Abstract
Objective
Levonorgestrel-releasing intrauterine system (LNG-IUS) has been shown to be an effective treatment for patients with abnormal uterine bleeding (AUB) in many Western studies. The purpose of study was to examine the effectiveness of LNG-IUS in the treatment of Indian women with AUB.
Methods
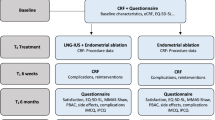
We conducted a retrospective observational study of 70 women diagnosed with AUB and treated with LNG-IUS insertion between February 2010 and 2014 at the Department of Gynecology of Sree Narayana Institute of Medical Sciences. Baseline endometrial biopsies were done before insertion of LNG-IUS, and outpatient follow-up with symptom diary was undertaken at 3-month intervals after insertion of LNG-IUS.
Results
Primary outcome in the two treatment groups was significantly greater among women assigned to levonorgestrel-IUS than among those assigned to usual treatment (mean difference in scores over the course of 1 year 13.4 points; 95 % confidence interval [CI] 9.9–16.9; P < 0.001). All six domains of the MMAS favored the levonorgestrel-IUS at every time point (P < 0.001) with the use of a test for trend.
Conclusion
In conclusion, our study showed that both the levonorgestrel-IUS and usual medical treatments reduced the adverse effect of menorrhagia on women’s lives over the course of 2 years, but the levonorgestrel-IUS was the more effective first choice, as assessed by the impact of bleeding on the women’s quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy menstrual bleeding (HMB) is clinically defined as menstrual blood loss (MBL) that is subjectively considered to be excessive by the woman and interferes with her physical, emotional, social and material quality of life.
MBL has been estimated indirectly by combining information of sanitary protection usage, flooding, clots, duration of menstruation and the woman’s personal opinion of her menstrual loss. Although this tends to be inaccurate, it is easy to undertake in clinical practice. There is substantial discordance between objective measures of menstrual blood loss and women’s perception of the amount of bleeding [1, 2]. Only about half the women with menorrhagia who present to healthcare providers have blood loss greater than the traditional clinical threshold of 80 ml per menstrual cycle [1]. Measurement of the hemoglobin level in menstrual blood collected in sanitary protective materials is inconvenient and often unacceptable for women [3]. Diary-based assessments of bleeding [4] also fail to reflect women’s experience of what is burdensome for them [5]. Clinical guidelines now advocate a shift in emphasis from the amount of menstrual blood loss to the more patient-centered definition of heavy menstrual bleeding that interferes with a woman’s physical, emotional and social life [3, 5].
In the early 1990s, it was estimated that at least 60 % of women presenting with HMB would have a hysterectomy as the first-line treatment. The majority of these hysterectomies occurred in women without structural uterine pathology. The number of hysterectomies for heavy MBL is now decreasing. This decline can be partially attributed to increased uptake of Mirena LNG-IUS and endometrial ablation treatments. NICE guideline has stated that while Mirena LNG-IUS is preferable to other medical treatments (tranexamic acid, NSAIDs, COC), this recommendation is based on indirect evidence [3].
Several non-hormonal and hormonal medical treatments are available for women with HMB. Since 2009 in the USA, and earlier in Europe, the levonorgestrel-releasing intrauterine system (levonorgestrel-IUS) (Mirena, Bayer HealthCare) has been available to treat this problem. Although developed as a contraceptive, the levonorgestrel-IUS also reduces menstrual blood loss [6]. In 2007, UK guidelines [3] introduced the option of the levonorgestrel-IUS for menorrhagia on the basis of limited evidence [7]. Updated meta-analyses, including the results of nine small, randomized trials (involving a total of 783 women) of the levonorgestrel-IUS as compared to non-hormonal and hormonal treatments, showed that the levonorgestrel-IUS resulted in a greater reduction in menstrual blood loss at 3–12 months of follow-up [6, 7]. However, it is not clear whether these short-term benefits persist, particularly since the rates of discontinuation of the levonorgestrel-IUS are as high as 28 % at 2 years [8], and the effects of this therapy on bleeding-related quality of life are not known.
The Effectiveness and Cost-Effectiveness of Levonorgestrel-Containing Intrauterine System in Primary Care against Standard Treatment for Menorrhagia (ECLIPSE) trial was a pragmatic, multicenter, randomized trial that compared the clinical effectiveness of the levonorgestrel-IUS with that of usual medical treatment in the primary care setting. Indirect comparison has shown that LNG-IUS generates more quality-adjusted life years (QALY) than other medical treatments (tranexamic acid, NSAIDs, COCP) and at a lower cost. Therefore, LNG-IUS is the recommended first-line treatment for HMB.
