put into the bag (Fig. 3). Once uterus or all fibroids are in, edges of the mouth of the bag are held closed from assistant’s to surgeon’s side. Lastly, lateral edge of the mouth close to the morcellation port is held and brought out, pulling a part of the bag outside. Mouth of the bag is pulled out of the abdomen (Fig. 4). Next, duodenum-shaped tail is inserted in a railroad fashion into the primary cannula (Fig. 5). Thus, the mouth end is out of the left lower port and the tail end is out of the primary port. This tail end has an opening, and 10-mm cannula is inserted through it (Fig. 6). Once pneumoperitoneum is created, CO2 through the primary cannula distends the bag. Optics is passed which showed the specimen in the bag. The entire peritoneal cavity and abdominal structures are out of the bag. Morcellator of 15 mm with blunt trocar is introduced through left lower port, after removing trocar and introducing grasper. The entire specimen is morcellated (Figs. 7, 8, 9).
Morcellator is removed, and primary cannula is withdrawn. A knot is tied at the tail end of bag below the opening through which the optics is passed (Fig. 10). Pneumoperitoneum is deflated, and by pulling the mouth of the bag from left lower port, the entire bag is removed (Fig. 11). In all 365 cases, bag integrity was checked with 1.5litre of diluted methylene blue instilled in the bag (Fig. 12). Hemostasis is checked and morcellation port is closed with port closure needle.
All patients were discharged in 48 h, and follow-up was done clinically, with sonography and MRI after 6 months and 1 year in 85% cases. No case of residual tumour, leiomyosarcoma or leiomyomatosis was found.
Results
Parameters such as duration of surgery, time for insertion of bag, morcellation of tissues, removal of bag, blood loss, complications, conversion to open surgery (Table 2, 3, 4, 5) and histopathologic findings were analyzed. There were no cases of leiomyosarcoma, 2 cases of symplastic tumor with no malignancy were noted. In both Group A and Group B, parameters like duration of surgery, blood loss and complications were studied and it was noted that contained morcellation added safety.
Laparoscopic contained bag morcellation for myomas and uterus with large myomas were evaluated. In myomectomy group both conventional and in bag laparoscopic morcellation were comparable in terms of duration of the surgery and blood loss. When all cases (hysterectomy and myomectomy combined together) and cases of hysterectomy with large fibroid were studied it was noted that laparoscopic in bag morcellation took less operative time and there was statistically significant difference in operative time. No case of leiomyosarcoma was found in our study of 720 cases of myomas or uterus with large myomas.
Demographic and clinical characteristics of patients, both in group A and in group B have been presented (Table 5).
Overall, laparoscopic contained electromechanical morcellation took 17 min of average time for insertion of bag, putting specimen in the bag and removal of bag, regardless of size, weight and number of fibroids. Overall time was saved as scattering or spillage of any material was avoided. In the study group, bag was cut due to technical issue in two patients followed by conventional morcellation and only in one case open surgery was needed to remove very large fibroids.
It was observed that multiple fibroids as many as 17 of variable size and weight, as big as 20 cm and very large uterus with fibroids up to 30 weeks weighing till 2100 g, can be accommodated in the bag for contained morcellation (Table 5).
Discussion
Thousands of women have benefited from minimally invasive laparoscopic surgery in many countries. Few cases of accidental leiomyosarcoma should not be the reason for patients of fibroids who are at low risk of leiomyosarcoma to not have access to the benefits of minimal access surgery.
Issue of laparoscopic morcellation has been put under caution and warning without giving adequate opportunity to find a solution. There is no debate that even one case of accidental leiomyosarcoma morcellated could increase the spread of tumour affecting patient safety in terms of morbidity and post-operative lifespan, but it is not the same for myoma. There is a myth that morcellation shortens the lifespan, but it is applicable for leiomyosarcoma and has no relevance for myomas.
Considering the high incidence of fibroids irrespective of the age of the woman and the number of laparoscopic myomectomies done globally huge benefit of minimal access surgery is imparted to women, reported cases of leiomyosarcoma [6] have been statistically insignificant. No definitive methods of diagnosing leiomyosarcoma are currently available. In the given situation, young women in reproductive age group needing myomectomy and also elderly women needing hysterectomy for large fibroids will be deprived of MIS and will be forced to do open surgery which has more morbidity, hospital stay and expenses.
The steps of both methods of myomectomy—open or laparoscopic, are same, viz. injecting diluted vasopressin, dissection of fibroids. Further dissected fibroids that are separated, come in contact with surrounding structures. Even during closure if we are closing the sarcoma or cancerous bed with needle and suture which touches all tissues, it can spread myoma, sarcomatous or malignant cells. In both, there can be residual leiomyosarcoma which has already spread in blood. This pre-existent spread cannot be prevented.