Methods
Patients
Women between 25 and 50 years of age who presented to their primary care physicians with menorrhagia involving at least three consecutive menstrual cycles were eligible to participate. Women were excluded if they intended to become pregnant over the next 2 years, were taking hormone replacement therapy or tamoxifen, had intermenstrual bleeding (between expected periods) or postcoital bleeding or findings suggestive of fibroids (abdominally palpable uterus equivalent in size to that at 10–12 weeks of gestation) or other disorders, or had contraindications to or a preference for either the levonorgestrel-IUS or usual medical treatments. Women with heavy, irregular bleeding were ineligible unless the results of endometrial biopsy were reported to be normal; no further investigations were mandated by the protocol. For diagnosis, we performed transvaginal sonography (TVS) and outpatient endometrial biopsy. The methods of endometrial biopsy were endometrial sampling with a catheter (66.6 %), D&C (25 %). All patients provided written informed consent.
Randomization
A minimized randomization procedure was used to achieve balance between the groups with respect to age (<35 years or ≥35 years), body mass index (BMI; the weight in kilograms divided by the square of the height in meters) (≤25 or >25), duration of symptoms (<1 year or ≥1 year), need for contraception (yes or no) and menorrhagia alone or menorrhagia accompanied by menstrual pain.
Study Interventions and Compliance
Eligible women who provided written informed consent were randomly assigned to either the levonorgestrel-IUS or usual medical treatment. Usual treatment options included mefenamic acid, tranexamic acid, a combined estrogen–progestogen or medroxyprogesterone acetate injection and were chosen by the physician and patient on the basis of contraceptive needs or the desire to avoid hormonal treatment [3, 9]. The particular medical treatment to be used was specified before randomization. Subsequently, treatments could be changed (from one medical treatment to another, from the levonorgestrel-IUS to medical treatment or from medical treatment to the levonorgestrel-IUS) or could be discontinued because of a perceived lack of benefit, side effects, referral for endometrial ablation or hysterectomy, or other reasons, according to usual practice [3, 9].
Outcome Measures and Follow-Up
The primary outcome measure was the condition-specific Menorrhagia Multi-Attribute Scale (MMAS) [10, 11], which is designed to measure the effect of menorrhagia on six domains of daily life (practical difficulties, social life, psychological health, physical health, work and daily routine, and family life and relationships). Summary scores, which range from 0 (severely affected) to 100 (not affected), were assessed at 3, 6, 12 and 24 months. The MMAS has a high degree of reliability and internal consistency [11], has good content and construct validity [11, 12], is responsive [13, 14] and is acceptable to respondents [10, 11, 13, 14].
Secondary outcome measures included general health-related quality of life and sexual activity. To assess quality of life, we used three instruments: the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36), version 2 (with scores ranging from 0 [severely affected] to 100 [not affected]).The validated Sexual Activity Questionnaire measures pleasure (with scores ranging from 0 [lowest level] to 18 [highest level]), discomfort (with scores ranging from 0 [greatest] to 6 [none]) and frequency (assessed relative to perceived usual activity as an ordinal response) [15]. Scores were obtained before randomization and by mail at 6 months, 1 and 2 years after randomization. Data were collected from participating clinicians regarding all serious adverse events, defined as adverse events that resulted in death, disability or hospitalization. Patients were also asked to report any hospitalizations and adverse events leading to discontinuation of the study drug.
Study Oversight
Study oversight was provided by an independent steering committee and an independent data and safety monitoring committee, whose three reviews of interim data provided no reason to modify the trial protocol on the basis of pragmatic stopping criteria. The study was conducted in accordance with the protocol. The manufacturers of the levonorgestrel-IUS and other therapeutic agents used in the study were not involved in any aspect of the trial.
Statistical Analysis
The study was designed for 90 % power (at P < 0.05) to detect small-to-moderate (0.3 SD) differences in the primary outcome at any one time point. This required an enrollment of 70 patients. Primary analyses were performed according to the intention-to-treat principle. All available data were included in this analysis. Changes from baseline scores within treatment groups were compared with the use of paired t tests. Effect sizes are presented with 95 % confidence intervals and two-sided P values.
Results
Patients and Follow-Up
Between February 2010 and August 2015, a total of 70 women with menorrhagia were randomly assigned to either the levonorgestrel-IUS 35 women or usual medical treatment (35 women) (Table 1).
In the women assigned to usual medical treatment, the initial prescription was for mefenamic acid, tranexamic acid or a combination of the two drugs. Women in the levonorgestrel-IUS group were almost twice as likely as those in the usual treatment group to still be receiving their assigned treatment at 2 years. The most common reasons cited for discontinuation of the levonorgestrel-IUS were lack of effectiveness (37 %) and irregular or prolonged bleeding (28 %). The most common reason for discontinuation of usual medical therapy was lack of effectiveness (53 %). There was no significant difference between the two groups in the frequency of serious adverse events.
Primary Outcome
Total scores on the MMAS improved significantly in both groups at 6 months and at 1 year, as compared to baseline scores Fig. 1.
Primary outcome in the two treatment groups include responses to the individual domains of the survey, but improvements in these scores were significantly greater among women assigned to levonorgestrel-IUS than among those assigned to usual treatment (mean difference in scores over the course of 1 year 13.4 points; 95 % confidence interval [CI] 9.9–16.9; P < 0.001). All six domains of the MMAS favored the levonorgestrel-IUS at every time point (P < 0.001 with the use of a test for trend).
In a sensitivity analysis that excluded women who crossed over from the assigned treatment to the other study treatments, improvement with the levonorgestrel-IUS, as compared to usual medical treatment, increased (mean difference in scores over the course of 1 year 17.8 points; 95 % CI 14.1–21.5; P < 0.001). Other sensitivity analyses yielded results that were not materially different from the results of the primary analysis (P < 0.001 for all comparisons).
In subgroup analyses, there was a significant interaction between treatment and BMI (P = 0.004). The benefit of the levonorgestrel-IUS was greater in women with a BMI above 25 (16.7 MMAS points; 95 % CI 12.6–20.9; P < 0.001) than in those with a BMI of 25 or less (5.4 MMAS points; 95 % CI −1.0 to 11.8; P = 0.10). This finding appeared to be attributable to the superior outcome with usual medical treatment in leaner women. Improvements with the levonorgestrel-IUS were similar in both subgroups. None of the other tests for subgroup interaction were significant.
General Quality of Life and Sexual Activity
SF-36 domains were generally significantly improved from baseline in both groups at all time points, although the scores for women in the levonorgestrel-IUS group were better than for those in the usual treatment group in seven of the eight domains in the analysis over all time points.
The mental health was the only domain for which there were no significant differences between Scores on the Quality of Life and Sexual Activity at Baseline and over 2 years. The improvements appeared to be greatest at 6 months but had lessened by the 2-year follow-up assessment. No significant differences were seen between treatments with respect to the EQ-5D instrument; scores were significantly improved from baseline in both groups at 2 years but not at earlier assessments. Nor did the treatments differ significantly with respect to the scores for the pleasure, discomfort and frequency domains of the Sexual Activity Questionnaire.
Surgical Interventions
The frequency of surgical interventions for heavy menstrual bleeding within 2 years did not differ significantly between the two groups. Hysterectomy was performed in 6 % of the women in each group (P = 0.44).
Discussion
The LNG-IUS releases a therapeutic daily dose of levonorgestrel (20 μg/day) for 5 years (Fig. 2) [16]. This results in the high local LNG concentrations that cause uniform suppression of endometrial proliferation, inactive histology, thin epithelium and decidualization of the stroma. LNG-IUS decreases the menstrual blood loss and pain by the suppression of endometrial proliferation. Kriplani et al. [17] evaluated the efficacy, acceptability and possible side effects of LNG-IUS for menorrhagia and concluded that LNG-IUS is an effective and well-accepted option in the medical management of menorrhagia. A significant decrease in the mean number of bleeding days at 1 month was observed in women with menorrhagia, and the decrease continued with treatment duration.
The LNG-IUS inhibits endometrial proliferation, thickens cervical mucus and suppresses ovulation. The licensed and non-licensed uses are indicated in the table.
The main side effect, often cited as the reason for discontinuation, is erratic spotting. This tends to subside 3–6 months from insertion. After 1 year of usage, there is a 71–95 % reduction in objectively measured MBL and around 50 % women have amenorrhea.