Widening the belly button or colpotomy or vaginal morcellation of large specimen can spread sarcoma or malignancy as the tissue and blood touch healthy tissues or peritoneal cavity.
The whole blame of spread of cancerous cells is put on laparoscopic morcellation mainly due to spinning effect of tissues morcellated, which can spread sarcoma or even lead to leiomyomatosis.
With all these issues, our study involved obtaining an acceptable technique. Laparoscopic contained morcellation in 365 cases of fibroid or large uterus with fibroids over 3 years was compared to a similar group of patients with conventional electromechanical morcellation done in 355 cases over 3 years earlier.
As seen, the technique of contained morcellation proves to be acceptable, safe and can be mastered by gynaecologists globally.
All the parameters are comparable with safety. Laparoscopic morcellation can be made acceptable in contained fashion.
Laparoscopic radical hysterectomy with node dissection is accepted [7] wherein tissue may be positive for cancer cells. MRI-guided focused ultrasound is accepted [8] as a treatment modality for fibroids without ruling out the possibility of leiomyosarcoma; similarly uterine artery embolization is also accepted.
Few study designs have focused on uterine cancers including accidental cancer in hysterectomy, myomectomy, uterine prolapse other than just myoma removal or hysterectomy for myoma uterus, wherein leiomyosarcoma is a surprise finding. These falsely exaggerate the possibility of leiomyosarcoma incidence, which were actually other uterine cancers too. These other uterine cancers can be picked up by Pap smear, ultrasonography, Color Doppler, curettage, aspiration cytology or hysteroscopy. The data are highly biased and of poor quality [9]. Our study is focused only on presumed myoma with accidental finding of leiomyosarcoma, which is specific and there were no documented leiomyosarcoma in the 720 cases included.
A meta-analysis reveals that the rate of leiomyosarcoma was 1 in 2000, restricting to the 64 studies; projected incidence of leiomyosarcoma was 1 in 8300 [9].
One Austrian multi-institutional study by Mayerhofer et al. [10] of 71 cases of leiomyosarcoma revealed that none developed from a confirmed myoma. Thus, leiomyosarcoma is primarily a separate tumour thought to be arising from myoma, which is a myth.
Genetic differences between fibroids and leiomyosarcomas indicate that leiomyosarcomas do not result from the malignant degeneration of fibroids. Cluster analysis of 146 genes found that the majority of genes are down-regulated in leiomyosarcomas but not in fibroids or myometrium. Comparative genomic hybridization did not find specific anomalies shared by fibroids and leiomyosarcomas [11].
Pritts et al. [12] found that, out of 2582 unsuspicious laparoscopic myomectomies, there was no case of leiomyosarcoma, but 7 cases of atypical myomas were found in 1216 subjects, aged 18–45 years. Prevalence of leiomyosarcoma was 0% and atypical myoma was 0.6%.
In a prospective study on peritoneal washings before and after morcellation [13], 21 patients had laparoscopic myomectomy; washings were collected 3 times, first on primary vision, second after removal of myoma and closure and third after uncontained power morcellation.
Cell block histology detected spindle cells in 6 post-morcellation samples; 3 had spindle cells detected on the post-myomectomy closure samples.
In 6 of 7 cases (85.7%) cases of in bag morcellation system used during laparoscopic hysterectomy [14], surgery was successful, and morcellated specimens ranged from 205 g to 638 g (median 413). Average time associated to bag use was 16.2 ± 7.65 min (median 14 min). Spread of spindle cells were detected in two cases after uncontained morcellation, but not after in-bag morcellation.
In one of our study of 21 cases of laparoscopic in-bag [4] morcellation of fibroid and uterus (14 hysterectomy specimens with maximum weight of 1.4 kg and 7 myomectomy specimens with fibroids ranging from 4 to 7 cm in size) the technique was safe and useful.
Conclusion
With the US FDA restriction on laparoscopic morcellation in 2014, there is a concern especially for morcellation of myoma or uterus with myomas globally in high-volume advanced laparoscopic surgery units.
Total Health Care Centre in India did a comparative study over 6 years, evaluating acceptability of laparoscopic contained morcellation (365 cases) versus laparoscopic conventional morcellation (355 cases) in women presumed to have myomas or uterus with myomas from 13 May 2012 to 14 August 2018.
In cases of Laparaoscopic myomectomy, conventional or in bag contained morcellation operating time and blood loss was comparable. However, in Laparoscopic Hysterectomy for large fibroids, in bag morcellation had less operating time which was statistically significant. There was no case of Leiomyosarcoma or mortality in either group.
It was observed that for specimen weighing up to 2100 g, with multiple fibroids (up to 17 in number) and maximum diameter of 20 cm, contained morcellation was possible.
Whether a myoma develops or transforms to leiomyosarcoma is unwarranted and undocumented. Further the current available modalities may not be able to diagnose leiomyosarcoma, but surely all other uterine cancers can be diagnosed by simpler means.