The results of this trial show that levonorgestrel-IUS, as compared to usual medical therapies for menorrhagia, leads to greater improvement in women’s assessments of the effect of heavy menstrual bleeding on their daily routine, including work, social and family life, and psychological and physical well-being.
At baseline, the women were substantially affected by heavy menstrual bleeding, as assessed with the use of condition-specific (MMAS) and general (SF-36) health-related scales. The scores improved significantly over a period of 2 years in both the levonorgestrel-IUS group and the usual treatment group. However, improvements in average scores and residual symptoms for all six MMAS domains were greater with the levonorgestrel-IUS than with usual medical treatment. The average between-group difference in the overall MMAS score over 1 years of follow-up was 13.4 points, with greater improvement in the levonorgestrel-IUS group than in the usual treatment group—a difference that was both statistically significant and clinically meaningful. The between-group difference was more than 0.5 SD, which is the minimum clinically important difference identified in a systematic review of studies reporting such data for health-related quality-of-life measures [11]. A 13.4-point difference represents a change in two or three MMAS domains: from being substantially to minimally affected by menorrhagia (e.g., from frequent to occasional disruptions of work and daily routine) or from being minimally affected to being unaffected (e.g., from experiencing some strain in family life to experiencing no strain in family life). The between-group difference reported here is also greater than that reported in an observational study comparing women who did and those who did not undergo surgery for menorrhagia [18].
The strengths of our randomized trial include its size (larger than prior trials of treatments for heavy menstrual bleeding), the multicenter design, the inclusion of patients ethnically representative of the UK population, the relatively low rates of loss to follow-up and the assessment of outcomes over a period of 2 years rather than 6 or 12 months, as in previous studies [13, 14]. In addition, previous trials have focused on the reduction in menstrual blood loss, which does not reflect the full effect of menorrhagia on women’s lives [13, 14]. In contrast, our primary outcome measure was the patient-reported, psychometrically valid, condition-specific MMAS, which better reflects women’s personal experience of the burden of menorrhagia. Interference with the quality of life, rather than perceptions of heavy menstrual bleeding itself, appears to be the primary factor in women’s decision to seek treatment [19].
Some limitations of our study should be noted. The range of options available for medical treatment complicates any efforts to compare the levonorgestrel-IUS with individual agents. However, the choice among the various agents is representative of current clinical practice.
The improvement from baseline in the average MMAS score at 6 months in the usual treatment group, which was sustained throughout the years of follow-up, was not explained by a switch in treatment, since similar improvements were noted when crossovers to the levonorgestrel-IUS were excluded from the analyses. The higher rate of discontinuation in the usual treatment group than in the levonorgestrel-IUS group could reflect greater symptom relief with levonorgestrel-IUS, but another possible explanation is that discontinuation of usual medical treatment does not require consultation. Nonetheless, at 2 years, 36 % of women in the levonorgestrel-IUS group had had the system removed, generally owing to lack of effectiveness or to irregular or prolonged bleeding, which are well-recognized reasons for discontinuing the levonorgestrel-IUS [20, 21]. This proportion is consistent with the proportions of women who discontinued levonorgestrel-IUS treatment in smaller trials that compared it with hysterectomy [22] (31 % of 117 women at 12 months).
In subgroup analyses, the levonorgestrel-IUS appeared to be less beneficial in women with a BMI of 25 or less than in those with a BMI of more than 25, an observation that was explained by an apparently greater efficacy of usual medical treatments in the leaner women. This analysis was one of several subgroup analyses and should be interpreted with caution, since the findings may be explained by chance and require confirmation.
We expected fewer surgical interventions in the levonorgestrel-IUS group, but rates were similarly low in the two groups. This finding may reflect the eligibility criteria for the trial, since women who had fibroids or other disorders were excluded.
Finally, given the long natural history of menorrhagia, study outcomes need to be assessed over a period that is longer than 2 years; additional intention-to-treat analyses are planned at 5 and 10 years.
In conclusion, our study showed that both the levonorgestrel-IUS and usual medical treatments reduced the adverse effect of menorrhagia on women’s lives over the course of 2 years, but the levonorgestrel-IUS was the more effective first choice, as assessed by the impact of bleeding on the women’s quality of life.
References
Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol. 1999;82:73–6.
Warner P, Critchley HO, Lumsden MA, et al. Referral for menstrual problems: cross sectional survey of symptoms, reasons for referral, and management. BMJ. 2001;323:24–8.