Our study of contained morcellation protects the interest of a few million women who are bereaved of the benefit of laparoscopic minimal access surgery, forcing to resort to open surgery or widening the minimal access port.
One cannot club all uterine cancers in the same group to prevent morcellation of myoma proved to be non-cancerous. The error of some to morcellate uterus harbouring cancer or leiomyosarcoma has nothing to do with morcellation of myoma proved to be non-cancerous by all available current modalities.
Further, our study also questions that how MRI-guided focused ultrasound and uterine artery embolization are accepted by US FDA as treatment modalities even without tissue diagnosis. Even laparoscopic surgery for uterine and early cervical cancers can be accepted even though we are dissecting tissues with possible malignant cells.
In our study, no case of leiomyosarcoma was found in patients with myomas. Contained electromechanical morcellation for myomas or uterus with myomas is acceptable.
The authors strongly believe and request all major gynaecological endoscopy organizations of the world, focused on women’s health to rise up to the expectation of protecting women’s right for minimal access surgery for myomas, as they deserve.
References
Steiner RA, Wight E, Tadir Y, Haller U. Electrical cutting device for laparoscopic removal of tissue from the abdominal cavity. Obstet Gynecol. 1993;81(3):471–4.
Food and Drug Administration. FDA discourages use of Laparoscopic power morcellation for removal of uterus or uterine fibroids. Food Drug Adm. 2014;17:4.
Cohen SL, Wang KC, Gargiulo AR, et al. Vasopressin administration during laparoscopic myomectomy: a randomized controlled trial. J Minim Invasive Gynecol. 2015;22(6S):S39.
Trivedi PH, Patil SS, Parekh NA, et al. Laparoscopic morcellation of fibroid and uterus in-bag. J Obstet Gynecol India. 2015;65(6):396–400.
Sheth SS. An approach to vasicouterine peritoneum through a new surgical space. J Gynaecol Surg. 1996;12(2):135–40.
Harlow BL, Weiss NS, Lofton S. The epidemiology of sarcomas of the uterus. J Natl Cancer Inst. 1986;76(3):399–402.
Hilaris GE, Tsoubis T, Konstantopoulos V, Pavlakis K. Feasibility, safety, and cost outcomes of laparoscopic management of early endometrial and cervical malignancy. JSLS. 2009;13(4):489–95.
Shen S, Fennessy F, McDannold N. Image-guided thermal therapy of uterine fibroids. Semin Ultrasound CT MRI. 2009;30(2):91–104.
Pritts EA, Parker WH, Brown J, et al. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids needs a systematic review. J Minim Invasive Gynecol. 2015;22(1):26–33.
Mayerhofer K, Obermair A, Windbichler G, et al. Leiomyosarcoma of the uterus: a clinicopathologic multicenter study of 71 cases. Gynecol Oncol. 1999;74(2):196–201.
Quade BJ, Wang TY, Sornberger K, et al. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosom Cancer. 2004;40:97–108.
Pritts EA, Vanness DJ, Olive DL. Prevalence of occult leiomyosarcomas and atypical leiomyomas after laparoscopic morcellation of leiomyomas in reproductive-age women. Gynecol Surg. 2015;12(3):165–77.
Pados G, et al. Peritoneal washings after power morcellation in laparoscopic myomectomy: a pilot study. HumReprod. 2017;32(10):2036–41.
Rimbach S, Holzknecht A, Schmedler C, et al. Austria first clinical experiences using a new in-bag morcellation system during laparoscopic hysterectomy. Arch Gynecol Obstet. 2016;294(1):83–93.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We, the authors, Prakash H. Trivedi, Soumil Trivedi and Sandeep Patil, state that there is no conflict of interest or any financial disclosures.
Ethical Approval
All procedures followed were in accordance with the ethical standards of the responsible committee, and informed consent was obtained from all patients for being included in the study.
Human and Animals Rights
This article does not contain any studies with animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr. Prakash H. Trivedi is the Scientific Director of Dr. Trivedi’s Total Health Care Pvt. Ltd. & Aakar IVF Centre, Professor and the Head of Department of Obstetrics and Gynaecology, Rajawadi Hospital; Dr. Soumil Trivedi is a Speciality Medical Consultant (SMC) in Rajawadi Hospital; Consultant in Dr. Trivedi’s Total Health Care Pvt. Ltd. & Aakar IVF Centre. Dr. Sandeep Patil is a DNB; FMAS Consultant & Partner in Yashadaa Hospital, Bhandup Mumbai
Rights and permissions
About this article
Cite this article
Trivedi, P.H., Trivedi, S. & Patil, S. Laparoscopic In-Bag Morcellation Compared with Conventional Morcellation of Myomas and Uterus with Myomas. J Obstet Gynecol India 70, 69–77 (2020). https://doi.org/10.1007/s13224-019-01273-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-019-01273-9