National Collaborating Centre for Women’s and Children’s Health. Heavy menstrual bleeding. London: Royal College of Obstetricians and Gynaecologists; 2007 (guideline CG44).
Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97:734–9.
Santer M, Wyke S, Warner P. What aspects of periods are most bothersome for women reporting heavy menstrual bleeding? Community survey and qualitative study. BMC Womens Health. 2007;7:8.
Endrikat J, Vilos G, Muysers C, et al. The levonorgestrel-releasing intrauterine system provides a reliable, long-term treatment option for women with idiopathic menorrhagia. Arch Gynecol Obstet. 2012;285:117–21.
Stewart A, Cummins C, Gold L, et al. The effectiveness of the levonorgestrel-releasing intrauterine system in menorrhagia: a systematic review. BJOG. 2001;108:74–86.
Middleton LJ, Champaneria R, Daniels JP, et al. Hysterectomy, endometrial destruction, and levonorgestrel releasing intrauterine system (Mirena) for heavy menstrual bleeding: systematic review and meta-analysis of data from individual patients. BMJ. 2010;341:c3929.
Frick KD, Clark MA, Steinwachs DM, et al. Financial and quality-of-life burden of dysfunctional uterine bleeding among women agreeing to obtain surgical treatment. Womens Health Issues. 2009;19:70–8.
Shaw RW, Brickley MR, Evans L, et al. Perceptions of women on the impact of menorrhagia on their health using multi-attribute utility assessment. Br J Obstet Gynaecol. 1998;105:1155–9.
Pattison H, Daniels JP, Kai J, et al. The measurement properties of the menorrhagia multi-attribute quality-of-life scale: a psychometric analysis. BJOG. 2011;118:1528–31.
Matteson KA, Boardman LA, Munro MG, et al. Abnormal uterine bleeding: a review of patient-based outcome measures. Fertil Steril. 2009;92:205–16.
Habiba M, Julian S, Taub N, et al. Limited role of multi-attribute utility scale and SF-36 in predicting management outcome of heavy menstrual bleeding. Eur J Obstet Gynecol Reprod Biol. 2010;148:81–5.
Protheroe J, Bower P, Chew-Graham C, et al. Effectiveness of a computerized decision aid in primary care on decision making and quality of life in menorrhagia: results of the MENTIP randomized controlled trial. Med Decis Mak. 2007;27:575–84.
Thirlaway K, Fallowfield L, Cuzick J. The Sexual Activity Questionnaire: a measure of women’s sexual functioning. Qual Life Res. 1996;5:81–90 (Erratum, Qual Life Res 1997;6:606).
Sitruk-Ware R, Inki P. The levonorgestrel intrauterine system: long-term contraception and therapeutic effects. Womens Health (Lond Engl). 2005;1:171–82.
Kriplani A, Singh BM, Lal S, et al. Efficacy, acceptability and side effects of the levonorgestrel intrauterine system for menorrhagia. Int J Gynaecol Obstet. 2007;97:190–4.
Habiba M, Julian S, Taub N, et al. Limited role of multi-attribute utility scale and SF-36 in predicting management outcome of heavy menstrual bleeding. Eur J Obstet Gynecol Reprod Biol. 2010;148:81–5.
Shapley M, Blagojevic M, Jordan K, et al. The spontaneous resolution of heavy menstrual bleeding in the perimenopausal years. BJOG. 2012;119:545–53.
Shaw RW, Symonds IM, Tamizian O, et al. Randomised comparative trial of thermal balloon ablation and levonorgestrel intrauterine system in patients with idiopathic menorrhagia. Aust N Z J Obstet Gynaecol. 2007;47:335–40.
Ewies AA. Levonorgestrel-releasing intrauterine system—the discontinuing story. Gynecol Endocrinol. 2009;25:668–73.
Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273–27724.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I declare that I have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Dr. Georgy Joy Eralil, MBBS, MS DGO, MRCOG, is working as an associate professor in Department of Obstetrics and Gynaecology, Sree Narayana Institute of Medical Sciences.
Appendix
Rights and permissions
About this article
Cite this article
Eralil, G.J. The Effectiveness of Levonorgestrel-Releasing Intrauterine System in the Treatment of Heavy Menstrual Bleeding. J Obstet Gynecol India 66 (Suppl 1), 505–512 (2016). https://doi.org/10.1007/s13224-016-0865-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13224-016-0865-3